
Pharmaco-Audiology Vigilance and Highly Active Antiretroviral Therapy (HAART) in South Africa: Ototoxicity Monitoring Pursued
*Corresponding Author(s):
Khoza-Shangase KDiscipline Of Speech Pathology And Audiology, School Of Human & Community Development, Faculty Of Humanities, University Of The Witwatersrand, Johannesburg, South Africa
Tel:0823390605,
Fax:0865536055
Email:Katijah.khoza@wits.ac.za
Abstract
Keywords
INTRODUCTION
Advances in clinical as well as psychosocial management of patients with HIV/AIDS have dramatically changed since the early stages of the pandemic, where treatment strategies did not appear to have had a positive influence on patients’ lives. The field of HIV/AIDS has advanced to such an extent that it has become acceptable and expected to focus energies on the quality of life issues surrounding HIV/AIDS and its treatments, rather than the sole focus on saving and sustaining life. It is within this context that research into sensory disabilities associated with HIV/AIDS is receiving more attention by academics as well as the medical fraternity. Within the head and neck, audiological manifestations including hearing loss and vestibular symptoms require attention to achieve clearer categorization of the symptomatology as well as sequellae of treatment options. Hence, the current researcher’s support of Friedman and Noffsinger’s [1] belief that as primary professionals in hearing health care, audiologists have a duty to inform not only themselves; but also other relevant health-care professionals about this issue, hence the current study.
Due to the fact that patients with HIV/AIDS are living longer because of the positive effects of HAART, it has become crucial that effects of HIV/AIDS and its treatments on the auditory system are better understood. It is well understood that the face of the HIV/AIDS pandemic has and continues to change internationally due to the discovery of antiretroviral drugs [2]. These advancements in treatment have also led to improvements in the medical field with people who have HIV/AIDS surviving for extended periods of time experiencing toxic-related morbidity that has influences on quality of life indicators [2]. There has always been a concern, nonetheless, that HIV-associated auditory disorders may be well under-reported; and this is thought to have possibly been due to the fact that even though hearing loss and dizziness are often the initial symptoms of underlying auditory system disease, these may not have been reported by patients prior to HAART because many patients focused on the life-threatening complications of the HIV disease rather than on quality of life issues [3]. In South Africa, where roll-out of HAART only occurred as recently as 2004, and where currently universal HAART coverage has still not been achieved, this under-reporting of auditory symptoms may still be highly prevalent. It is likely that reporting of quality of life complications will only be evidenced once universal coverage is attained for the population infected.
According to the research literature, auditory abnormalities associated with HIV/AIDS and its treatments have been reported in persons with varying degrees of HIV infection, as well as in treatment naïve as well as patients on antiretroviral treatment [4-8]. Causes of these abnormalities can be direct [4-7] as well as indirect [6-8]; although this distinction is not always clear and consistent. The direct or primary effects are reportedly due to the fact that the virus is neurotropic in nature and commonly manifests itself neurologically [4-7]. The indirect and/or iatrogenic causes are linked to opportunistic infections which require suppressive therapy, thereby leading to ototoxicity [6-8]. This evidence is based on numerous clinical, and mostly medically oriented studies that have demonstrated the occurrence of hearing loss and other auditory manifestations in HIV/AIDS. This evidence demonstrates that auditory manifestations may be one of the challenges that the population will have to deal with; therefore over and above management of the known side effects of ARVs, research into the identification and monitoring of all other manifestations of the disease is required. It is important to state that a majority of these findings are mainly from developed countries where the presentation and management of HIV/AIDS varies to that in developing countries, suggesting a need for more research into this area particularly since the numbers of adults living with HIV/AIDS in developing countries such as South Africa continues to remain high, and also because the context is different. With regard to auditory manifestations, both identification and monitoring of ototoxicity require rigorous research to enhance the patients’ quality of life, particularly since internationally; a link has been established between ARVs and ototoxicity [6-10].
Due to the fact that opportunistic infections are common in HIV/AIDS, use of a drug regimen that often involves potentially ototoxic medications [9,10] is not unusual. This therefore increases the likelihood of ototoxicity in this population; with particular contribution of the use of experimental antiretroviral drugs with undocumented or unknown side effects [8]. In South Africa, ototoxic drugs that are often used in the treatment of tuberculosis as an opportunistic infection increase the potential for a drug-induced hearing loss in this population [10]. Although the side-effects of many antiretroviral drugs are yet to be determined, HIV-infected individuals are often prescribed medications as a prophylaxis or treatment of opportunistic infections that have been long associated with the development of audiological and vestibular changes [8,11-14]. Antineoplastic medications such as vincristine, antifungal agents including amphotericin B, flucytosine and ketoconazole, immune modulators, aminoglycoside antibiotics, erythromycin, and Azidothymidine (AZT) are all widely prescribed in the management of HIV and are all reported to be associated with significant ototoxicity [7,8,11-14]. These medications are associated with hearing loss, tinnitus and vertigo. Frequently administered medications for PCP (Pentamidine, TMP/SMX, Primaquine) may cause tinnitus, vertigo, dizziness, auditory disturbances, deafness, decreased hearing, hearing loss, and otalgia [8]. Moreover, the use of experimental medications with relatively unknown toxicity as well as the use of ototoxic drugs, such as anti-Tuberculosis (TB) medications, in combination adds to the overall effect on hearing [15].
