
Pericytes and Resident Perivascular Macrophages Play a Key Role in the Development of Enlarged Perivascular Spaces in Obesity, Metabolic Syndrome and Type 2 Diabetes Mellitus
*Corresponding Author(s):
Melvin R. HaydenDepartment Of Internal Medicine, Endocrinology Diabetes And Metabolism, Diabetes And Cardiovascular Disease Center, University Of Missouri School Of Medicine, One Hospital Drive, Columbia, MO 65211, United States
Tel:573 346 3019,
Email:mrh29pete@gmail.com
Abstract
Pericyte(s) (Pcs) and resident perivascular macrophages (rPVMΦs) are positioned perfectly in the neurovascular unit (NVU) and perivascular spaces (PVS) to facilitate metainflammation that results in brain endothelial cell activation and dysfunction and neuroinflammation. Their positions within the NVU allow intimate contact with one another between the NVU and PVS as follows: Brain endothelial cells (BECs) and the Pcs via their shared basement membrane and physical contact peg-socket junctions with N cadherins and gap junctions Cx43; Pcs and intimate contacts with rPVMΦs residing in the PVS. Additionally, rPVMΦs have intimate contact with the astrocyte endfeet (ACef) that form the outermost membrane of PVS. Importantly, ACef have intimate contact with BECs that have intimate physical contact with neuronal axons and dendrites to complete NVU coupling. The multiplicity of intimate contacts of NVU cells allow for continuous crosstalk communications to provide brain homeostasis. While each of the cells of the NVU play important roles in the development of enlarged perivascular spaces (EPVS), this review focuses on the Pcs and rPVMΦs and discusses each of the intimate contacts and their functional significance in detail with numerous illustrations and transition electron microscopic images to demonstrate their role in the development of EPVS. EPVS are known to be biomarkers for cerebral small vessel disease and impaired glymphatic system waste clearance. While the importance of EPVS in mixed dementia, vascular contributions to cognitive impairment and dementia that result in high economic and psychosocial cost to the global community are unquestionable, the focus in this manuscript is on the how the triad of obesity, metabolic syndrome, and type 2 diabetes mellitus has on the development of EPVS.
Keywords
Astrocytes; Blood-brain barrier; Enlarged perivascular spaces; glymphatic system; Microglia; MRI; Pericytes; Perivascular macrophages; Perivascular spaces; Small vessel disease
Abbreviations
AC: astrocyte; ACef, astrocyte end-feet; AGE/RAGE, advanced glycation end products/receptor for advanced glycation end products; AQP4, aquaporin-4; BBB, blood–brain barrier; BEC(s), brain endothelial cell(s); BECact/dys, brain endothelial cell activation/dysfunction; BG, basal ganglia; BM, basement membrane; CAA = cerebral amyloid angiopathy; CADASIL = cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CBF = cerebral blood flow; CCVD, cerebrocardiovascular disease; CBF, cerebral blood flow; Cl, capillary lumen. CNS, central nervous system; CR, capillary rarefaction; CSF, cerebrospinal fluid; CSO, centrum semiovale; DVS, dural venous sinus; EPVS, enlarged perivascular spaces; GS = glymphatic space; HTN, hypertension; ISF, interstitial fluid;; ISF, interstitial fluid; ISS, interstitial space; late-onset Alzheimer’s disease; LPS, lipopolysaccharide; lpsEVexos, lipopolysaccharide extracellular vesicles; MetS, metabolic syndrome; MGCs, microglia cells; MMPs, matrix metalloproteinases; MRI, magnetic resonance imaging; MW, metabolic waste; NO, nitric oxide; MW = metabolic waste; MRI, magnetic resonance imaging; NVU, neurovascular unit; Pc, pericyte; Pcfp, pericyte foot process; PVS, perivascular spaces; PVS/EPVS, perivascular space/enlarged perivascular space; rPVMΦ, resident perivascular macrophages; SAS, subarachnoid space; sLPS, soluble lipopolysaccharide; rPVMΦ, reactive perivascular macrophage; SVD, small vessel disease; T2DM, type 2 diabetes mellitus; TEM, transmission electron microscopy; TIA, transient ischemic attack; WMH, white matter hyperintensities.
Introduction
Perivascular spaces (PVS) and enlarged perivascular spaces (EPVS) (PVS/EPVS) or Virchow-Robin spaces have multiple structural and functional importance. PVS/EPVS are fluid filled spaces that ensheathe the precapillary arterioles (transporting CSF) and postcapillary venules (transporting primarily interstitial fluid mixed with lesser amounts of CSF) in the brain [1, 2, 3, 4]. Precapillary arteriole PVS are known to deliver cerebrospinal fluid (CSF), while postcapillary venules are known for the anatomical transcytosis of proinflammatory leukocytes and their clearance of interstitial fluid (ISF) and metabolic waste (MW) from the interstitial spaces (ISS) via the PVS that serve as a conduit for the glymphatic system (GS) that bathe the parenchymal neurons (Figs. 1, 2) [5, 6].
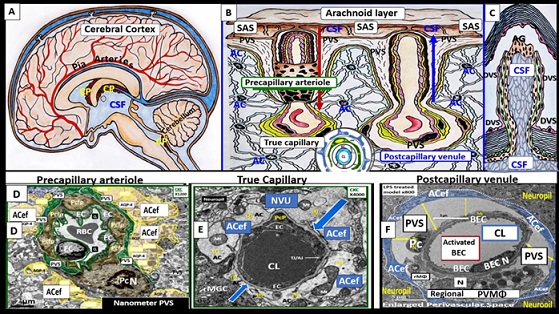
Figure 1. A collage of cerebrospinal fluid (CSF) and interstitial fluid (ISF) bathing the brain and transmission electron micrographs of precapillary arteriole, true capillary, and postcapillary venule with its perivascular spaces (PVS). Panels A, B, and C illustrate the CSF being delivered from the subarachnoid space (SAS) throughout the central nervous system (CNS) and parenchymal neurons via the perivascular spaces that ensheathe the pia arteries and precapillary arterioles to the true capillaries where solutes and fluids are delivered and then the postcapillary venules and veins to carry the interstitial fluid (ISF) and metabolic waste to the SAS and CSF for disposal in panels B and C. Panel D illustrates a precapillary arteriole and note the pseudo-colored golden yellow astrocyte endfeet (ACef) that tightly abut the pseudo colored green perivascular space (PVS). Panel E illustrates a true capillary wherein the ACef tightly abut the basement membrane (BM) of the mural neurovascular unit (NVU) endothelial cells (EC) and pericyte (Pc) cells. Panel F depicts an enlarged PVS as denoted by the space demarked by yellow double arrows and note the ACef have detached and separated from the BM of the mural cells. Modified images provided by CC 4.0 [8]. AC = astrocyte; ACef = astrocyte endfeet; BM = basement membrane; CL = capillary lumen; EC = brain endothelial cell; Pc = pericyte; Pcef = pericyte endfeet; RBC = red blood cell; WM = waste material.
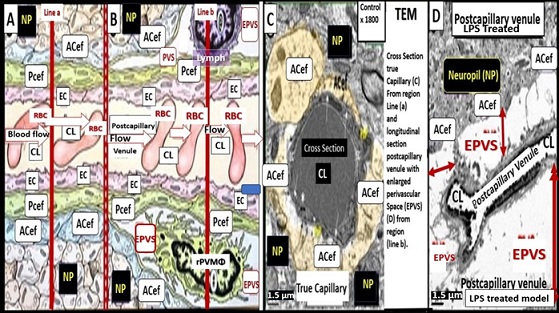
Figure 2. Illustration depicting the transition from normal capillaries with no perivascular space at the level of the true capillary to a postcapillary venule with a normal perivascular space (PVS) that transitions to a postcapillary venule with an enlarged perivascular space (EPVS) with supportive transition electron micrographs (TEMs) in panels C and D. Panels A and B are an illustration to demonstrate the transition from a true capillary without a perivascular space in panel A that transitions to the postcapillary venule with a normal perivascular space (PSV) to the postcapillary venule with enlarged perivascular spaces (EPVS). Panel C demonstrates a normal control true capillary in cross section without a perivascular space that is responsible for the delivery of nutrients, solutes, and oxygen and note how the pseudo-colored golden yellow astrocytes (ACs) and their endfeet (ACef) tightly abut the mural cells (endothelial cells (ECs) and pericytes (Pcs). Panel D depicts an elongated postcapillary venule with obvious EPVS in a longitudinal section and note how the ACef have detached and separated from the NVU mural endothelial cells (ECs) and pericytes (Pcs) basement membranes (BMs) to create the EPVS. Scale bars C and D equal 1.5 μm. ACef = astrocyte endfeet; CL = capillary lumen; EC = brain endothelial cell; EPVS = enlarged perivascular spaces; Pcef = pericyte endfeet-foot processes; LPS = lipopolysaccharide; Lymph = lymphocyte; NP = neuropil; RBC = red blood cell: WM = waste material.
