
Performance of a Mixed Rearing of Parachanna obscura (Gunther, 1861) Post-larvae and Natural Prey Dominated By Copepods and Rotifers in a Semi-Controlled Environment
*Corresponding Author(s):
Nana Towa AlgrientDepartment Of Forestry, Faculty Of Agronomy And Agricultural Sciences, University Of Dschang, Po Box: 222 Dschang, Cameroon
Email:algrient@yahoo.fr
Abstract
With a view to diversify species for a sustainable aquaculture and the preservation of biodiversity, the study of the performance of a mixed rearing of Parachanna obscura post-larvae and zooplankton was carried out. Thus, 117 Parachanna obscura with an average weight of 0.46 ± 0.11 g and an average total length of 5 ± 0.5 cm were reared in basins containing zooplankton. The post-larvae were divided into three treatments T1 (rotifers), T2 (copepods) and T3 (rotifers and copepods). Data on zooplankton were collected every 7 days and those on post-larvae every 15 days. The results relating to the dynamics of zooplankton in the environment were affected by the type of association. Thus, the highest zooplankton density was observed at T1 (287485ind/l). No significant difference was obtained between the treatments in terms of survival rate and cannibalism. However, the highest values of all the growth characteristics of Parachanna obscura were recorded in cultured post-larvae subjected to the T2 treatment. Parachanna obscura post-larvae seem to show a preference for copepods.
Keywords
Mixed-rearing; Parachanna Obscura; Post-larvae; Zooplankton
Introduction
The development of fish farming through the diversification of farmed species is one of the sustainable solutions in aquaculture. This diversification of species must go through the domestication of endogenous species in order not only to preserve endogenous species and limit certain environmental risks, but also to best contribute to the requirements of local markets. One of the potential species for aquaculture in Africa (Nigeria, Cameroon etc.) is the snakehead fish Parachanna obscura for its high-quality flesh and high nutritional value. African snakehead, P. obscura flesh is very much appreciated by African consumers. It has high economic value for aquaculture since it has better growth rate (2 g/day), few bones, tasty flesh, accepts high stocking density and can use the atmospheric oxygen for respiration [1-4]. However, its production nowadays depends more than 95% on capture in the natural environment. Thus, in order to reduce the pressure exerted on Parachanna obscura in waterways, it is necessary to contribute to its domestication. One of the stages of this domestication involves the study of its food ethology and its functional characteristics in captivity, especially the rearing phase in a semi-controlled environment. Because one of the main obstacles to the development of aquaculture is the availability of fingerlings. The optimal conditions for the success of larval rearing must be studied both in the wild and in captivity. The work of Couternay and Williams [5] showed that in the natural environment, the juveniles of Parachanna obscura feed on insects, shrimps and zooplankton, in particular copepods. Zooplankton is a set of animal microorganisms playing a key role in the development and growth of larvae and juvenile fish due to its high protein content (54%) [6,7]. Zooplankton, in particular rotifers, cladocerans, copepods (especially Cyclopoidae) are the groups most consumed by freshwater fish larvae. But the consumption or preference of each group of zooplankton may vary depending on the availability of prey and also the species of fish and their size.
Despite the studies carried out on the protein and lipid requirements [8] and the carbohydrate requirements [9] of P. obscura fry, no study has focused on the ethology of post-larvae and the dynamics of zooplankton in mixed culture. It is in this perspective that this work was initiated with the general objective of contributing to the improvement of the production of Parachanna obscura and in particular of fry. More specifically, to evaluate the effect of post-larvae of Parachanna obscura on the dynamics of prey (Rotifers and Copepods) and the influence of the types of prey on their growth performance, survival rate and cannibalism.
Material And Methods
Study period and area
The trial was conducted from May to July 2022 (in 35 days) at the Applied and Research Farm (FAR) and at the Ichthyology and Applied Hydrobiology Unit of the Faculty of Agronomy and of Agricultural Sciences of the University of Dschang. The city of Dschang is located in the agro-ecological zone of the Western Highlands - Cameroon (5°36' and 5°44' LN; 10°06' and 10°22' LE; altitude 1392 -1396 m). The annual rainfall varies between 1500 and 2000 mm, with a rainy season which extends from March to November and a dry season from November to March.