As mentioned earlier, in South Africa, one of the most frequently administered treatments to the HIV/AIDS population is that of TB treatment, where TB cases have dramatically increased over the past decade [16,17]. This upsurge in TB cases seems to continue, largely due to co-infection with the HIV, and with the emergence of drug resistant TB [17]. This co-occurrence of HIV/AIDS and TB has important clinical implications for the audiologist with regard to the possible association between TB treatment [18,19] and HAART. This implication is even more pronounced where the TB and HIV infected patient also happens to be under chronic noise exposure; which can act synergistically to exacerbate auditory symptoms. The concern with TB treatment is because some of the drugs used in the treatment of TB fall under the umbrella term ‘aminoglycosides’, which are known to be ototoxic in nature. Examples of these aminoglycosides include amikacin, gentamicin, kanamycin, netimicin, paromomycin, streptomycin, tobramycin, and apramycin [19].
As far as HIV/AIDS treatment is concerned; the impact of medications on hearing function have been reported, although not extensively, with Nucleoside analogue Reverse Transcriptase Inhibitors (NRTIs). Although a variety of adverse effects have been attributed to NRTIs, only a small number of cases of ototoxicity have been reported in the literature; particularly from developing country contexts. Case studies [20] have been reported where ototoxicity has been noted; although with a number of confounding variables including advanced age, occupational noise exposure, and prior hearing loss. One should note that not all of the currently reviewed studies utilized sensitive ototoxicity monitoring protocols such as extended high-frequency audiometry and/or otoacoustic emissions. Furthermore, some of these studies also did not follow longitudinal research designs that could have allowed the researchers to investigate within-subject changes, but they rather followed cross sectional methodology designs. In addition, the reports that other factors such as age, drug interactions, concomitant noise exposure, and so on may have an influence on the ototoxicity of HAART should be taken into consideration when reviewing the effects of HAART on hearing. Several other international cases of ototoxicity have been reported in HIV-infected patients who had been treated with zalcitabine [21-23], didanosine [24], zidovudine [20], and combinations of zidovudine and didanosine [25], stavudine and lamivudine [15], stavudine, lamivudine, didanosine, and hydroxyurea [15], and post exposure prophylaxis with stavudine, lamivudine, and nevirapine [26]. Furthermore, a study of 99 HIV-infected individuals on HAART revealed that hearing loss was common in their sample. This hearing loss was mostly associated with being 35 or older and with a history of ear infection, and there was a trend toward an association with documented receipt of therapy with antiretroviral drugs in the preceding 6 months [27]. Moreover, previous cross-sectional studies and case reports have shown an association between hearing loss and NRTI therapy [15,27,28]. There have been two case reports of hearing loss in persons receiving HAART regimens that included NRTIs and a second class of antiretroviral drugs; one with a NNRTI (Nevirapine) and one with a Protease Inhibitor (PI) (lopinavir/ritonavir) each combined with NRTIs, (both these subjects were also receiving stavudine and lamivudine) [20,26,29].
As earlier mentioned, while ototoxic hearing loss has been described in HIV-infected people, there have been extremely limited prospective studies [30]. Hence there still needs to be extensive investigations to clearly establish and confirm this relationship. The study by Schouten et al., [30] investigated hearing changes longitudinally in treatment-naïve HIV-infected patients following initiation of regimens containing NRTIs; and the results revealed changes in hearing levels at all frequencies at 32 weeks following commencement of HAART. The changes were however not attributed to treatment with ZVD and ddI; even after taking into account noise exposure, immune status and age. The results of this prospective pilot study did not support the view that treatment with nucleoside antiretroviral drugs damages hearing. These results were consistent with the report from the Adult/Adolescent Spectrum of HIV Disease Project Group that demonstrated no association between hearing loss and drugs used in the treatment of HIV; but were contrary to findings from other cross-sectional studies and case reports that indicated ototoxicity to be common in this population [27,31,32].
Reviewed studies have several criticisms that can be levelled against them when it comes to their methodological designs. Firstly, most studies did not incorporate Otoacoustic Emissions (OAEs) as part of their monitoring battery, and this could have had a significant impact on their results since OAEs have been shown to be sensitive to cochlear damage in ototoxicity monitoring. Secondly, most included small sample sizes; mostly case reports, and small sample sizes significantly reduces the strength of the study in terms of the ability to generalize the findings. Additionally, small sample sizes limit the power of the studies in detecting a difference and also limit their ability to accurately interpret results. Thirdly, and understandably; there were no control groups. Finally, extended high-frequency audiometry did not form part of the test battery in most. Extended high-frequencies have been reported to be finely tuned to the effect of damaging environmental factors such as noise and ototoxic drugs [12].
