
Photodegradation Products and their Analysis in Food
*Corresponding Author(s):
Joshka VerduinVant Hoff Institute For Molecular Sciences, Analytical Chemistry Group, University Of Amsterdam, Amsterdam, Netherlands
Tel:+31 205256642,
Email:joshka.verduin@gmail.com / joshka.verduin@student.uva.nl
Abstract
Graphical Abstract
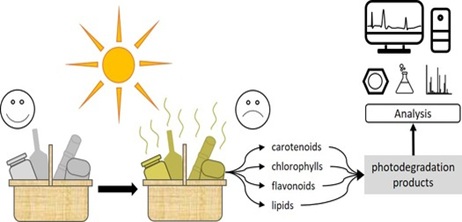
Food spoilage and corresponding waste of packaging material is a serious problem in modern society. Food products can be degraded due to different factors, of which one of them is light-induced degradation. This process can cause the colour of food products to fade or decrease the quality of the product. Moreover, nutrients can be lost or harmful degradation products can be formed. Due to the complex composition of food products, the process of photodegradation is not fully understood and appropriate analysis methods are not fully developed yet. Therefore, this work reviewed the photodegradation of different food components (i.e. carotenoids, chlorophylls, flavonoids and lipids) and the analysis of such photodegradation products. The photodegradation reactions involved were found to be photo-oxidation, -isomerisation, hydrogen transfer, hydrogen abstraction and photolysis, with a great variety of photodegradation products. Moreover, the analytical strategies to analyse these in the best possible way were critically reviewed. In general, no other approaches than standard methods have appeared to be used for the analysis of photodegradation studies. (Ultra) high performance liquid chromatography has been found to be the most commonly-used separation technique, with UV and MS as standard detection methods. For specific applications, GC can be used for the separation and unconventional detectors can be used for the detection of the photodegradation species. Examples listed in this review were the electron spray resonance detector for monitoring radical reactions, the vacuum ultraviolet detector for identifying volatiles and high-resolution MS for accurately characterizing the photodegradation species. Using the information and advices given in this review, a better understanding of the nature of the photodegradation species is gained together with strategies to properly analyse these. Nonetheless, due to the complex composition of food products and the influence of other factors than light, more extensive research on this topic still needs to be performed.
Keywords
Chemical analysis; Degradation products; Food spoilage; Photodegradation
INTRODUCTION
In modern society, most consumers desire healthy, unprocessed foods without the addition of synthetic compounds. At the same time, they want food to have long shelf-lives, both for their own ease as well as to avoid unnecessary food spoilage. However, the shelf-lives of food products are not infinite, as foodstuffs can degrade, hence spoil, in numerous ways. Generally, there are three ways in which food can spoil: physically, microbially and chemically [1,2]. Factors involved with chemical spoilage are pH, temperature, light and oxygen, of which the latter two are included in the scope of this study. Even though foodstuffs are thermodynamically instable, proper processing and packaging can slow down its degradation [3,4]. Light is one of the factors inducing chemical spoilage of food [1]. Foodstuffs exposed to light can break down into unwanted degradation products. These compounds can cause discolouration and form off-flavours and undesired odours, making the food less appetising [5,6]. Another concern is that photodegradation can decrease the quality of food, for instance by breaking down vitamins and other essential nutrients [7,8]. A simple solution to prevent light-induced degradation of foodstuffs would be the usage of packaging that completely blocks the foodstuffs from all light. Notwithstanding, consumers prefer clear packages so they can see what they buy [6,7]. Thus, in order to protect food from light-degradation and to meet the demands of the consumers, first a proper understanding of the nature of the photodegradation products must be established. However, due to the very complex nature of the composition of foodstuffs, photodegradation pathways of many foods are challenging to determine. Another problem is that even though there is information available on this topic, it is usually considered as too time-consuming and expensive to perform this type of analysis. Therefore, this review focuses on providing an overview on
• The different types of photodegradation reactions;
• The nature of the degradation products that are commonly being formed during the photodegradation of food components of interest;
• The analytical strategies to analyse and characterise these degradation products in the best possible way.
Classes of photochemical reactions
There are multiple ways in which molecules can interact with light. When light is being absorbed by a molecule, its energy can either be transferred into heat or induce a chemical reaction [9]. The latter is called a photochemical reaction as it is a reaction that is triggered by light, i.e. a photon is being absorbed to electronically excite a molecule. For this type of reaction to take place, two requirements have to be met:
• The photon energy should be sufficiently high to be absorbed;
• The energy must be high enough to break and form bonds [10]. Photochemical reactions can be divided into two types: direct or photosensitised reactions (Figure 1).