Animal material
- Origin of post-larvae
A total of 117 Parachanna obscura post-larvae with an average weight of 0.46 ± 0.11 g and a total length of 5 ± 0.5 cm were used (Figure 1). They were captured by fish farmers in the wild, more specifically in streams of the Sangmélima area. They were transported in a perforated 20l plastic container to the FAR of the University of Dschang. These post-larvae were acclimatized for 14 days in concrete tanks.
 Figure 1: Parachanna Obscura post-larva.
Figure 1: Parachanna Obscura post-larva.
- Origin and collection of zooplankton: Rotifers and Copepods
The zooplankton was collected in a dirivated pond of the farm of the University of Dschang according to the methodology described by Agadjihouédé et al. [10]. To this end, 200 liters of water were collected and filtered using a plankton net with a 50 µm mesh opening. Two liters of sample was retained and transported to the Ichthyology and Applied Hydrobiology laboratory for identification, isolation and counting of the two groups of zooplankton (Rotifers and Copepods) which will be used to seed the production medium. After homogenization of the previously filtered sample, a sub-sample of 10 ml was taken using a calibrated pipette and introduced into a Petri dish 90 mm in diameter squares with 5 mm sides. The identification of rotifers and copepods was based on the observation of morphological characters (body shape, size, presence or absence of antennae, abdomen, appendix, shell).
Rotifer and copepod species were isolated using a pipette and introduced into jars containing zooplankton-free water (Figure 2). Then a sub-sample of isolated rotifers and copepods was fixed in 5% formalin to count the density of each group to be seeded in the production tanks.
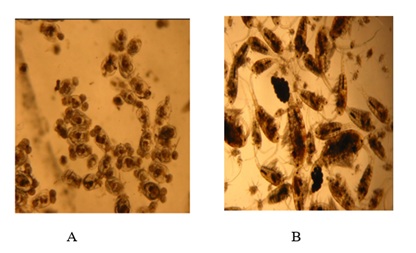 Figure 2: Culture of rotifers (A) and copepods (B).
Figure 2: Culture of rotifers (A) and copepods (B).
Conduct of the study
The test was carried out in 12 plastic tanks (circular shape, volume 30 L, depth 0.4 m) randomly arranged in a greenhouse (2 m high, 2.5 m wide and 8 m long) made of white polyane (Figure 3A). Each of the tanks (Figure 3B) received 20 L of water from the borehole (not containing zooplankton). At the start of the study, the tanks were fertilized with pig manure at a dose of 10 g/20 L as the base fertilization [11] and a third (1/3) of this dose every week as maintenance fertilization. The pig manure was tied in a mosquito net and immersed in water in order to reduce the deposit and/or suspended solids in the water.
 Figure 3: Greenhouse (A) and production tanks (B).
Figure 3: Greenhouse (A) and production tanks (B).
Two days after fertilization, each tank received 400 ml of the phytoplankton collected in a fertilized tank. A sample of phytoplankton water was observed under a binocular magnifying glass in order to be reassured of the absence of zooplankton. Five days after phytoplankton inoculation, each production tank received one of the following rotifer and copepod seeding densities: T1: 1 246 individuals of Rotifer (R); T2: 1 246 individuals of Copepods (C) and T3: 623 individuals of Rotifers (R) + 623 individuals of Copepods (C). Seven days later, the tanks were stocked with the post-larvae of Parachana obscura at a rate of 0.65 ind/litre, i.e., 13 post-larvae.
Note that among the groups of zooplankton collected (rotifers and copepods) a few eggs of cladocerans and ostracods were found in our samples. The presence of these two other groups was confirmed by their development one week after inoculation of the basins. But, despite their presence, the development of rotifers and copepods was dominant in the tanks until the end of the study.
Data collection and characteristics studied
The main data collected related to the physicochemical characteristics of water, rotifers and copepods, and the growth performance of Parachanna obscura.