PVS are considered not to be enlarged if they are not visible on T-2 weighted magnetic resonance images (MRI). However, PVS are considered to be enlarged (EPVS) when they can be identified by T-2 weighted MRIs and EPVS are approximately two-three millimeters in diameter [4, 7]. EPVS are recognized as important structural remodeling changes in various neurologic diseases and are currently known as biomarkers for cerebral small vessel disease (SVD) and vascular dementia (VaD), which are also known to be associated with lacunar stroke and white matter hyperintensities (WMH) [3, 7, 8, 9, 10, 11, 12]. Importantly, EPVS associate with advancing age, hypertension, lacunes, microbleeds, intracerebral hemorrhages, cerebrocardiovascular diseases with transient ischemic episodes and stroke, SVD, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADISIL), cerebral amyloid angiopathy (CAA), obesity, metabolic syndrome (MetS), type 2 diabetes mellitus (T2DM), WMH, late-onset Alzheimer’s disease (LOAD), sporadic Parkinson’s disease, and non-age-related multiple sclerosis [2, 3, 4, 7, 8, 9, 10, 13, 14, 15, 16, 17]. Further, our global population is already one of the oldest in history and additional aging is expected to increase in the coming years as our global population continues to age [8, 18, 19]. Additionally, EPVS are related to extracranial atherosclerosis, cerebromacrovascular, and cerebromicrovascular disease in addition to age-related neurodegenerative diseases such as LOAD and sporadic Parkinson’s disease. EPVS are located primarily in the basal ganglia (BG) and the centrum semiovale (CS0); however, they have also been identified in the hippocampus, midbrain, and the frontal cortex {Fig. 3) [4, 9, 20].
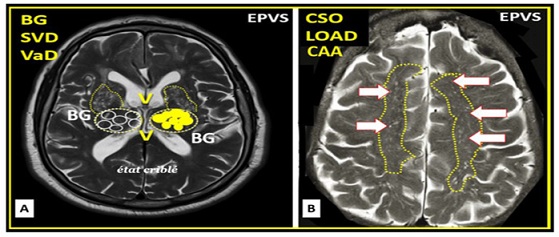 Figure 3. Magnetic resonance imaging (MRI) comparisons of basal ganglia (BG) enlarged perivascular spaces (EPVSs) to centrum semiovale (CSO) EPVSs. Panel (A) depicts the paired EPVSs within the BG that are traced in open circles on the left and masked yellow circles on the right BG. Additionally, note the faint white spaces within the paired dashed lines just above the paired BG structures. This MRI image is from a 75 y/o male status post-stroke, recovered with small vessel disease. Panel (B) depicts the paired elongated oval structures outlined by yellow dashed lines to enclose multiple white enlarged perivascular spaces. Note the open white arrows outlined in red pointing to prominent EPVSs. MRI image from a 79 y/o female with history of transient ischemic attacks. Importantly, note that BG EPVSs strongly associates with cerebral small vessel disease (SVD) in Panel (A) and that CSO EPVSs strongly associates with late-onset Alzheimer’s disease and cerebral amyloid angiopathy (CAA) in Panel (B). Permission to reproduce this image by author is by CC 4.0 [20].
Figure 3. Magnetic resonance imaging (MRI) comparisons of basal ganglia (BG) enlarged perivascular spaces (EPVSs) to centrum semiovale (CSO) EPVSs. Panel (A) depicts the paired EPVSs within the BG that are traced in open circles on the left and masked yellow circles on the right BG. Additionally, note the faint white spaces within the paired dashed lines just above the paired BG structures. This MRI image is from a 75 y/o male status post-stroke, recovered with small vessel disease. Panel (B) depicts the paired elongated oval structures outlined by yellow dashed lines to enclose multiple white enlarged perivascular spaces. Note the open white arrows outlined in red pointing to prominent EPVSs. MRI image from a 79 y/o female with history of transient ischemic attacks. Importantly, note that BG EPVSs strongly associates with cerebral small vessel disease (SVD) in Panel (A) and that CSO EPVSs strongly associates with late-onset Alzheimer’s disease and cerebral amyloid angiopathy (CAA) in Panel (B). Permission to reproduce this image by author is by CC 4.0 [20].
Notably, it has been determined that EPVS in the CSO may have a greater association with amyloid beta pathology [21], and that EPVS of the BG are more indicative of arteriolosclerosis, hypertensive arteriopathy, diabetes mellitus, hyperlipidemia, prior stroke, lacunes, deep microbleeds, and SVD [22, 23, 24]. Also, EPVS have been determined to be a marker for an increased risk of cognitive decline and dementia independent of other small vessel disease markers over a four-year period [25]. EPVS are known to exist in at least three major subtypes based on the regions of their occurance as follows: Type I PVS/EPVS are located along lenticulostriate arteries that enter the BG sometimes referred to as État criblé (a collection of multiple radiolucent 1-5 millimeter of EPVS frequently found in the BG in T-2 weighted MRIs); type II are present along the path of perforating medullary arteries to enter cortical gray matter around high convexities that extend into the white mater and are associated with CSO regions; type III are located in the midbrain and surround the penetrating branches of the collicular and assessory collicular arteries [26]. Recently, Paradise et al., have shown that EPVS are a marker for an increased risk of cognitive decline and dementia, independent of other small vessel disease markers [27]. Further, this group has also suggested that EPVS should no longer be thought of as just an incidental finding associated with aging but also a biomarker for SVD and cognitive impairment, dementia, and a biomarker of impaired waste clearance in the brain [28]. Multiple mechanisms are thought to be involved in the development of EPVS, which include the following: (1) increased fluid and neurotoxic proteins that enter the PVS due to BBB dysfunction/disruption due to increased permeability; (2) increased fluid inflow to the PVS due to ACef dysfunction, detachment, separation and aquaporin-4 dysfunction with decreased water uptake allowing the accumulation of water in the PVS; (3) stalling or obstruction of the PVS conduit or impaired glymphatic efflux due to inflammation and the accumulation of excess leukocytes with phagocytosis and accumulation of excessive phagocytic debris, oxidative stress, and activation of increased MMPs, which result in stagnation, stalling, and/or varying degrees of PVS conduit glymphatic system obstruction of the waste removal mechanisms; (4) arteriole or venule vascular stiffening and/or spiraling of arterioles that are associated with decreased vascular pulsatility, which results in decreased fluid flow within the PVS contributing to PVS enlargement; (5) atrophy or loss of surrounding neurons and their axons [2, 3, 8, 9, 10, 11, 15, 16, 28]. Further, EPVS do not develop all at once but are thought to be associated with a sequence of events and exist as an evolutionary spectrum such that they develop over time to result in SVD, neuroinflammation, impaired cognition and neurodegeneration (Fig. 4) [8, 29].