It is for the aforementioned reasons that the current study was conceptualised. It was deemed important to conduct longitudinal studies in developing countries such as South Africa; although challenges are anticipated in such contexts. These challenges include firstly, the fact that the nature of the HIV/AIDS disease and the population being studied may preclude complete control over confounding variables; and secondly, securing decent sized comparison groups may be difficult, thereby preventing randomized matching of participants; and thirdly, extended high-frequency audiometry which does not form part of the routine audiological test battery may influence the type of results found; and this influence could reflect in clinical changes in the extended high-frequencies depicted on the audiogram being entirely missed. Lastly, the length of time for which the audiologic monitoring can occur due to attrition may be too short to allow for clinical hearing loss possibly caused by HAART to manifest and therefore be detected on the audiogram; because of the migrating nature of patients in the public health care sector. Nonetheless, such longitudinal studies of patients on various regimens of HAART need to be performed. These need to be conducted in order to determine if any hearing changes occur during the period when the patients are receiving HAART; hence the current study. It is also important that assessment measures such as DPOAEs and extended high frequency audiometry, which are sensitive to cochlear changes, form part of the methodologies employed [33]. The current study therefore aimed to monitor the auditory status in two groups of adults with AIDS receiving HAART–two different regimens (Group A: nevirapine, 3TC-lamivudine, and D4T-stavudine; and Group B: AZT-azidothymidine, 3TC, and Efavirenz) in hospital outpatient clinics in Johannesburg, Gauteng, South Africa. The anticipated significance of the study besides adding to the published evidence on ototoxicity from developing countries; was to aid in guiding audiological clinical management of patients with HIV/AIDS in order to enhance their quality of life.
MATERIAL AND METHODS
Research aims and objectives
Specific objective: To longitudinally assess hearing function in AIDS infected adults on HAARTThe null hypothesis was that the participants’ hearing function before and after antiretroviral drug-use would remain the same. The alternative hypothesis was that it would not remain the same, that is, participants would present with changes in their auditory function [34-36].
Design of the study: The design adopted in the current study was a repeated measures, quasi-experimental design with pre- and post-treatment testing [37]. The advantage of the chosen design was the fact that it is purported to be the best design where there are practical and ethical barriers to conducting randomized controlled trials [38]. The aim was to assess and monitor the hearing function before and during antiretroviral treatment-with measures taken before commencement of ARVs, and at every three months interval; with the last analysed measures taken at 15 months following initiation of treatment. The antiretroviral medications and other therapies were the independent variables, with the audiological measures (otoscopy, impedance audiometry, pure tone audiometry, otoacoustic emissions) being dependent variables.
Participants: A total sample of 41 in Group A (mean age=34.6yrs; median=33yrs) and 33 in Group B (mean age=33.6yrs; median=33yrs) participated in the current study. The patients selected for this study were recruited from the hospital’s Adult HIV/AIDS clinics. Patients attending these Johannesburg based clinics have already been diagnosed with HIV/AIDS and are seen there for general medical management as well as antiretroviral treatment and monitoring. At the time of the study all patients with CD4+ counts below 250 cells/mm3 had access to ARV treatment, at these clinics-and this is the group that was targeted for the current study. Because the sample was restricted to a part of the population that was readily available, a nonprobability convenience sampling technique was adopted [37,39].
For participant selection criteria; although the researcher strongly believed that having strict inclusion criteria would create an artificial environment; not authentically representative of the adult HIV population in the context of the study; the researcher still believed that it was crucial to have some degree of control over variables which are known to have potential confounding effects on the results of the study (e.g. advancing age, noise exposure, history of TB treatment, syphilis, and so forth) [37,39,40]. These participant inclusion and exclusion criteria that were adopted following baseline testing are depicted in Table 1.
| Inclusion Criterion | Present |
| HIV/AIDS positive serology | Yes |
| On one of the 2 identified ARVs regimen | Yes |
| Age between 18 and 50 years | Yes |
| Alert and oriented | Yes |
| Normal pure tone audiometry (thresholds better or equal to 25dBHL) at baseline | Yes |
| Resides in Johannesburg, Gauteng for the duration of the study | Yes |
| Exclusion Criterion | Present |
| Noise exposure | No |
| Recent (less than 3 years) or current history of treatment for TB and radiotherapy | No |
| Positive clinical or serological evidence of syphilis | No |
| Middle ear pathology | No |
| Presence of tinnitus | No |
| Recent (less than 3 years) history of previous ARV use | No |
Table 1: Summary of participant Inclusion and Exclusion Criteria following baseline measures.
RESEARCH PROCEDURES AND MATERIALS
Following infection control measures proposed by Kemp and Roeser [41], all testing was conducted in a sound-proof booth. Pure tone audiometry testing followed by Distortion Product Otoacoustic Emission (DPOAE) measurements for all participants was undertaken and systematically recorded.