- Evaluation of the physicochemical characteristics of water
Data on the physicochemical characteristics of the water (Table 1) were collected in situ. The temperature, pH, and conductivity were measured every 2 days between 6 and 8 a.m. using a multi-parameter brand "Water Quality Tester". Nitrites and nitrates were measured every 14 days using the strips.
|
Characteristics |
Treatments |
||
|
T1 |
T2 |
T3 |
|
|
TM (°C) |
24.94±0.12 |
24.87±0.13 |
24.87±0.14 |
|
pH |
8.01±0.67 |
7.58±0.53 |
7.99±0.34 |
|
Cond (ppm) |
51.00±1.20 |
49.80±0.60 |
51.20±1.50 |
|
NO3- (mg/L) |
1<NO3<3 |
1<NO3<3 |
1<NO3<3 |
|
NO2- (mg/L) |
0<NO2<2 |
0<NO2<2 |
0<NO2<2 |
Table 1: Physicochemical parameters of water in the environment.
Collection of rotifers and copepods
Zooplankton were collected every 7 days. A one-liter water sample was collected and filtered through a 50 µm mesh plankton net. Then, a 10 mL sub-sample concentrated in zooplankton was retained, labelled, fixed in 5% formalin and stored in 30 ml plastic bottles for quantitative and qualitative analyzes in the laboratory.
In the laboratory, after homogenization of the 10 mL of sample taken, a sub-sample of 5 mL was taken using a calibrated pipette and introduced into a Petri dish 90 mm in diameter. Counting was done using a binocular magnifying glass (Motic with 2X objective).
- Collecting Parachanna obscura growth data
Data relating to the growth of Parachanna obscura were collected every 15 days on 1/3 of the number of fish in each tank during control fishing.
- Characteristics studied
(1) Density (D) of rotifers and copepods
It is defined as the numerical abundance of organisms in an ecosystem. It is calculated using the following Cacot et al. [12] formula:
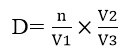
Where, n= number of individuals counted v1= volume of filtrate sampled; v2 = volume of the concentrated filtrate; v3 = volume of filtered water
(2) Daily production (P) of copepods and rotifers
P = (Nt – No)/t,
Where, No = initial number (L) and Nt = final number (L), t = duration of production.
(3) Growth characteristics of Parachanna obscura post-larvae
a. Weight gain (g)
It is used to assess weight gain during breeding. It is calculated by the following formula:
WG = Wf-Wi or Wi=Initial average weight (g) and Wf= Final average weight (g)
b. Specific growth rate
It is used to assess the weight gained by the fish each day as a percentage of its live weight. It is calculated by the following formula:
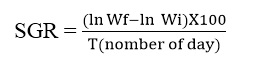 Where, ln= natural logarithm
Where, ln= natural logarithm
c. Condition factor K
This factor gives a good idea of the body condition of the fish, i.e., the relative importance of body mass in relation to its length. It is calculated by the following formula:
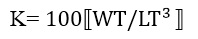
Where, WT = Total weight (g) LT = Total length (cm)
d. Survival rate (SR)
Survival rate = ((Initial number of fish-mortality) / (Initial number of fish)) X 100
e. Cannibalism rate (CR)
The rate of cannibalism (CR) makes it possible to evaluate cannibalism of type 1 and 2 according to the formulas:
CR1= 100 × (Number of mutilated dead fry)/ (Initial total number)
CR2= 100 × (Number of fry having disappeared)/ (Initial total number)
f. Mortality rate (MR)
It was calculated to assess mortality according to the formula:
MR= 100 × (Number of dead fry)/ (Initial total number)
Statistical analysis
The data collected were recorded, organized and coded in an Excel 2010 spreadsheet. They were subjected to the analysis of variance (ANOVA) with 1 factor. Duncan's test at the 5% threshold was used to separate means in the event of a significant difference between the treatments. Analyzes were performed using SPSS (Statistical Package for Social Sciences) version 21.0 statistical software.
Results
Effect of Parachanna obscura post-larvae on prey dynamics
The effect of Parachanna obscura post-larvae on prey dynamics is summarized (Table 2) and illustrated (Figure 4). It emerges that the density as well as the daily production of prey were significantly (p < 0.05) highest in treatment T1; but otherwise, comparable to those of the T3 treatment.