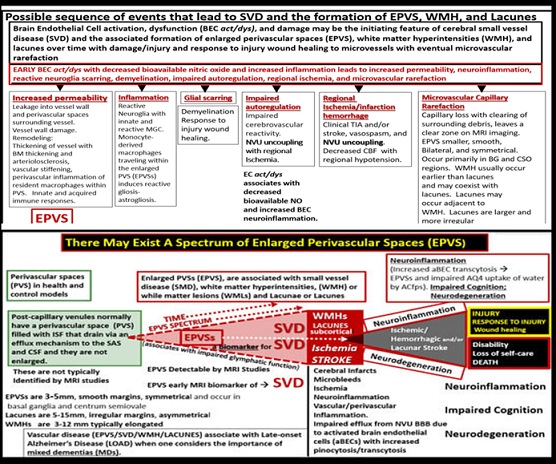
Figure 4. A probable sequence of events and a continuum of remodeling, which results in a spectrum regarding the development of enlarged perivascular spaces (EPVS) over time. There exists a sequence of remodeling changes and events in the development of enlarged perivascular spaces, white matter hyperintensities, lacunes and small vessel disease. Image provided with permission by CC 4.0 [8, 29]. ACfp = astrocyte endfeet; BBB = blood–brain barrier; BEC = brain endothelial cell; BEC act/dys = brain endothelial cell activation and dysfunction; BG = basal ganglia; BM = basement membrane; CBF = cerebral blood flow; CSF = cerebrospinal fluid; CSO = centrum semiovale; ISF = interstitial fluid; LOAD = late-onset Alzheimer’s disease; MGC = microglia cell; mm = micrometer; MRI = magnetic resonance imaging; NO = nitric oxide; NVU = neurovascular unit; PVS = perivascular spaces; spaces; SAS = subarachnoid space; TIA = transient ischemic attack; WMH = white matter hyperintensities.
The Obesity, MetS and T2DM triad is associated with the development of EPVS and may contribute to accelerated brain aging and injury [8]. Notably, the MetS is known to increase the risk for developing cerebrocardiovascular disease with both macro-and microvascular disease; arteriolosclerosis and extracranial and cranial atherosclerosis as well as T2DM [29, 30]. The MetS has multiple risk factors and variables that would contribute to EPVS and it is known that T2DM increases the risk for late-onset Alzheimer’s disease (LOAD) as well as other neurodegenerative diseases including age-related Parkinson’s disease (Fig. 5) [29].
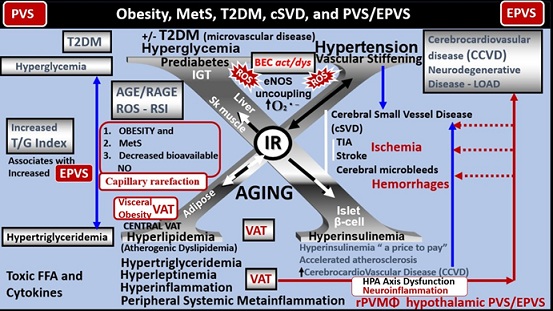
Figure 5. Metabolic syndrome (MetS), cerebral small vessel disease (SVD) and perivascular spaces (PVS)/enlarged perivascular spaces (EPVS). There are four arms to the central X in this illustration that points to hyperlipidemia (lower left), hyperinsulinemia of insulin resistance (IR) (lower right), essential hypertension (upper right), and hyperglycemia (upper left). It is currently known that EPVS are a biomarker of SVD, cognitive decline and dementia, and possibly impaired glymphatic system waste removal. Importantly, visceral adipose tissue (VAT), increased triglyceride/glucose index (TG index), and hypertension associate with SVD. Each of these four arms is either directly or indirectly associated with EPVS and SVD. Notably, the triad of obesity, MetS, and decreased bioavailable nitric oxide (NO) are known to associate with capillary rarefaction. Also, note how metainflammation (the chronic production of sterile peripheral induced inflammation) primarily by VAT in obesity, MetS and T2DM contributes to the development of hypothalamic pituitary axis (HPA) axis dysfunction due to neuroinflammation that is partially induced by the resident perivascular macrophage (rPVMΦ) in the hypothalamic regions and cerebrocardiovascular disease (CCVD), SVD, TIA, stroke, microbleeds, hemorrhages, and neurodegeneration (red arrows straight and dashed lines). This modified image is provided with permission by CC 4.0 [8, 29] AGE = advanced glycation end-products; RAGE = receptor for AGE; AGE/RAGE = advanced glycation end-products and its receptor interaction; BECact/dys = brain endothelial cell activation and dysfunction; eNOS = endothelial nitric oxide synthase; FFA = free fatty acids–unsaturated long chain fatty acids; IGT = impaired glucose tolerance; LOAD = late-onset Alzheimer’s disease; O2 •− = superoxide; ROS = reactive oxygen species; RSI = reactive species interactome; Sk = skeletal; T2DM = type 2 diabetes mellitus; TG Index = triglyceride/glucose index; TIA = transient ischemia attacks.
Javierre-Petit et al., has recently demonstrated that in addition to cerebral infarcts EPVS burden was associated with diabetes independently of other neuropathologies in a cohort of 654 individuals from a community-based older adults [31]. Capillary rarefaction (CR) in the brain (loss of capillaries) has recently been found to be associated with an increase in obesity, MetS, and T2DM [8. 31, 32, 33]. Recently, Schulyatnikova and Hayden have hypothesized that capillary rarefaction may leave an empty space within the PVS that is subsequently filled with interstitial fluid [8]. This loss of capillaries within the PVS may allow for an increase in total percentage fluid volume within the PVS when the capillary undergoes rarefaction and may contribute to the development of EPVS (Fig. 6) [8].
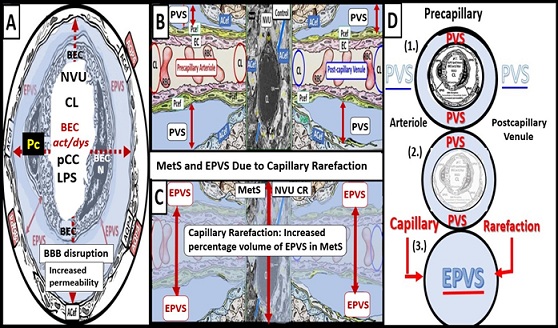
Figure 6. Cross and longitudinal sections representitive of pre- and postcapillary arterioles and venules with an encompassing surrounding perivascular space (PVS). Panel A depicts a cross section of a capillary surrounded by a PVS (solid double red arrows) and its increase in total volume to become an enlarged perivascular space (EPVS) (dashed double red arrows), which represents capillary rarefaction. Panel B demonstrates a control longitudinal capillary that runs through an encompassing PVS (light blue). Panel C depicts capillary rarefaction in a longitudinal view and note how the volume of the PVS increases it total volume once the capillary has undergone rarefaction (double red arrows). Panel D depicts the progression of a normal precapillary arteriole and postcapillary venule PVS to an EPVS once the capillary has undergone rarefaction allowing for an increase total percentage volume of the PVS (1.- 3.). Image provided with permission by CC 4.0 [8]. ACef = astrocyte endfeet; AQP4 = aquaporin 4; BEC = brain endothelial cells; BECact/dys = brain endothelial cell activation and dysfunction; CL =capillary lumen; EC = endothelial cell; lpsEVexos = lipopolysaccharide extracellular vesicle exosomes; NVU = neurovascular unit; Pcef = pericyte endfeet.
CR is known to occur in multiple clinical situations, including: aging, hypertension, obesity, MetS, T2DM, SVD, and LOAD. Also, there are multiple proposed mechanisms that may co-occur to result in CR, including: oxidative – redox stress, inflammation, BECact/dys and loss, Pc dysfunction and loss, impaired angiogenesis (increased ratio of antiangiogenic factors/proangiogenic factors), microvessel ischemia with emboli or hemorrhage, decreased microvessel shear stress, increased microvessel tortuosity, and in some cases increased transforming growth factor beta [34, 35]. While this mechanistic hypothesis for possible expansion of PVS due to CR is plausible, more research will be required for it to gain support as a mechanism for increased EPVS.