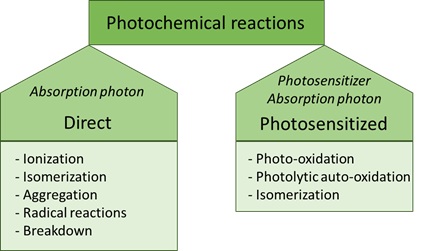
Figure 1: Classes of photochemical reactions.
Direct photochemical reactions
A direct photochemical reaction is a reaction in which a photon is absorbed, inducing a chemical reaction and thereby modifying a molecule [11]. In this type of reaction, light is directly influencing the molecule. The energy of the absorbed photon and the structure of the reacting molecule determines what type of reactions will take place after a direct photochemical reaction. Examples of direct photochemical reactions are ionisation, isomerisation, radical dissociation, aggregation or degradation into other components [10]. Also, secondary reactions can take place via reactive intermediates. These can react further, usually via thermal processes, to form the final reaction product (s).
Photosensitised reactions
In contrast to the direct photochemical reaction, a photosensitised reaction requires another compound to facilitate the interaction between a molecule with light. Without presence of this so-called photosensitiser, the molecule would not react with light, hence no photochemical reaction would take place [7]. This photosensitiser is a chromophore that contains conjugated double bonds [12]. Photosensitisers are commonly present in foodstuffs, with examples being vitamins and chlorophylls. The electrophilic character of photosensitisers makes them susceptible to different reaction mechanisms, including photo-isomerisation and photo-oxidation (Figure 2).
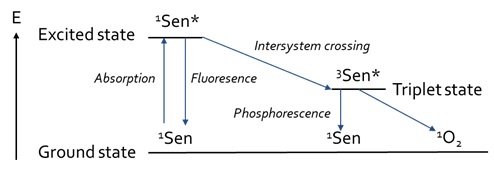
Figure 2: Jablonski-diagram of the processes involved in photosensitized reactions, based on Min and Boff [13].
Photo-oxidation
During photosensitisation, the photosensitiser is first being excited after absorbance of a photon. Then, a photo-oxidation reaction can occur under the presence of oxygen. Naturally, molecular oxygen occurs in the triplet state [14]. However, oxygen can also be formed into the singlet state, in which the antibonding π*-orbitals are located into one orbital, instead of two separate orbitals. The resulting vacant orbital contributes to the very electrophilic character of this allotrope of oxygen, making it highly reactive towards double bonds [13]. There are various ways in which singlet oxygen can be formed [13]. Photosensitisers can form singlet oxygen under the influence of both oxygen and light [14]. Either visible or UV-light can be absorbed by the sensitiser to form excited singlet sensitiser [13]. The excited singlet sensitiser can either fall back to the ground state via fluorescence, or it can also fall back to a lower energy state via Internal Conversion (IC) (Figure 2) by losing heat. Furthermore, it can be transferred to an excited triplet state via Intersystem Crossing (ISC), after which it can fall back to the ground state via either phosphorescence or by reacting with another compound via type-I or type-II mechanisms (Figure 3).
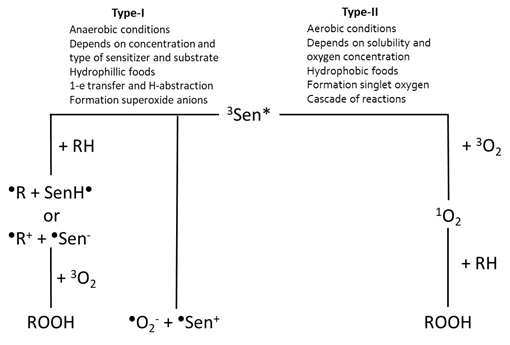
Figure 3: Overview of the photo-oxidation reactions via the type-I and type-II mechanisms, based on Min and Boff [13].