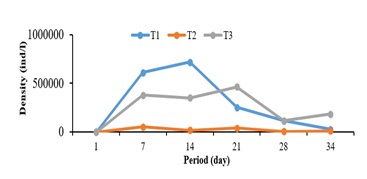 Figure 4: Evolution of the density of the type of natural prey in culture with post-larvae of Parachanna obscura.
Figure 4: Evolution of the density of the type of natural prey in culture with post-larvae of Parachanna obscura.
|
Characteristics |
Treatments |
||
|
T1 |
T2 |
T3 |
|
|
Initial density (ind/L) |
62 |
62 |
62 |
|
Final densité (ind/L) |
287 485a |
22 430b |
248 541ab |
|
P (ind/L/j) |
10 265a |
799b |
8 874ab |
Table 2: Prey density and production in mixed rearing with Parachanna obscura.
a,b; the means with the same letters are not significantly different (p>0.05).
The evolution of prey density according to the type of association with Parachanna obscura post-larvae generally shows a different trend and pace of evolution between the densities recorded in the T1, T2 and T3. treatments (Figure 4). Regardless of the treatment considered, a drop in density phase was observed from the 21st day. However, the density curve of the T2 treatment (copepods) remained the lowest whatever the period of the study.
Note: T1 = Rotifers; T2 = Copepods; T3= Rotifers +Copepods
Effect of prey type on growth performance of Parachanna obscura post-larvae
The influence of the type of culture on the growth characteristics of the post-larvae of Parachanna obscura (Table 3) appears that no significant difference (p>0.05) was recorded between the treatments for all characteristics considered. However, the highest values of all the characteristics were recorded in cultured post-larvae subjected to the T2 treatment.
|
Growth characteristics |
Treatments |
||
|
T1 |
T2 |
T3 |
|
|
Wi (g) |
0.46 ± 0.11 |
0.46 ± 0.11 |
0.46 ± 0.11 |
|
Wf (g) |
0.73±0.12 |
0.77±0.10 |
0.71±0.24 |
|
WG (g) |
0.25±0.10 |
0.30±0.51 |
0.28±0.74 |
|
SGR (%/j) |
1.63±1.28 |
1.83±0.21 |
1.51±0.72 |
|
TL (cm) |
5.17±0.60 |
6.40±1.49 |
5.87±0.98 |
Table 3: Growth characteristics of post-larvae of Parachanna obscura.
T1 = Rotifers; T2 = Copepods; T3= Rotifers +Copepods
Evolution of average weight and total length
The evolution of the average weight and the total length of the post-larvae of Parachanna obscura (Figure 5) generally shows a trend of regular and comparable evolution between the post-larvae subjected to the different treatments. However, the highest values for both average weight and total length were recorded with treatment T2 (copepods) whatever the period of the study and the lowest at the end of the study with treatment T1(rotifers).
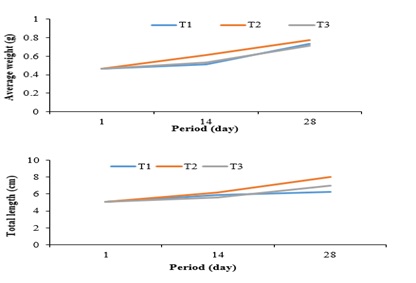 Figure 5: Evolution of the average weight and total length of Parachanna obscura post-larvae.
Figure 5: Evolution of the average weight and total length of Parachanna obscura post-larvae.
Note: T1 = Rotifers; T2 = Copepods; T3= Rotifers +Copepods.
Evolution of the weight gain of the post-larvae of Parachanna obscura
The weight gain of the post-larvae of Parachanna obscura according to the type of prey (Figure 6) showed that from the 1st to the 14th day, the weight gain was higher in the post-larvae subjected to the T2 treatment. (copepods) and lower in those exposed to T1 treatment (rotifers). On the other hand, the opposite was recorded from the 15th to the 28th day.
Survival, mortality and cannibalism performance of Parachanna obscura post-larvae
The survival (ST), mortality (TM) and cannibalism (TC) rates of Parachanna obscura post-larvae summarized (Table 4) show that no significant difference (p>0.05) was observed between the different treatments.