Pericyte(s) (Pc) cells and brain endothelial cell(s) (BECs) are the two mural cells that are essential to form the multicellular neurovascular unit (NVU) consisting of BECs, Pcs, astrocytes and their endfeet (ACef), perivascular microglia cell(s) (PVMGCs) and resident perivascular macrophages (rPVMΦs), and neurons [36], which are important in the development of EPVS. Pcs extend their elongated pericyte foot processes (Pcfp) that encircle BECs and communicate via physical contact peg sockets and gap junctions connexins with BECs and are also in intimate association with rPVMΦ. Pcs are uniquely positioned within the NVU and make physical and intimate connections with BECs, rPVMΦs, and ACef [3, 36]. Pcs are multifunctional and known to process signaling, integrate and coordinate signals from BECs, rPVMΦs, and neurons to complete the NVU and provide for NVU coupling to assist in increasing cerebral blood flow (CBF) in regions of increased neural activity, and signaling [36, 37]. Pcs also generate multiple functional responses critical for central nervous system functions in both health and disease. These functions include the regulation and maintenance of the blood brain barrier (BBB), BBB permeability, angiogenesis, NVU capillary hemodynamic responses, and clearance of metabolic waste including neurotoxins, hemodynamic responses including NVU coupling via ACef that connect to neurons and control microvascular cerebral blood flow (CBF) via NVU coupling, and importantly neuroinflammation [37, 38]. Notably, Pcs have been thought to act as pluripotent mesenchymal stem cells and are capable of lifting from the NVU niche and migrating to regions of CNS injury [37]. The unique structural localization of Pcs and their foot processes that are interspersed or sandwiched between the BEC BMs of the NVU and ACef and the PVS and its outermost ACef place them in pivotal position to regulate the inflammatory responses of the CNS in the immediate region of the NVU PVS in addition to the CNS neuronal parenchyma [39, 40].
rPVMΦs play a key and important role in the development of EPVS. Resident perivascular macrophages (rPVMΦs) reside within the PVS and similar to the CNS microglial cells (MGCs), in that, both are derived from the yolk sack play an important role in the development of EPVS (Figs.1F, 7) [41, 42].
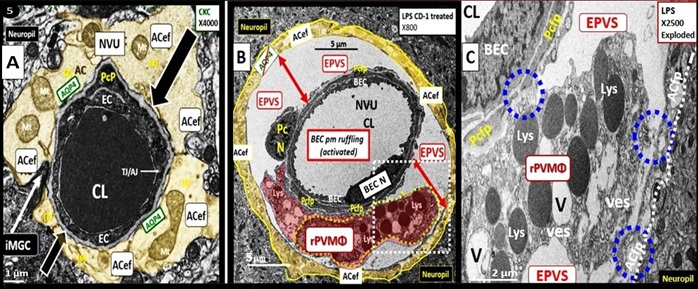
Figure 7. Enlarged perivascular space (EPVS) and resident-reactive perivascular macrophage (rPVMΦs) in a postcapillary venule compared to a true capillary. Panel A demonstrates a normal true capillary in a 20-week-old female C57B6/J control model and note how the ACef tightly abut the shared basement membrane (open black arrows) of the brain endothelial cell (BEC) and pericyte foot process (PcP). Panel B depicts an EPVS with a prominent rPVMΦ (pseudo-colored red) in a 20-week-old lipopolysaccharide (LPS)-treated CD-1male model and note how the astrocyte endfeet-foot processes (ACfp) are markedly separated from the capillary mural cells (BEC and Pc) (red double arrows). Panel C depicts the rPVMΦs in an exploded image with intimate contact with the Pcfps basal lamina and the rPVMΦ intimate contact with basal lamina of the ACef (outermost boundary of the EPVS abluminal lining) (dashed blue circles). Modified images provided with permission by CC 4.0 [29]. AQP4 = aquaporin 4; Lys = lysosomes; Mt = mitochondria; N = nucleus; NVU = neurovascular unit; V =vacuoles; ves = vesicles.
TEM images have consistently shown that rPVMΦs are located within the PVS between the luminal mural cells and the outermost basal lamina of the ACef or glial limitans and the brain parenchyma as depicted in figure 5B [42, 43, 44]. As one reviews the literature on rPVMΦ, the term border-associated macrophages (BAMs) is frequently discussed and these BAMs are now thought to be rPVMΦ since they have been shown to reside within the PVS by TEM studies [42, 43, 44]. rPVMΦs are known to facilitate BBB integrity, promote glymphatic drainage, and exert immune function such as phagocytosis and serve as antigen presenting cells within the PVS to facilitate neuroinflammation once it is initiated since they are key components of the PVS and CNS-resident immune system [41].
The PVS As An Anatomical Crossroad And Space That Provide Multicellular Crosstalk To Facilitate The Development Of EPVS
Neurological disorders and diseases are known to have heterogenous pathogenesis, with multiple overlapping contributions of vascular, immune, and neuronal mechanisms of brain injury. PVS/EPVS in the brain represent a crossroad intersection where those mechanisms interact [16], in addition to providing a conduit for the key anatomical component of the glymphatic pathway/system (GS) [5], which plays a crucial role in waste clearance of interstitial fluid that has been shown to be linked to neurodegenerative disease [16]. This neuroinflammation occurs initially in the PVS that has become enlarged (EPVS) due to the obstruction of the PVS/glymphatic system due to the accumulation of cells and cellular debris due to excessive neuroinflammation that occurs within the PVS of precapillary arterioles and postcapillary venules [16]. These PVS provide a niche space for the ongoing inflammatory processes, which occur due to the extensive crosstalk between activated and dysfunctional BECs with proinflammatory leukocytes and rPVMΦ that are initially activated via peripheral metainflammation (sterile peripheral inflammation primarily produced by the extensive visceral adipose tissue) associated with obesity, MetS, and T2DM. These activated BECs undergo extensive crosstalk with adjacent Pcs that are in direct physical cell-cell contact via peg sockets, gap junction Cx43, and N-Cadherins. In turn, these reactive Pcs undergo extensive crosstalk communication with the PVS rPVMΦs and these rPVMΦ undergo extensive crosstalk communication with incoming proinflammatory leukocytes that are passed into the PVS via diapedesis through the activated BECs to eventually travel throughout the CNS [45]. These incoming proinflammatory leukocytes provide the oxidative stress and phagocytosis that activate MMP 2-9 that are capable of degrading the outer boundary of the perivascular space glia limitans to allow these now proinflammatory leukocytes to enter the CNS interstitial spaces (ISSs) to affect local, regional, and generalized neurons to instigate neuroinflammation with impaired synaptic function, neurodegeneration, and impaired cognition. Thus, the PVS and their subsequent enlargement act as the crossroad for extensive crosstalk communication between activated BECs, Pcs, rPVMΦs, incoming leukocytes, and ACef to allow leukocytes to pass into the interstitium to result in CNS neuroinflammation. Thus, PVS represent a crossroad where cerebrovascular, neuroinflammatory, and neurodegenerative mechanisms, of brain injury converge and interact (Fig. 8) [16, 45].
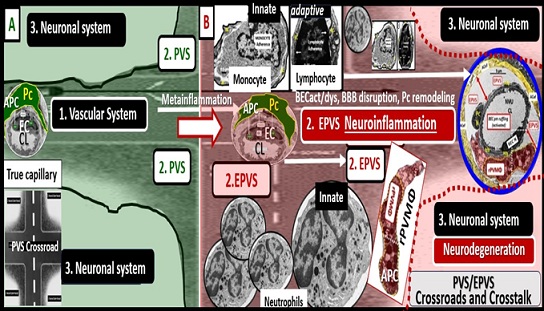
Figure 8. The perivascular spaces/enlarged perivascular spaces (PVS/EPVS) serve as an anatomical crossroad or intersection for the vascular, neuroinflammatory, and neuronal systems. These three systems interact and allow for multiple cellular signaling crosstalk communication associated with the metainflammation of obesity, metabolic syndrome (MetS), and type 2 diabetes mellitus (T2DM) to result in impaired cognition and neurodegeneration. Panel A demonstrates the normal appearing PVS in control models with the green background. Note the highway PVS crossroad icon in lower left panel from which this figure was constructed. Panel B depicts the EPVS with its resident reactive perivascular macrophage (rPVMΦ) and leukocytes (neutrophils, monocytes, and lymphocytes) that have undergone diapedesis via paracellular or transcytotic routes via the activated BECs to enter the EPVS and comprise step 1 of the 2-step process of leukocytes entering the neuropil interstitial space (ISS). These leukocytes not only undergo cellular crosstalk with the activated BECs but also crosstalk with one another as well as the resident perivascular macrophage (rPVMΦ) within the PVS/EPVS, the pericyte (Pc), and the astrocyte endfeet (ACef) to result in EPVS, impaired cognition, and neurodegeneration. It is important to note that both the Pc and the rPVMΦ are known to be antigen presenting cell(s) (APCs). Additionally, the reactive leukocytes are capable of generating a huge amount of reactive oxygen species - oxidative stress and secretion of matrix metalloproteinases 2, 9 that are capable of degrading the outermost boundary of the PVS/EPVS ACef basal lamina or glia limitans to allow for the second-step for leukocyte entry into the neuropil interstitial spaces to result in neuroinflammation and subsequent neurodegeneration. The PVS/EPVS anatomical crossroad along with its multiple cellular crosstalk can therefore result in a self-perpetration or vicious cycle of brain injury and response to injury wound healing to result in neuroinflammation and neurodegeneration with impaired cognition. Additionally, it is important to note that the PVS forms the conduit for the glymphatic system to deliver metabolic waste and toxins from the interstitial fluid and provides the crosstalk communication necessary for neuroinflammation to develop within the PVS/EPVS. The increased neuroinflammation that occurs within the PVS/EPVS will develop considerable metabolic waste debris that will slow and cause delayed efflux to the interstitial fluid and cerebrospinal fluid to result in further dilation of the PVS/EPVS. BBB = blood-brain barrier; BECact/dys = brain endothelial cell activation/dysfunction; CL = capillary lumen; EC = brain endothelial cells; EPVS = enlarged perivascular space; Pc = pericytes; PVS = perivascular space; rPVMΦ = resident reactive perivascular macrophage(s).