The type-I mechanism occurs more likely under anaerobic conditions [9] and the reaction rate is determined by the concentration and type of the photosensitiser and the substrate [13]. Due to the more hydrophobic nature of oxygen, this type of mechanism occurs more often in hydrophilic food as there is less oxygen present. Examples of food components reacting via the type-I mechanisms include amines and quinones [14]. In the type-I mechanism, one-electron transfer and hydrogen-abstraction are the two reactions caused by the direct interaction between the excited triplet sensitiser and the substrate [6,9]. During H-abstraction, the excited triplet sensitiser donates or accepts H-atoms to induce free radical (chain) reactions [7,14]. During electron transfer, the excited triplet sensitiser reacts with oxygen to form superoxide anions, which occurs in less than 1% of the cases [13]. More often this reaction with oxygen occurs via the type-II mechanism, which is most favourable under aerobic conditions [9]. Since oxygen is better soluble under hydrophobic conditions, the type-II mechanism occurs more often in hydrophobic foods and lipid-water mixtures, such as milk [13]. Its reaction rate is determined by the solubility and the concentration of oxygen in the food. During the type-II mechanism, excited triplet sensitiser can also react with triplet oxygen to form singlet oxygen and singlet sensitiser, which is the dominant (>99%) reaction. The formed singlet oxygen is reactive towards electron-rich compounds, leading to additional reactions of, for instance, double bonds [8]. This results in the formation of hydroperoxides at the place at which the double bond was initially located. This reactive and electrophilic singlet oxygen reacts further with electron-rich compounds to form hydroperoxides. This highly-reactive singlet oxygen reacts with a variety of species to induce degradation, hence decreasing the quality of the food [15].
Photolytic auto-oxidation and photo-isomerisation
Another type of a photo-oxidation reaction is photolytic auto-oxidation, in which lipids are degraded into free radicals due to light exposure [3,14]. The auto-oxidation reactions itself are caused by metal-ions, heat and light that remove a hydrogen atom from a lipid. This leads to formation of free radicals and subsequently hydroperoxide formation [16]. These hydroperoxides further induce free radical chain reactions. Examples of food components that can degrade via this reaction are carotenoids. Carotenoids are very light-sensitive species, so the reaction with atmospheric oxygen occurs rather easily [17]. Besides degrading into different compounds, the molecule can also isomerise into a different conformation via photo-isomerisation. These two reaction mechanisms are in competition with each other and are determined by various parameters, such as the presence of a catalyst, the light intensity and the temperature [18]. An example in food is the all-trans configuration of β-carotene isomerising into different cis-conformations under the presence of light [18,19].
Photodegradation products
The composition of food is complex and the individual ingredients of a food product itself are already difficult to analyse, let alone a mixture of all these compounds. Therefore, this review focuses on a selection of compounds that are commonly present in foodstuffs. Compounds of interest that will be included within the scope of this research are carotenoids, chlorophylls, flavonoids and lipids. Since all these types of compounds are susceptible to light-induced degradation reactions, it is important to understand which type of reactions are involved in each of these compound classes.
Carotenoids
Carotenoids originate from the photosynthetic tissues of microorganisms, plants and algae [17]. These conjugated compounds are very non-polar, meaning that they can be found in hydrophobic parts of a cell, such as the core. Despite its stability towards pH and heat, carotenoids are very light-sensitive due to the electron-rich nature of the chromophore [5]. There are multiple ways in which carotenoids can degrade, including auto-oxidation, thermal degradation, photodegradation and photo-isomerisation, of which the latter two are light-dependent [5,17]. Carotenoids tend to isomerise from the all-trans configuration to different cis-configurations under the influence of heat and light [20]. This isomerisation is not harmful, in fact, it possibly has multiple health benefits. For example, Levin et al. showed that 9-cis β-carotene quenches singlet oxygen in fatty acids better than the all-trans isomer, although no difference in the activity of the two isomers has been found by Liu et al. [21,22]. Moreover, in 2015 Relevy et al. showed that the 9-cis isomer could prevent the formation of plaques in arteries [23]. One of the functions of carotenoids in living cells is protecting the cell from DNA and lipid damage from singlet oxygen reactions, meaning that carotenoids function as a quencher for singlet oxygen [17]. Furthermore, carotenoids can also minimise the occurrence of lipid oxidation reactions. In addition, carotenoids can protect organisms, such as plants, against reactions with sensitive species with the so-called inner-filter effect [8]. During the inner-filter effect, light is absorbed by a certain compound, such as a carotenoid, to prevent excitation of another compound [9].