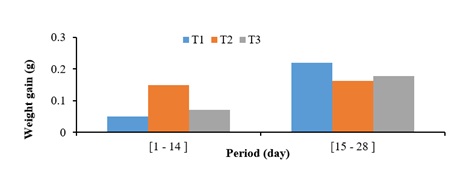 Figure 6: Average weight gain of post-larvae of Parachanna obscura according to the type of prey.
Figure 6: Average weight gain of post-larvae of Parachanna obscura according to the type of prey.
|
Characteristics |
Treatments |
||
|
T1 |
T2 |
T3 |
|
|
SR (%) |
75.66±23.11 |
76.66±25.77 |
76.00±24.51 |
|
MR (%) |
8.33±8.02 |
9.33±10.06 |
9.00±9.00 |
|
CR (%) |
16.00±15.09 |
14.00±16.37 |
15.33±15.50 |
Table 4: Survival, mortality and cannibalism performance of Parachanna obscura post-larvae.
T1 = Rotifers; T2 = Copepods; T3= Rotifers +Copepods
SR= Survival rate, MR= mortality rate and CR= cannibalism rate
Discussion
The mastery of larval rearing is a key step for the success of the domestication of species in aquaculture. The results show that the density of copepods and rotifers cultured with post-larvae of Parachanna obscura varied from one treatment to another with a phase of colonization and decolonization. Despite the presence of Parachanna obscura post-larvae in the rearing medium, an increase in prey density was recorded until day 14 in the T1 treatment and on the 21st day in the T3 treatment. These results could be linked to the development cycle of zooplankton, in particular rotifers. Indeed, it has been demonstrated by several authors [6] that zooplankton in culture medium always present a phase of colonization followed by that of decolonization. The low density of copepods in the T2 treatment could be justified by the fact that they were more appreciated and consumed by the post-larvae of Parachanna obscura. The decrease in prey density at T3 treatment could conclude that the post larvae of Parachanna obscura also consume rotifers.
This observation confirms that P. obscura is an opportunistic predator that feeds on all available prey in the environment in which it is located [13]. But in our breeding environment, Parachanna obscura seems to have a preference for large natural prey such as copepods. These results corroborate those of Gogbe et al. [13] who observed the presence in the stomach contents of P. obscura the presence of zooplankton. The very low densities of natural prey after the 21st day despite maintenance fertilization would result from heavy consumption by post-larvae.
The results showed that the dominance of a particular type of natural prey (rotifers or copepods) in the culture medium of the post-larvae of Parachanna obscura did not significantly affect the growth characteristics. However, the highest mean weight and total length values were obtained from Parachanna obscura post-larvae subjected to the copepod-dominated prey treatment. This result confirms the hypothesis specified above that Parachanna obscura presents a high consumption of copepods which have a large size compared to rotifers. The high weight gain after 2 weeks at T1 is explained by the high density of rotifers present (287.485 ind/l) linked to their high recolonization rates in the first 6 days [6]. The decline in the growth characteristics of the post-larvae of Parachanna obscura at the T2 treatment from the 15th day would be due to the low density of copepods necessary for their diets.
The survival rate of Parachanna obscura post-larvae observed at the end of the test is less than 100% observed by Kpoguè et al. [9] in a controlled environment in larvae. This result can be explained first of all by the conditions of the breeding environment, namely the physicochemical characteristics of the environment, in this case the pH which varied from 7.77 to 8.04 was higher than the tolerance value of Parachanna obscura (6.5–7.5) as reported by Riehl and Baensch [14].
This increase in pH would be due to the action of phytoplankton which consumes huge amounts of C02 in the environment. This difference could also be explained by the low quantity of food (zooplankton) in the medium, since the high mortalities obtained between the 14th and the 28th day of the test are in phase with the drop in the density of zooplankton in the environment.
The high rate of cannibalism after 15 days would also be due to the low density of prey in the rearing environment and therefore would have stimulated aggressive behavior between individuals.
Conclusion
In sum, prey dynamics were influenced by the presence of Parachanna obscura post-larvae. Thus, rotifers despite their decrease over time were higher in the rearing environment showing that the post-larvae of Parachanna obscura consume all zooplankton prey but have a preference for copepods.