Thus, the vascular, neuroimmune, and neuronal systems can develop a pathological interplay, which can create a conducive environment capable of promoting a self-perpetration of brain injury mechanisms across different neurological regions of the CNS and neurological diseases, including those that are primarily thought of as neurodegenerative, neuroinflammatory or cerebrovascular diseases [16].
The PVS/EPVS provide a safe sanctuary space region to harbor the incoming proinflammatory leukocytes due to the NVU BBB disruption with increased permeability due to obesity, MetS, and T2DM as well as other possible clinical diseases. There is plenty of incoming proteinaceous waste material being taken up by the PVS. The PVS acts as a conduit space of CNS GS drainage that occurs between the ISF and the contents of postcapillary PVS efflux conduit for human and rodent models CNS metabolic toxic waste removal that is now widely accepted in the literature [5, 8, 20, 46, 47]. Thus, the postcapillary venule PVS serves as the anatomical conduit for the GS efflux of metabolic waste [48]. The accumulated leukocytes that reside within the PVS storage santuary will have plenty of opportunity to phagocytose this proteinaceous waste debris to eventually result in PVS neuroinflammation with stalling of PVS efflux waste removal of ISF flow even to the point of PVS obstruction with downstream enlargement and EPVS [5, 16, 25, 49, 50]. Recently, Mendes et al., were able to show that in obese high-fat-diet fed mice (C57BL6) that this induced proinflammatory rPVMΦs in the hypothalamus helps to explain the HPA axis dysfunction found in obesity, MetS, and T2DM (Figs. 5, 9) [51].
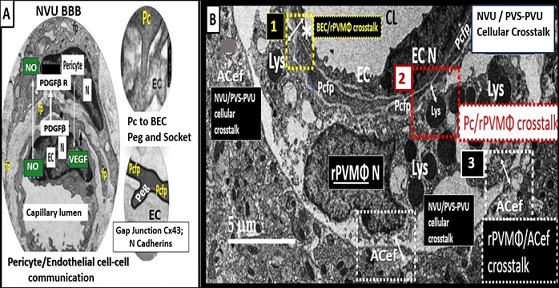
Figure 9. Perivascular spaces (PVS) and enlarged PVS (EPVS) provide a santuary space to serve as a crossroad for multicellular crosstalk between brain endothelial cell(s) (BECs), pericyte(s) (Pcs) and pericyte foot processe(s) (PCfps), resident perivascular macrophage(s) (rPVMΦs), leukocytes, and astrocyte endfeet (ACef). Panel A demonstrates the neurovascular unit (NVU) with its blood-brain barrier/interface (BBB), as a result of the BECs tight and adherens junction(s) (TJ/AJs). The BECs and encircling Pc and its Pc foot processes (Pcfp) have a unique cell-cell direct physical contact for cell-cell communication via its peg socket morphology and phenotype along with its N-cadherin junctions and its gap junction protein connexin 43 (Cx43). Panel B depicts the PVS/EPVS with its cellular contents of a rPVMΦ. Importantly note that there are three close intimate cell-cell contact regions for cellular crosstalk including 1. BEC/rPVMΦ (yellow boxed-in dashed lines and asterisk); 2. Pcfp/rPVMΦ (red boxed-in dashed lines; 3. rPVMΦ/ACef (white boxed-in dashed lines). Thus, this figure identifies the Pc and its foot processes along with the rPVMΦ as key cells residing within the PVS/EPVS – perivascular unit (PVU) to provide for this extensive crosstalk communication between NVU BECs, Pcef, rPVMΦs, and ACef. Double arrows depict this cell-cell crosstalk communication.
Reactive Juxtavascular Microglia Cells (rJVMGCs), Neuroinflammation, and Enlarged Perivascular Spaces (EPVS)
When neuroinflammation is discussed, the CNS resident immune microglia cell(s) (MGC) most often comes to mind and is discussed extensively in the literature [52, 53, 54, 55, 56]; however, in this narrative review the focus has been primarily on the rPVMΦ that reside within the PVS by TEM studies. This is not only because PVS and EPVS are important [2, 8, 57] but also because both MGCs and PVMΦs have been rapidly gaining interest over the past decade [58]. Additionally, Xie et al., revealed that a bibliometric analysis linked brain related diseases with rPVMΦs and also pointed to the interest of reactive peripheral macrophages in visceral adipose tissue and vascular diseases in obesity, MetS, and T2DM as current hotspots in research [58]. Notably, CNS rJVMGCs could play a concurrent role along with rPVMΦs in PVS-induced neuroinflammation and enlargement [59]. For example, rJVMGCs are capable of promoting NVU BBB disruption allowing the diapedesis of leukocytes into the PVS [60] and further, neurotoxic insults are capable of inducing both rJVMGCs and reactive astrocytes (rACs) [61, 62, 63]. Also, rPVMGCs that lie outside of the PVS in the CNS parenchyma are known to be concurrently associated with ACs when peripheral cytokines/chemokines are chronically increased as in metainflammation associated with obesity, MetS, and T2DM [61, 62, 63]. Additionally, rACs and rPVMΦs would be capable of increasing CNS-derived proinflammatory cytokines/chemokines as well as reactive oxygen, nitrogen, sulfur species to result in an increased activity of the reactive species interactome (RSI), which are known to increase the secretion of matrix metalloproteinases (MMPs-2, 9) and contribute to BBB disruption [64, 65]. These MMPs would be capable of contributing to the degradation of the ACef basal lamina (glia limitans) to allow the breaching of the PVS by proinflammatory leukocytes to complete the 2nd step of the 2-step process of CNS neuroinflammation [45, 59]. Notably, Zeng et al., recently demonstrated that EPVS severity was associated with the progression of tauopathy in LOAD and that rMGCs neuroinflammation mechanisms mediated this relationship of EPVS and tauopathy [66].
Current And Evolving Technologies To Observe The Perivascular Spaces (PVS)
In 2019 Sepehrband et al., examined and summarized the current and evolving technologies to observe PVS [67]. The current clinical methods now include identification and quantification of PVS as the number of visible PVS by visual hand-counting methods or axial slices of T2-weighted images that has the highest number of PVS in the region of interest and then utilizing the Wardlaw’s scale as previously demonstrated (Fig. 3) [4, 68, 69]. As one uses the Wardlaw’s scale, the observer selects a representative axial slide for each region to be studied. If EPVS are observable in the midline or midbrain, it is automatically given a score of one, otherwise it is assigned a score of zero. When observing the BG and CSO, each EPVS and the region is rated according to five-point rating scale as follows: zero equaling no EPVS found; one equaling one to 10; two equaling 11-20; three equaling 21 to 40; five equaling more than 40 EPVS. For example, earlier (Figs. 3A, 3B) each have a Wardlaw’s scale score of five by visual hand-counting methods. This five-point rating is known to have higher inter observer and test-retest reliability. However, it has been used to objectively link EPVS to neurological diseases and glymphatic dysfunction as a maker of disease [68]
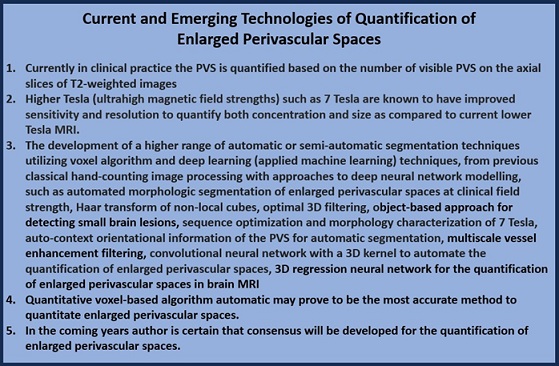
This method utilizing Wardlaw’s scale is known to be laborious and prone to errors, therefore; efforts to improve efficiency and accuracy have been made by using a wide range of automatic or semi-automatic segmentation techniques, from classical image processing approaches to deep neural network modelling that utilize complex algorithms (Box 1) [70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81].