Case history and otoscopy
Impedance audiometry
Pure tone audiometry
Distortion product otoacoustic emissions
Test parameters: Diagnostic/High frequencyStimuli:Intensity level L1-L2=10dB (e.g. L1=65dB, L2=55dB)Ratio f2/f1=1.22Frequency range 750 to 8000 HzThe presence of the DPOAE was determined by comparing the amplitude of the DPOAE to that of the noise floor to calculate the size of the emission. A Distortion Product (DP)-amplitude that exceeded the noise floor by at least 7dB across all frequencies measured was regarded as indicative of a normally functioning cochlea [33,40]. The size of the emission at the different frequencies measured was then monitored over the four testing sessions.
VALIDITY AND RELIABILITY
DATA ANALYSIS AND STATISTICAL PROCEDURES
Firstly, statistical comparison was done basing the results on the average change from baseline. Each frequency’s mean change from baseline for the ears individually was computed and then combined for both pure tone audiometry and distortion product otoacoustic emissions. Repeated-measures analysis of variance [34] was used to compare the mean change from baseline from session to session. In the analysis of the DPOAE data, the baseline DPOAE levels in decibels SPL for each f2 value tested were compared with the corresponding session 2, 3 and 4 measurements. The signal-to-noise difference was used as the measure of DPOAE amplitudes. For pure tone audiometry data, the baseline thresholds in decibels HL for each frequency were compared with the corresponding session 2, 3 and 4 results as well. To statistically test the hypothesis, a threshold P value (alpha) of 0, 05 was selected, [47]. Lastly, as part of statistical analysis of the data, a post-test in the form of the Tukey-Kramer multiple-comparison post-test was conducted. This test was conducted to compare pairs of group means so as to identify where, precisely, statistically significant changes occurred along the time continuum [40] (baseline to 15 months)-if they did.
Secondly, for the purposes of the current study, clinical significance (changes that are deemed significant enough to indicate structural and functional changes on the ear–as observed on the audiogram) over and above statistical significance was examined. The current researcher supports the school of thought that maintains that assessing an intervention’s effect should not only focus on the statistical significance of the findings, but should also focus at the clinical relevance or importance of these outcomes [40,48]. For pure tone testing, most often a change of 10dB at one or more frequencies is commonly taken to be indicative of some significant change [44]; andthis was the protocol followed in the current study [40]. As far as DPOAEs are concerned, change is only regarded as significant in DPOAE measures if there is a change of at least more than 6dB in DPOAE level between consecutive measures [49,50]. Absence of clinically significant changes in DPOAEs confirm intact cochlear functioning and the absence of evidence of cochlea damage, even microcochlear pathology–which is often evident on OAEs long before being depicted on the pure tone audiogram [33,40].
Table 2 provides a summary of all collection material and test procedures used in this study.
| Equipment | Function | Pass Criteria | Fail Criteria |
| Case history form | Gather important case history data | Refer to inclusion and exclusion criteria | Refer to inclusion and exclusion criteria |
| Welch Allyn Otoscope | Visual inspection of the ear | Clear outer ear with normal and tympanic membrane | Obstruction; abnormal tympanic membrane,pathologies of the outer and middle ear |
| AZ26 Interacoustictympanometer | Middle ear functioning assessment | Type A tympanogram | Other tympanograms but type A |
| AC40 Diagnostic audiometer | Conventional pure tone audiometry (250 - 8000Hz)Extended High-Frequency audiometry (10, 12 and-16kHz) | Thresholds at and better than 25dBHL and no Air-Bone Gap | Thresholds worse than 25dBHL |