Growth performance of Parachanna obscura post-larvae was not significantly affected by prey type. However, the greatest weight and length gains were recorded in post-larvae subjected to the copepod-dominated environment.
Survival, cannibalism and mortality rates were not affected by prey type. Nevertheless, the highest survival rate was observed in post-larvae subjected to the environment dominated by copepods.
References
- Micha JC (1974) Fish populations study of Ubangui river: Trying local wild species for fish culture. Aquaculture 4: 85-87.
- Victor R, Akpocha BO (1992) The biology of snakehead, Channa obscura (Gunther), in a Nigerian pond under monoculture. Aquaculture 101: 17-24.
- Bassey AU, Ajah PO (2010) Effect of three feeding regimes on growth, condition factor and food conversion rate of pond cultured Parcahanna obscura (Gunther, 1861) (Channidae) in Calabar, Nigeria. Turkisch Journal of Fishenies and Aquatitic Sciences 10: 195-202.
- Bolaji BB, Mfon TU, Utibe DI (2011) Preliminary study on the aspects of the biology of snakehead fish Parachanna obscura (Günther) in a Nigerian wetland. African Journal of Food and Agriculture Nutrition Development 11: 4708-4717.
- Couternay WR, Williams JD (2004) Snakeheads (Pisces, Channidae): A Biological Synopsis and Risk Assessment.
- Agadjihouédé H, Bonoun CA, Montchowui E, Laleye PA (2011) Recherche de la dose optimale de fiente de volaille pour la production spécifique de zooplancton à des fins piscicoles. Cahiers Agricultures 20: 247-260.
- Nana TA (2018) Effets des doses de la fiente de poule et de lisier de porc sur la biodiversité et la dynamique des populations de phytoplancton et de zooplancton en étang en zone soudano-guinéenne d’altitude de l’Ouest Cameroun.
- Kpoguè GNSD, Ayanou AG, Toko II, Mensah AG, Fiogbe DE (2013) Influence of dietary protein levels on growth, feed utilization and carcass composition of snakehead, Parachanna obscura (Günther, 1861) fingerlings. International Journal of Fisheries and Aquaculture 5: 71-77.
- Kpoguè GNSD, Dakpogan BH, D’almeida AFM, Vodounnou JVDS, Aissetche G, et al. (2018) Effet de la densité de mise en charge sur les performances zootechniques et la production chez les alevins de Parachanna obscura élevés en milieu contrôlé. Journal of Applied Biosciences 128: 12883-12890.
- Agadjihouédé H, Montchowui E, Chikou A, Laleye PA (2010) Libération comparée de sels dans l’eau par la minéralisation de l’azola, la bouse de vache, la fiente de volaille et les sons de riz et de mais utilisés en pisciculture. International Journal of Biological and Chemical Sciences 5:1883-1897.
- Songmo B, Nana TA, Efole ET, Tchoumboue (2018) Influence of pig dung dose on zooplankton productivity in microcosm. International Journal of Fisheries and Aquatic Research 3: 28-34.
- Cacot P, Mikolasek O, Nguenga D (2007) Contribution à l’amélioration de la production d’alevins au Cameroun: Essais de reproduction et d’élevage de nurserie avec Clarias gariepinus et deux autres espèces. CIRAD Rapport de Mission effectuée du 7.
- Gogbe ZM, Blahoua KG, Djadji ELG, et N’Douba V (2017) Habitudes alimentaires de Parachanna obscura (Günther, 1861) dans un lac de barrage hydroélectrique ouest-africain: lac d’Ayamé 2, Côte d’Ivoire. Afrique Science 13: 248-260.
- Riehl R, Baensch HA (1991) Aquarien Atlas. Band. 1. Melle: Mergus, Verlag für Natur-und Heimtierkunde. Germany 992.
Citation: Algrient NT, Alida DF, Léclair SB, Emmanuel KTJ, Thomas EE (2023) Performance of a Mixed Rearing of Parachanna obscura (Gunther, 1861) Post-larvae and Natural Prey Dominated By Copepods and Rotifers in a Semi-Controlled Environment. J Aquac Fisheries 7: 076.
Copyright: © 2023 Nana Towa Algrient, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