When the PVS become visible in MRI images in white matter regions such as in the BG and CSO regions they represent pathological features of brain vasculature damage, which also indicate obstruction of the CSF/ISF flow and associated impairment of waste efflux of clearance to the SAS/CSF and systemic vascular system (Fig. 2) [82Sepehurband]. Currently, the most widely accepted and adopted technology involve the utilization of Wardlaw’s scale [4Wadlaw, 69Pham]. For a more in-depth discussion of the current and evolving technologies to observe enlarged perivascular) readers are urged to read the following paper by Pham et al, [69].
Conclusions
EPVS have been previously noted for decades, but frequently overlooked and were initially thought to be of uncertain pathophysiology [9]. However, EPVS are currently emerging as important aberrant morphological findings in association with multiple clinical diseases and aging. Some have even suggested that it is now uncontested that PVS play critical roles in not only maintaining homeostasis but also priming neuroinflammation as illustrated in figure 8 [82, 83].
In this narrative review, the first paragraph of the introduction discusses the perivascular spaces (PVS) and enlarged perivascular spaces (EPVS) (PVS/EPVS) or Virchow-Robin spaces that have multiple structural and functional importance; the second paragraph discusses the obesity, MetS and T2DM triad that is associated with the development of EPVS that may contribute to accelerated brain aging and the injury role and the association of obesity, MetS, and T2DM in the development of EPVS; while the third paragraph discusses the key role of Pc cells in the development of EPVS; the fourth paragraph discusses the key role that rPVMΦs play in the development of EPVS. Section 2. discusses the importance of the PVS and the EPVS as a regional anatomical crossroads and spaces that provide for multicellular crosstalk to facilitate the development of EPVS as well as functioning as a repository space for leukocytes that have undergone diapedesis across the NVU BBB due to BECact/dys. Section 3. discusses the current and emerging technologies of quantitating EPVS.
Importantly, the key roles of Pcs and rPVMΦ were explored in more depth as they relate to neuroinflammation and the development of EPVS than in most other papers that have reviewed EPVS. As our knowledge regarding the development of EPVS continues to grow and we better understand how they are important in their associated clinical disease states we will undoubtedly continue to make new findings regarding their development and progression. For example, how might we be able to slow or prevent PVS enlargement and how might EPVS associate with impaired glymphatic waste removal, impaired cognition, neuroinflammation, and neurodegeneration?
While this narrative review parallels many of the referenced publications regarding PVS/EPVS and their development, the author has utilized multiple TEM images and multiple illustrations in order to aid in the understanding of not only structural remodeling but also the functional changes associated with the development of EPVS. More research in this field is necessary and it is obvious that this field is growing rapidly with many different hot spots being explored along the way, especially in regards to the postcapillary venule, which is the conduit for the glymphatic system of interstitial fluid waste efflux and removal to the CSF and systemic circulation. Additionally, there is an exponential expansion of research studies in the glymphatic pathway-system field of study, which utilizes the postcapillary venule perivascular space and its growing expansion in this research field of study.
M.R.H. is totally responsible for the Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation, Resources, Data Curation, Writing—Original Draft Preparation, Writing—Review and Editing, Visualization, Supervision, Project Administration, and Funding Acquisition. Author has read and agreed to the published version of the manuscript.
Funding
Author has not received grants from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The tissues provided for the representative electron microscopic images utilized in this manuscript were all approved in advance by the University of Missouri Institutional Animal Care and Use Committee (No. 190), and animals were cared for in accordance with National Institutes of Health guidelines and by the Institutional Animal Care and Use Committees at the Harry S. Truman Memorial Veterans Hospital and University of Missouri, Columbia, MO, USA, and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data and materials will be provided upon reasonable request.
Acknowledgments
The author would like to acknowledge Tatyana Shulyatnikova for the contributions of the artistic illustrations and editing of this manuscript. The author would also like to acknowledge DeAna Grant Research Specialist of the Electron Microscopy Core Facility at the NexGen Precision Health Research Center, University of Missouri, Columbia, Missouri. The author also acknowledges the William A. Banks Lab at the VA Medical Center-Seattle, Washington for their kind support.
Conflicts of Interest
Author declares no conflicts of interest.
References
- Zhang ET, Inman BE, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J. Anat.1990;170:111–123.
- Bown CW, Carare RO, Schrag MS, Jefferson AL. Physiology and Clinical Relevance of Enlarged Perivascular Spaces. 2022;98;107–117. doi: 10.1212/WNL.0000000000013077
- Brown R, Benveniste H, Black SE, Charpak S, Dichgans M, Joutel A, Nedergaard M, Smith KJ, Zlokovic BV, Wardlaw JM. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc. Res. 2018;114:1462–1473. doi: 10.3389/fneur.2022.844938
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12: 822–838. doi: 10.1016/S1474-4422(13)70124-8
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748
- Yu L, He X, Li H, Zhao Y. Perivascular Spaces, Glymphatic System and MR. Front. Neurol. 2022;13:844938. doi: 10.3389/fneur.2022.844938
- Francis F, Ballerini L, Wardlaw JM. Perivascular spaces and their associations with risk factors, clinical disorders and neuroimaging features: A systematic review and meta-analysis. Int J Stroke. 2019;14(4):174749301983032. doi: 10.1177/1747493019830321
- Shulyatnikova T, Hayden MR. Why are Perivascular Spaces Important? Medicina (Kaunas). 2023;59(5):917. doi: 10.3390/medicina59050917
- Doubal FN, Maclullich AMJ, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged Perivascular Spaces on MRI Are a Feature of Cerebral Small Vessel Disease. Stroke. 2010;41(3):450-454. doi: 10.1161/STROKEAHA.109.564914
- Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. Vascular dysfunction-The disregarded partner of Alzheimer's Disease. Alzheimer’s Dement. 2019;15(1):158-167. doi: 10.1016/j.jalz.2018.07.222
- Loos CMJ, Klarenbeek P, van Oostenbrugge RJ, Staals J. Association between Perivascular Spaces and Progression of White Matter Hyperintensities in Lacunar Stroke Patients. PLoS One. 2015; 10(9): e0137323.doi: 1371/journal.pone.0137323
- Benjamin P, Trippier S, Lawrence AJ, Lambert C, Zeestraten E, Williams OA, Patel B, Morris RG, Barrick TR, MacKinnon AD, Markus HS. Lacunar Infarcts, but Not Perivascular Spaces, Are Predictors of Cognitive Decline in Cerebral Small-Vessel Disease. 2018 Mar;49(3):586-593. doi: 10.1161/STROKEAHA.117.017526
- Arba F, Quinn TJ, Hankey GJ, Lees KR, Wardlaw JM, Ali M, et al. Enlarged perivascular spaces and cognitive impairment after stroke and transient ischemic attack. Int J Stroke. 2016;13(1). doi: 10.1177/174749301666609
- Bokura H, Kobayashi S, Yamaguchi S. Distinguishing silent lacunar infarction from enlarged Virchow-Robin spaces: a magnetic resonance imaging and pathological study. J Neurol. 1998;245(2):116-122. doi: 10.1007/s004150050189
- Heier LA, Bauer CJ, Schwartz L, Zimmerman RD, Morgello S, Deck Large Virchow-Robin spaces: MR-clinical correlation. Am J Neuroradiol. 1989;10(5):929-936.