| Monitoring function | No 10dB threshold change at one or more frequencies over time | A change of 10dB at one or more frequencies over time | |
| Biologic Scout OAE machine | Diagnostic DPOAE measurement | Greater than 7dB DP amplitude at frequencies assessed | Less than 7dB DP amplitude |
| Ototoxicity monitoring | No DPOAE level change of more than 6dB between consecutive measures | Change of more than 6dB in DPOAE level between consecutive measures |
ETHICAL CONSIDERATION
RESULTS
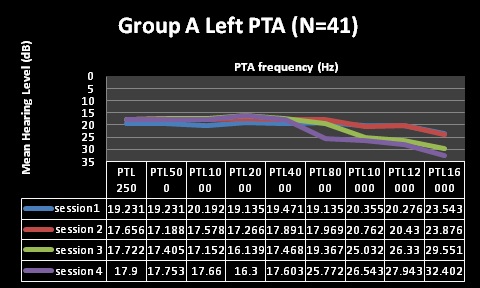
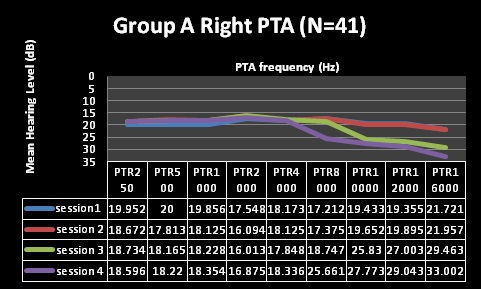
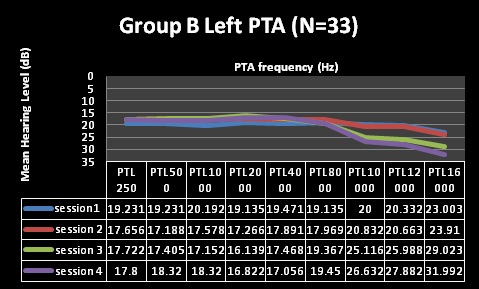
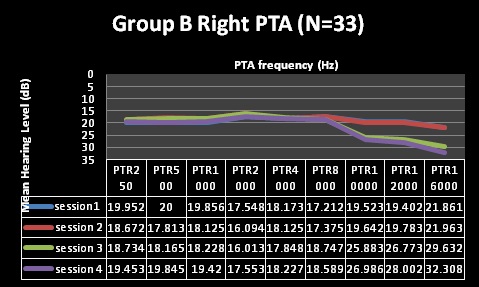
| Factor | Group A (N=41) | Group B (N= 33) |
| Pure Tone Audiometry (PTA) | Normal through first 2 testing sessions, changes at 10,12 and 16kHz at session 3, changes including 8kHzat session 4 | Normal through first 2 testing sessions, changes at 10,12 and 16kHzat session 3, and 4 |
| Clinical Analysis | Clinically significant changes @ 10, 12 and 16kHz | Clinically significant changes @ 10, 12 and 16kHz |
| (change of 10dB at one or more frequencies) | (change of 10dB at one or more frequencies) | |
| Statistical Analysis | Statistically significant changes at10, 12 and 16kHz (alpha was less than 0.05) | Statistically significant changes at 12 and 16kHz (alpha was less than 0.05) |
| Statistically non-significant changes at 8kHz | Statistically non-significant changes at 10kHz | |
| Factor | Group A (N=41) | Group B (N= 33) |
| Distortion Product Otoacoustic Emissions (DPOAEs) | Reduced/absent DPOAEs at session 2 (reduced),3 and 4 | Reduced/absent DPOAEs at session 2 (reduced), 3 and 4 |
| Clinical Analysis | Clinically significant changes at 6,8 kHz at session 3 and 4 | Clinically significant changes at 8 kHz at session 3 and 4 |
| (6 to 9dB change) | (6 to 9dB change) | |
| Statistical Analysis | Statistically significant changes (p<.001) at6 and 8kHz at session 2, 3and4 | Statistically significant changes (p<.001) at8kHz at session 2, 3 and4 |
Furthermore, DPOAE measures for both groups A and B (Table 3 and Figures 5-8) revealed commencement of decreasing cochlea functioning (as indicated by reduced DPOAE amplitudes); although still within normal limits with average DPOAE emission size being above the level regarded as indicative of normally functioning cochlea across all frequencies evaluated for sessions one and two. MANOVA tables indicated that these changes were not statistically significant (p>0.05). Furthermore, these changes were also not clinically significant. Change is only regarded as clinically significant in DPOAE measures if there is a change of at least 6dB in DPOAE level between consecutive measures [49,50]. At sessions 1 and 2 in both groups; none of the mean changes in DPOAE results were greater than 6dB–indicating that no clinical changes occurred in the cochlear function.
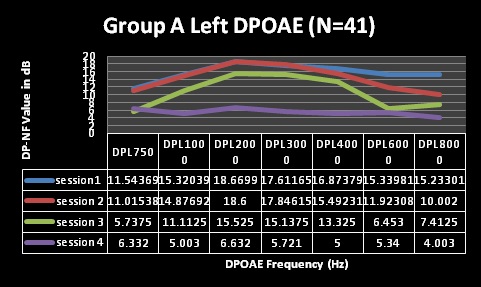
Figure 5: Changes in Distortion Product Otoacoustic Emissions (DPOAEs) per frequency over time (group A, left ear).
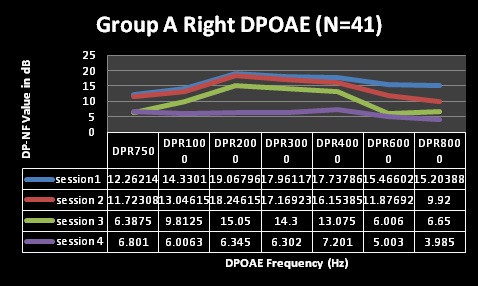
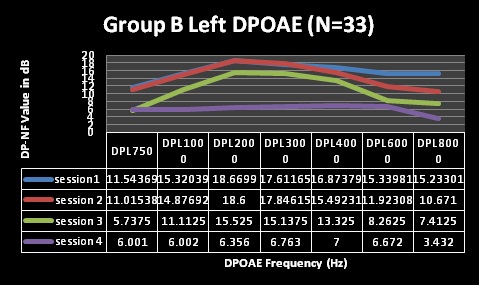

Despite the essentially normal hearing function in high frequencies at sessions 1 and 2, results of the DPOAE analysis in both groups revealed cochlea function to be abnormal from session 2 at 8 kHz in both groups with changes indicating declining DPOAE values at repeated measures. These changes were found to occur at all frequencies evaluated but were more clinically significant at the high frequencies (and 6 and 8 kHz in group A) at session 3 and 4. The DPOAE results at these two frequencies at session 3 were in fact below the norm in that the DPOAE value did not exceed the noise floor by at least 7 dB as expected in a normally functioning cochlea. Statistically, MANOVA [within group (time)] results indicated extremely significant (p < 0.001) for all frequencies assessed–implying that cochlear function changed after HAART initiation.
For the statistically significant results for DPOAEs in the ‘within group’ analysis, the Tukey-Kramer test indicated that, generally, the significant changes occurred between baseline measures and session 2 (at 3 months) for the lower frequencies, with the higher frequencies being significantly affected throughout the 4 sessions of testing.
DISCUSSION
These preventative measures would include the use of oto-protective agents as well as dose and medications considerations. When life-threatening illness necessitates treatment with ototoxic drugs, preserving the quality of the patients’ remaining life is customarily a treatment goal. Early detection of ototoxic hearing loss provides physicians with the critical information and opportunity necessary to minimize further impairment and, in some cases, prevent hearing loss from progressing to the point where permanent damage occurs. Although hearing loss is not regarded as a life-threatening condition, it does become a severe threat to essential quality of life indicators unless intervention occurs early during treatment. The adverse effects of a hearing loss on cognitive-linguistic skills and psychosocial behaviour are well documented, as well as the serious vocational, social, and interpersonal consequences for the patient [40]. One might argue that HAART plays a critical role in the treatment of a serious or life-threatening disease, that it offers such important therapeutic effects compared to the ototoxic side effects, that the ototoxicity risk can be considered to be of minor importance. However, the problem of ototoxic side effects is reported to be critical in developing countries, where highly effective and low-cost drugs are more easily prescribed without adequate monitoring [40].
This problem in compounded by the fact that patients on potentially ototoxic medication, without being audiologically monitored, may be exposed to other toxins such as noise exposure following treatment with ototoxic drugs which can act synergistically with the drugs that have not been fully cleared from the inner ear [40]. Increased susceptibility to hearing loss can continue for several months after completion of treatment or therapy with ototoxic drugs, and this might have a different meaning in the case of ARVs where the treatment is for life. Because of this concern, it becomes crucial that hearing conservation in the form of advising patients to avoid excessive noise exposure for at least six months following treatment is instituted. Additionally, provision of hearing aids to this population may require closer monitoring and detailed patient counselling with regards to careful monitoring and control of the hearing aid maximum output during HAART use [40].
The presence of significant changes on DPOAEs in the longitudinal follow up sessions in both groups mirrored previous findings [40] which indicated microcochlear pathology that was not necessarily indicated on pure tone audiometry, when basic audiometry (250Hz-8000Hz) was used. This microcochlear pathology was however not sub-clinical in the current study as it was depicted on the extended high-frequency changes identified in the current study. It is clear from the current study that based on the objective nature of DPOAE measures, subclinical auditory changes occurred as early as 3 months, with clinical changes clearly identifiable by 9 months following HAART initiation in both groups. These findings firstly, highlight the importance of the use of such sensitive measures (DPOAE) in monitoring the possible effects of toxins on the ear since DPOAE have been shown to have an arguably important role to play in this regard. Secondly, these findings reveal the crucial need for including extended high-frequencies in ototoxicity monitoring to be able to identify clinical changes on the audiogram early enough before these changes include frequencies important for speech perception.
The presence of extended high-frequency and DPOAE changes indicating changes in hearing status suggested hearing loss that could have possibly been due to medications used by participants in the two groups (Group A: nevirapine, 3TC-lamivudine, and D4T-stavudine; and Group B: AZT-azidothymidine, 3TC, and efavirenz). These results were consistent with the reports that have associated iatrogenic hearing loss with many of the drugs used to treat HIV/AIDS [11,14,40].
Although one cannot draw definitive conclusions that HAART regimens used in the current samples had a direct effect on hearing – because of the difficulty in controlling extraneous variables in such a population, and also because of the lack of a control group; findings are supported by previous evidence. Current findings are also consistent with ototoxic hearing loss in terms of nature and configuration of the loss on audiograms–where high frequencies are the primary initial site of clinical change. Controlling for extraneous variables in human subjects with AIDS is an impossible task, and the current researcher is of the strong opinion that isolating all the possibly contributing confounding variables may provide a more definitive answer with a clearer cause-and-effect relationship, but may not necessarily provide a practical, relevant, and context-sensitive finding. Within the South African AIDS population, it may be impossible to find participants who are only exposed to just one strict HAART regimen without any other medications prescribed for the numerous other conditions the patient might present with, including TB. Furthermore, the possible effects of the use of alternative/complimentary medications by a large majority of South Africans alongside HAART cannot be ignored. Current results are believed to be valuable evidence for the South African context, and provide more realistic and context specific implications, those of the importance of audiologists’ involvement in the drug development process, the audiologists’ inclusion in the treatment teams where ototoxic medications are prescribed, the need for establishment and implementation of ototoxicity monitoring protocols as part of routine clinical management of adults with HIV/AIDS, and the inclusion of both DPOAEs and extended high-frequency audiometry in ototoxicity monitoring protocols.