- Trolli F, Cipollini V, Moci M, Morena E, Palotai M, Rinaldi V, Romano C. Ristori G, Giubilei F, Salvetti M, Orzi F, Guttmann CRG, Cavallari M. Perivascular Unit: This Must Be the Place. The Anatomical Crossroad Between the Immune Vascular and Nervous System. Front Neuroanat. 2020;14: 17. doi: 3389/fnana.2020.00017
- Zhu YC, Tzourio C, Soumaré A, Mazoyer B, Dufouil C, Chabriat Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. 2010;41(11):2483-2490. doi: 10.1161/STROKEAHA.110.591586
- Hayden MR. Type 2 Diabetes Mellitus Increases The Risk of Late-Onset Alzheimer's Disease: Ultrastructural Remodeling of the Neurovascular Unit and Diabetic Gliopathy. Brain Sci. 2019;9(10):262. doi: 10.3390/brainsci9100262
- Gutierrez J, Rundek T, Ekind MSV, Sacco RL, Wright Perivascular Spaces Are Associated with Atherosclerosis: An Insight from the Northern Manhattan Study. AJNR Am J Neuroradiol. 2013; 34(9): 1711–1716. doi: 10.3174/ajnr.A3498
- Hayden MR. Brain Injury: Response to Injury Wound Healing Mechanisms and Enlarged Perivascular Spaces in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Medicina (Kaunas). 2023;59(7):1337. doi: 10.3390/medicina59071337
- Charidimou G, Boulouis MP, Frosch JC, Baron M Pasi JF, Albucher B, et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI–neuropathology diagnostic accuracy study. Lancet Neurol. 2022;21(8):714-725. doi: 10.1016/S1474-4422(22)00208-3
- Vilor-Tejedor N, Ciampa I Operto G, Falcón C, Suárez-Calvet M, Crous-Bou et al. Perivascular spaces are associated with tau pathophysiology and synaptic dysfunction in early Alzheimer's continuum. Alzheimers Res Ther. 2021 Aug 5;13(1):135. doi: 10.1186/s13195-021-00878-5
- Kapoor A, Gaubert A, Yew B, Jang JY, Dutt S, Li Y, et al. Enlarged perivascular spaces and plasma Aβ42/Aβ40 ratio in older adults without dementia. Neurobiol Aging. 2023 Aug;128:43-48. doi: 10.1016/j.neurobiolaging.2023.04.004
- Banerjee G, Kim HJ, Fox Z, Jäger HR, Wilson D, Charidimou A, Na HK, Na DL, Seo SW, Werring DJ. MRI-visible perivascular space location is associated with Alzheimer's disease independently of amyloid burden. Brain. 2017; 140(4):1107-1116. doi: 10.1093/brain/awx003
- Passiak BS, Liu D, Kresge HA, Cambronero FE, Pechman KR, Osborn KE, et al. Perivascular spaces contribute to cognition beyond other small vessel disease markers. 2019;92(12): e1309–e1321. doi: 1212/WNL.0000000000007124
- Rudie JD. Rauschecker AM, Nabavizadeh SA, Mohan S. Neuroimaging of Dilated Perivascular Spaces: From Benign and Pathologic Causes to Mimics. J neuroimaging. 2018;28(2): 139-149. doi: 1111/jon.12493
- Paradise M, Crawford JD, Lam BCP, Wen W, Kochan NA, Makkar S, Dawes L, Trollor J, Draper B, Brodaty H, Sachdev PS. Association of Dilated Perivascular Spaces With Cognitive Decline and Incident Dementia. 2021;96(11):e1501-e1511. doi: 10.1212/WNL.0000000000011537
- Okar SV, Hu F, Shinohara RT, Beck ES, Reich DS, Ineichen BV. The etiology and evolution of magnetic resonance imaging-visible perivascular spaces: Systematic review and meta-analysis. Front in Neuroscience. 2023;17:1038011 doi: 3389/fnins.2023.1038011
- Hayden MR. Brain Endothelial Cells Play a Central Role in the Development of Enlarged Perivascular Spaces in the Metabolic Syndrome. Medicina (Kaunas). 2023;59(6):1124. doi: 10.3390/medicina59061124
- Hayden MR. Overview and New Insights into the Metabolic Syndrome: Risk Factors and Emerging Variables in the Development of Type 2 Diabetes and Cerebrocardiovascular Disease. Medicina (Kaunas). 2023;59(3):561.doi: 10.3390/medicina59030561
- Javierre-Petit C, Schneider JA, Kapasi A, Makkinejad N, Tamhane AA, Leurgans SE, Mehta RI, Banes LL, Bennett DA, Arfanakis K. Neuropathologic and Cognitive Correlates of Enlarged Perivascular Spaces in a Community-Based Cohort of Older Adults. Stroke. 2020;51:2825–2833. doi: 10.1161/STROKEAHA.120.029388
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, et al. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:1339–1352. doi:10.1093/gerona/glu080
- Paavonsalo S, Lackman MH, KaramanS. Capillary Rarefaction in Obesity and Metabolic Diseases—Organ Specificity and Possible Mechanisms. Cells. 2020;9(12): 2683. doi: 10.3390/cells9122683
- Chantler PD, Shrader CD, Tabone LE, d’Audiffret AC, Huseynova K, Brooks SD, Branyan KW, Grogg KA, Frisbee JC. Cerebral Cortical Microvascular Rarefaction in Metabolic Syndrome is Dependent on Insulin Resistance and Loss of Nitric Oxide Bioavailability. Microcirculation 2015, 22, 435–445. doi: 10.1111/micc.12209
- van Dinther M, Voorter PHM, Jansen JFA, Jones EAV, van Oostenbrugge RJ, Staals J, Backes Assessment of microvascular rarefaction in human brain disorders using physiological magnetic resonance imaging. J Cereb Blood Flow Metab.2022;42(5): 718–737. doi: 10.1177/0271678X221076557
- Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat Neurosci. 2016;19(6):771–783. doi: 1038/nn.4288
- Dore-Duffy P, Andre Katychev A, Xueqian Wang X, Van Buren CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. . 2006;26(5):613-624. doi: 10.1038/sj.jcbfm.9600272
- Zheng L, Guo Y, Zhai X, Zhang YChen W, Zhu Z, Xuan W, Li P. CNS Perivascular macrophages in the CNS: From health to neurovascular diseases. Neurosci Ther. 2022;28(12):1908-1920. doi: 10.1111/cns.13954
- Rustenhoven J, Jansson D, Smyth LC , Dragunow Brain Pericytes As Mediators of Neuroinflammation. Trends Pharmocol Sci. 2017;38(3):291-304. doi: 10.1016/j.tips.2016.12.001
- Jansson D, Rustenhoven J, Feng S, Hurley D, Oldfield RL, Bergin PS, Mee EW, Faull RLM, Dragunow A role for human brain pericytes in neuroinflammation. J Neuroinflammation. 2014;11:104. doi: 10.1186/1742-2094-11-104
- Yang T, Guo R, Zhang F. Brain perivascular macrophages: Recent advances and implications in health and diseases. CNS Neurosci 2019;25(12): 1318–1328. doi: 10.1111/cns.13263
- Ide S, Yahara Y, Kobayashi Y, Strausser SA, Ide K, Watwe A, et al. Yolk-sac-derived macrophages progressively expand in the mouse kidney with age. 2020; 9: e51756. doi: 10.7554/eLife.51756
- Mato M, Ookawara S, Kurihara K. Uptake of exogenous substances and marked infoldings of the fluorescent granular pericyte in cerebral fine vessels. Am J Anat. 1980;157(3):329?332. doi: 10.1002/aja.1001570308
- Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126(12):4674?4689. doi: 10.1172/JCI86950
- Owens T, Bechmann I, Engelhardt B. Perivascular Spaces and the Two Steps to Neuroinflammation. Journal of Neuropathology & Experimental Neurology. 2008;67(12);1113–1121. https://doi.org/10.1097/NEN.0b013e31818f9ca8
- Yu L,Hu X, Li H, Zhao Y, Perivascular Spaces, Glymphatic System and MR. Front Neurol. 2022;13: doi:3389/fneur.2022.844938
- Nedergarrd M. Garbage truck of the brain. Science. 2013;28:340(6140).1529-1530. doi: 10.1126/science.1240514
- Hablitz LM, Nedergaard M. The Glymphatic System: A Novel Component of Fundamental Neurobiology. J Neurosci.2021;41(37): 7698–7711. doi: 1523/JNEUROSCI.0619-21.2021
- Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326(1):47–63.10.1016/0006-8993(85)91383-6
- Gouveia-Freitas K, Bastos-Leite Perivascular spaces and brain waste clearance systems: relevance for neurodegenerative and cerebrovascular pathology. Neuroradiology.2021; 63(10): 1581–1597. doi: 10.1007/s00234-021-02718-7
- Mendes,NF, Velloso LA. Perivascular macrophages in high-fat diet-induced hypothalamic inflammation. J 2022;19(1): 136. doi: 10.1186/s12974-022-02519-6
- Muzio L, Viotti A, Martino G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Neurosci. 15:742065. doi: 10.3389/fnins.2021.742065
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8 57–69. 10.1038/nrn2038
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms Underlying Inflammation in Neurodegeneration. 2010; 140(6): 918–934. doi: 10.1016/j.cell.2010.02.016
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006; 38, 333–347
- Streit WJ, Mrak RE, Griffin WST. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation.2004;1:14. doi: 1186/1742-2094-1-14
- Charisis S, Rashid T, Liu H, Ware JB, Jensen PN, Austin TR, et al. Assessment of Risk Factors and Clinical Importance of Enlarged Perivascular Spaces by Whole-Brain Investigation in the Multi-Ethnic Study of Atherosclerosis. JAMA Netw Open. 2023;6(4):e239196. doi: 10.1001/jamanetworkopen.2023.9196
- Xie L, Zheng L, Chen W,Zhai X, Yunlu Guo Y, Zhang Y, et al. Trends in perivascular macrophages research from 1997 to 2021: A bibliometric analysis. 2023;29(3):816-830. doi: 10.1111/cns.14034
- Sofroniew Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015; 16(5): 249–263. doi: 10.1038/nrn3898
- da Fonseca ACC, Matias D, Garcia C, Amaral R, Luiz Geraldo H, Freitas C, Souza Lima The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci.2014;8: 362. doi: 10.3389/fncel.2014.00362
- Liddelow SA,Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature.2017;541(7638): 481–487. doi: 1038/nature21029
- Verkhratsky A, Butt AM. Neuroglia: Function and Pathology. 1st; Academic Press. 125 London Wall, London EC2Y 5AS, United Kingdom 525 B Street. Copyright © 2023 Elsevier Inc. All rights reserved.