Current findings, confirm earlier findings by Khoza-Shangase [40] where ototoxicity of lamivudine-3TC, stavudine-D4T and efavirenz was monitored in a group of adults, with an additional new finding illustrating the usefulness of extended high-frequency audiometry in early identification of drug-induced hearing loss. Firstly, the importance of analysing both clinical and statistical significance in findings was re-affirmed. Current findings highlight the importance of including both these means of establishing significance in such longitudinal audiological studies, where one might find statistically non-significant but clinically significant changes, and vice versa–or the ideal where clinically and statistically findings are obtained.
CONCLUSION
It is important to interpret findings from the current study having taken cognizant of the identified methodological limitations. The main limitations of the current study included, firstly, that for ethical and practical reasons there was no control group, but this was partially overcome by the fact that each participant served as their own control within the longitudinal study design. Secondly, the nature of the HIV/AIDS disease and the population being studied precluded complete control over confounding variables that could have had an influence on the results such as interactions of ARVs with other therapies, especially traditional medicines which have been reported to be in widespread use. Nevertheless, current findings add to the growing evidence on links between HAART and ototoxicity, and aid in attempting to provide an answer to the question: Does HAART sound toxic? Current findings clearly demonstrate the ototoxic nature of HAART; and also present an argument for pharmaco-audiology vigilance when HAART is used in South Africa: a continued need for pursuing ototoxicity monitoring.
REFERENCES
- Friedman JL, Noffsinger D (1998) Hearin loss associated with HIV/AIDS: Social, cultural, and political issues. Seminars in Hearing 19: 205-214.
- Zapor MJ, Cozza KL, Wynn GH, Wortmann GW, Armstrong SC (2004) Antiretrovirals, Part II: focus on non-protease inhibitor antiretrovirals (NRTIs, NNRTIs, and fusion inhibitors). Psychosomatics 45: 524-535.
- Zuniga J (1999) Communication disorders and HIV disease. J Int Assoc Physicians AIDS Care 5: 16-23.
- McArthur JC (1987) Neurologic manifestations of AIDS. Medicine (Baltimore) 66: 407-437.
- Kallail KJ, Downs DW, Schertz JW (2008) Communication disorders in individuals with HIV/AIDS. Kansas Journal of Medicine.
- Bankaitis AE (1996) Audiological changes attributable to HIV. Audiology Today 8: 14-16.
- Lalwani AK, Sooy CD (1992) Otologic and neurologic manifestations of acquired immunodeficiency syndrome. The Otolaryngological Clinics of North America 25: 1183-1198.
- Bankaitis AE, Schountz T (1998) HIV-Related Ototoxicity. Seminars in Hearing 19: 155-163.
- Birchall MA, Wight RG, French PD, Cockbain Z, Smith SJ (1992) Auditory function in patients infected with the human immunodeficiency virus. ClinOtolaryngol Allied Sci 17: 117-121.
- Khoza-Shangase K, Mupawose A, Mlangeni NP (2009) Ototoxic effects of tuberculosis treatments: How aware are patients? African Journal of Pharmacy and Pharmacology 3: 391-399.
- Bankaitis AE, Keith RW (1995) Audiological changes associated with HIV infection. Ear Nose Throat J 74: 353-359.
- Campbell KCM (2007) Pharmacology and ototoxicity for Audiologists. United States: Thomson/Delmar Learning.
- Gold S, Tami TA (1998) Otolaryngological Manifestations of HIV/AIDS. Seminars in Hearing 19: 2.
- Kohan D, Hammerschlag PE, Holliday RA (1990) Otologic disease in AIDS patients: CT correlation. Laryngoscope 100: 1326-1330.
- Simdon J, Watters D, Bartlett S, Connick E (2001) Ototoxicity associated with use of nucleoside analog reverse transcriptase inhibitors: A report of 3 possible cases and review of the literature. Clin Infect Dis 32: 1623-1627.
- Clarke M, Dick J, Bogg L (2006) Cost-effectiveness analysis of an alternative tuberculosis management strategy for permanent farm dwellers in South Africa amidst health service contraction. Scand J Public Healt 34: 83-91.
- Aziz MA, Wright A, Laszlo A, De Muynck A, Portaels F (2006) Epidemiology of anti-tuberculosis drug resistance (the global project on anti-tuberculosis drug resistance surveillance): An updated analysis. Lancet 368: 2142-2154.
- Smith A, MacKenzie I (1997) Hearing loss from ototoxics. WHO Drug Information 11: 7-10.
- Cohn A (1981) Etiology and pathology of disorders affecting hearing. Medical audiology: Disorders of hearing. New Jersey: Prentice-Hall.
- Simdon J, Watters D, Bartlett S, Connick E (2001) Reply. Clin Infect Dis33: 2101-2102.
- Martinez OP, French MA (1993) Acoustic neuropathy associated with zalcitabine-induced peripheral neuropathy. AIDS 7: 901-902.
- Monte S, Fenwick JD, Monteiro EF (1997) Irreversible ototoxicity associated with zalcitabine. Int J STD AIDS 8: 201-202.
- Powderly WG, Klebert MK, Clifford DB (1990) Ototoxicity associated with dideoxycytidine. Lancet 335: 1106.
- Vogeser M, Colebunders R, Depraetere K, Van Wanzeele P, Van Gehuchten S (1998) Deafness caused by didanosine. Eur J ClinMicrobiol Infect Dis 17: 214-215.
- Christensen LA, Morehouse CR, Powell TW, Alchediak T, Silio M (1998) Antiviral therapy in a child with pediatric Human Immunodeficiency Virus (HIV) case study of audiologic findings. J Am AcadAudiol 9: 292-298.
- Rey D, LHeritier A, Lang JM (2002) Severe ototoxicity in a health care worker who received postexposure prophylaxis with stavudine, lamivudine, and nevirapine after occupational exposure to HIV. Clin Infect Dis 34: 417-418.
- Marra CM, Wechkin HA, Longstreth WT Jr, Rees TS, Syapin CL, et al. (1997) Hearing loss and antiretroviral therapy in patients infected with HIV-1. Arch Neurol 54: 407-410.
- McNaghten AD, Wan PC, Dworkin MS; Adult/Adolescent Spectrum of HIV Disease Project Group (2001) Prevalence of hearing loss in a cohort of HIV-infected patients. Arch Otolaryngol Head Neck Surg 127: 1516-1518.
- Williams B (2001) Ototoxicity may be associated with protease inhibitor therapy. Clin Infect Dis 33: 2100-2102.
- Schouten JT, Lockhart DW, Rees TS, Collier AC, Marra CM (2006) A prospective study of hearing changes after beginning zidovudine or didanosine in HIV-1 treatment-naïve people. BMC Infect Dis 6: 28.
- Khoza K, Ross E (2002) Auditory function in a group of adults infected with HIV/AIDS in Gauteng, South Africa. S Afr J Commun Disord 49: 17-27.
- Bateman C (2006) Taking uBhejane by the horn(s). S Afr Med J 96: 382-386.
- Hall JW (2000) Handbook of otoacoustic emissions. Singular Publishing Group.
- Newell R, Burnard P (2006) Research for evidence-based practice. USA: Blackwell Publishing.
- Rosenthal R, Rosnow RL (1991) Essentials of behavioural research: Methods and data analysis. New York: McGraw-Hill.
- Stein F, Cutlet SK (1996) Clinical research in Allied Health and Special Education. (3rd edn). London: Singular Publishing Group Inc.
- Devore JL (1999) Probability and statistics for engineering and the sciences. Pacific Grove, Carlifonia: Brooks/Cole Publishing Company.
- Grimshaw J, Campbell M, Eccles M, Steen N (2000) Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract 17 1: 11-16.
- Schiavetti N, Metz DE (2002) Evaluating research in communicative disorders. (4th edn). Boston: Allyn & Bacon.
- Khoza-Shangase K (2011) Highly active antiretroviral therapy: Does it Sound toxic? J Pharm Bioallied Sci 3: 142-153.
- Kemp RJ, Roeser RJ (1998) Infection Control for Audiologists. Seminars in Hearing 19: 195-203.
- Friedmann I, Arnold W (1994) Pathology of the ear. Journal of Pathology 174: 63.
- Martin FN, Clark JG (2003) Introduction to audiology. (8th edn). Boston: Allyn & Bacon.
- Ludman L, Wright T (1998) Diseases of the ear. London: Arnold Publishers.
- Campbell KC, Durrant J (1993) Audiologic monitoring for ototoxicity. OtolaryngolClin North Am 26: 903-914.
- Bess FH, Humes LE (1990) Audiology: The fundamentals. Baltimore: Williams & Wilkins.
- Devlin AS (2006) Research methods: Planning, conducting and presenting research. Belmont: Thomson/Wadsworth.
- Middel B (2002) Statistical significant change versus relevant or important change in (quasi) experimental design: Some conceptual and methodological problems in estimating magnitude of intervention-related change in health services research. International Journal of Intergrative Care 2: 1-21.
- Dreisbach LE, Long KM, Lees SE (2006) Repeatability of high-frequency distortion-product otoacoustic emissions in normal-hearing adults. Ear Hear 27: 466-479.
- Roede J, Harris FP, Probst R, Xu L (1993) Repeatability of distortion product otoacoustic emissions in normally hearing humans. Audiology 32: 273-281.
- General Principles including Research in Children, Vulnerable groups. South African Medical Research Council, International Collaboration and Epidemiology.
- Dorrington RE, Johnson LF, Bradshaw D, Daniel T (2006)The demographic impact of HIV/AIDS in South Africa. National and provincial indicators for 2006. Centre for Actuarial Research, South African Medical Research Council and Actuarial Society of South Africa, Cape Town.
Citation: Khoza-Shangase K (2014) Pharmaco-Audiology Vigilance and Highly Active Antiretroviral Therapy (HAART) in South Africa: Ototoxicity Monitoring Pursued. J AIDS Clin Res Sex Transm Dis 1: 001.
Copyright: © 2014 Khoza-Shangase K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