- Augusto-Oliveira M, Arrifano GP, Delage CI, Tremblay ME, Crespo-Lopez ME, Verkhratsky A. Plasticity of microglia. Biol. Rev. 2022;97:217–250. 217 doi: 10.1111/brv.12797
- Hayden MR. Hypothesis: Neuroglia Activation Due to Increased Peripheral and CNS Proinflammatory Cytokines/Chemokines with Neuroinflammation May Result in Long COVID. Neuroglia. 2021;2(1):7-35. https://doi.org/10.3390/neuroglia2010004
- Low RN, Low RJ, Akrami A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front Med (Lausanne) 2023 Mar 31;10:1011936.doi: 10.3389/fmed.2023.1011936
- Zeng Q, Li K, Luo X, Wang S, Xu X, Jiaerken Y, Liu X, Hong L, Hong H, Li Z, Fu Y, Zhang T, Chen Y, Liu Z, Huang P, Zhang M; for behalf of Alzheimer's Disease Neuroimaging Initiative (ADNI). The association of enlarged perivascular space with microglia-related inflammation and Alzheimer's pathology in cognitively normal elderly. Neurobiol Dis. 2022;170:105755. doi: 10.1016/j.nbd.2022.105755
- Sepehrband F, Barisano G, Sheikh-Bahaei N, Cabeen RP, Choupan J, Law M, Toga Image processing approaches to enhance perivascular space visibility and quantification using MRI. Sci Rep.2019; 9: 12351. doi: 10.1038/s41598-019-48910-x
- Paradise MB, Beaudoin MS, Dawes L, Crowford JD, Wen W, Brodaty H, Sachdev PS, Development and validation of a rating scale for perivascular spaces of 3T MRI. J Neurol Sci. 2020;409:116621. Doi: 10.1016/j.ins.2019.116621
- Pham W, Lynch M, Spitz G, O'Brien T, Vivash L, Sinclair B, Law M. A critical guide to the automated quantification of perivascularspaces in magnetic resonance imaging. Front Neurosci. 2022 Dec 14;16:1021311. doi: 10.3389/fnins.2022.1021311
- Boespflug EL, et al. MR Imaging–based Multimodal Autoidentification of Perivascular Spaces (mMAPS): Automated Morphologic Segmentation of Enlarged Perivascular Spaces at Clinical Field Strength. Radiology.2018;286:632–642. doi: 10.1148/radiol.2017170205
- Jung, E., Zong, X., Lin, W., Shen, D. & Park, S. H. Predictive Intelligence in Medicine. In International Workshop on PRedictive Intelligence In MEdicine11121, 18–25 (Springer, 20108)
- Ramirez J, et al. Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer’s disease patients and normal elderly from the Sunnybrook dementia study. J. Alzheimer’s Dis. 2015;43:415–424. doi: 10.3233/JAD-132528
- Wang X, et al. Development and initial evaluation of a semi-automatic approach to assess perivascular spaces on conventional magnetic resonance images. J. Neurosci. Methods.2016;257:34–44. doi: 10.1016/j.jneumeth.2015.09.010
- Hou Y, et al. Enhancement of Perivascular Spaces in 7 T MR Image using Haar Transform of Non-local Cubes and Block-matching Filtering. Rep. 2017;7:1–12. doi: 10.1038/s41598-016-0028-x ]
- Ballerini L, Lovreglio R, Hernandez MDCV, Ramirez J,MacIntosh BJ, Black SE, Wardlaw JM. Perivascular Spaces Segmentation in. Brain MRI Using Optimal 3D Filtering. Rep.2018;8:1–11. Sci Rep. 2018;8(1):2132. doi: 10.1038/s41598-018-19781-5
- Valdes Hernandez DC, et al. Towards the automatic computational assessment of enlarged perivascular spaces on brain magnetic resonance images: A systematic review. J. Magn. Reson. Imaging. 2013;38:774–785. doi: 10.1002/jmri.24047
- Descombes X, Kruggel F, Wollny G, Gertz HJ. An Object-Based Approach for Detecting Small Brain Lesions: Application to Virchow-Robin Spaces. IEEE Trans. Med. Imaging.2004;23:246–255. doi: 10.1109/TMI.2003.823061
- Zong X, Park SH, Shen D, Lin W. Visualization of perivascular spaces in the human brain at 7T: Sequence optimization and morphology characterization. 2016;125:895–902. doi: 10.1016/j.neuroimage.2015.10.078
- Park SH, Zong X, Gao Y, Lin W, Shen D. Segmentation of perivascular spaces in 7 T MR image using auto-context model with orientation-normalized features. 2016;134:223–235. doi: 10.1016/j.neuroimage.2016.03.076
- Frangi Alejandro F., Niessen Wiro J., Vincken Koen L., Viergever Max A. Medical Image Computing and Computer-Assisted Intervention — MICCAI’98.Berlin, Heidelberg: Springer Berlin Heidelberg; 1998. Multiscale vessel enhancement filtering; pp. 130–137.
- Dubost F, et al. 3D regression neural network for the quantification of enlarged perivascular spaces in brain MRI. Image Anal. 2019;51:89–100. doi: 10.1016/j.media.2018.10.008
- Sepehrband F,Barisano G , Sheikh-Bahaei N, Choupan J, Cabeen RP Lynch KM, Crawford MS, Lan H, Mack WJ , Chui HC, Ringman JM, Toga AW; Alzheimer’s Disease Neuroimaging Initiative. Volumetric distribution of perivascular space in relation to mild cognitive impairment. Neurobiol Aging, 2021;99:28-43. doi: 10.1016/j.neurobiolaging.2020.12.010
- Ineichen BV, Okar SV, Proulx ST, Engelhardt B, Lassmann H, Reich DS. Perivascular spaces and their role in neuroinflammation. 2022;110(21):3566-3581. doi: 10.1016/j.neuron.2022.10.024.
Citation: Hayden MR (2023) Pericytes and Resident Perivascular Macrophages Play a Key Role in the Development of Enlarged Perivascular Spaces in Obesity, Metabolic Syndrome and Type 2 Diabetes Mellitus. J Alzheimers Neurodegener Dis 9: 062.
Copyright: © 2023 Melvin R. Hayden, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