β-carotene
One well-known example of carotenoids is β-carotene. Under the influence of light, β-carotene isomerises from the commonly occurring all-trans configuration to different cis-configurations [18,19]. Besides photo-isomerisation, photodegradation reactions of β-carotene can also occur (Figure 4). For instance, under the influence of light β-carotene can undergo hydrogen-abstraction, forming a β-carotene radical [17]. Moreover, when excited state β-carotene falls back to the ground state, it can undergo reactions with radicals that were formed during the H-abstraction, leading to the formation of β-carotene radical cations. Furthermore, β-carotene can act as a single oxygen quencher [24]. The main degradation product of this quenching reaction is β-carotene-5,8-endoperoxide [8]. Other common degradation products are β-carotene 5,6-epoxide, β-apo-14’-carotenal, β-apo-10’-carotenal, β-apo-8’-carotenal, β-ionone and β-carotenone 5,8-endoperoxide [17]. β-carotene can also protect lipids against light-induced oxidation and it can protect riboflavin from photodegradation with the inner-filter effect [25]. The absorption maxima of β-carotene show that β-carotene can act as a filter against 405 and 436nm. It should be noted that even though exposure of food components at the non-absorbing wavelengths of β-carotene might prevent the photodegradation of the carotenoid itself, it also results in losing the beneficial protecting effects of the carotenoid when it is exposed to non-absorbing wavelengths (Figure 4).
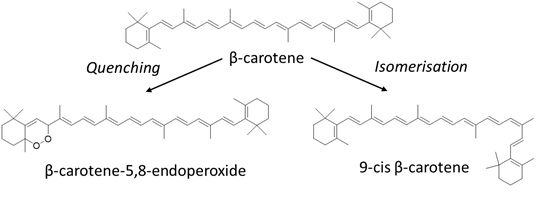
Figure 4: Due to photodegradation, β-carotene can degrade into different photodegradation products, such as β-carotene-5,8-endoperoxide via quenching or isomerise into 9-cis-β-carotene.
Lycopene
Another frequently abundant carotenoid in foods is lycopene. Just like β-carotene, lycopene can also act as an anti-oxidant and singlet oxygen quencher and its quenching properties are even better than that of β-carotene [26]. The end-groups of β-carotene consist of two closed aromatic rings (Figure 4), whereas these rings are open in the structure of lycopene. This structural difference was suggested to cause a slower degradation reaction of lycopene [27]. Lycopene also occurs naturally in the all-trans configuration but isomerises into different cis-configurations due to heat, chemical reactions and (visible) light, of which the photo-isomerisation is more probable than the other two causes [26]. During photosensitised oxidation of lycopene, singlet oxygen can be formed. The reaction between singlet oxygen and lycopene leads to formation of a cyclic peroxide [17]. This reactive intermediate can break into degradation products apo-6’-lycopenal and a small amount of oxygenated species. Whereas no safety hazards have been reported for these compounds, the structural difference of these compounds can lead to discolouration or even colour loss.
Chlorophylls
Chlorophylls are involved in the vital process of photosynthesis by absorbing the incoming light [28]. Furthermore, chlorophyll is involved in the charge distribution and electron transport in cellular reactions [29]. As a large variety of food products contain ingredients that are plant-based, chlorophyll is a highly abundant compound in the diet. Its structure is composed of a porphin ring with a phytol group and a magnesium ion bound at its centre [30]. This group is non-polar and facilitates the stacking interactions, coordination of metals, and the formation of linear polypyrrols and heteroaromatic rings. Chlorophyll consists of chlorophylls A and B and due to its bright yellow/green colours, chlorophyll is also being used as a food dye. As consumers utilise the vivid green colour as a criterion for good quality, it is important that light degradation of food colorants is prevented to satisfy the demands of consumers. There are multiple factors that can induce degradation of chlorophyll, such as light and the presence of enzymes and micro-organisms [31]. Multiple studies showed that the light-induced degradation of chlorophyll occurs via the process of photo-oxidation [32-34]. Besides chlorophyll A and B, plant-based products can also contain other chlorophyll compounds. For instance, Thron et al. showed that in an olive oil sample both chlorophyll A and B, as well as the degradation product pheophytin were present [34]. This is due to loss of the central magnesium-atom via demetallation or so-called ‘pheophytinisation’ [35]. The pheophytins are more stable than chlorophyll, however, these itself are susceptible to light, leading to photosensitised oxidations [34]. The study of Thron et al. has also showed that wavelengths below 400nm have a bigger impact on the photosensitised reactions of the chlorophyll derivatives than longer wavelengths. This means that coloured packaging material, green in this example, could be used to filter out damaging wavelength-ranges (Figure 5).
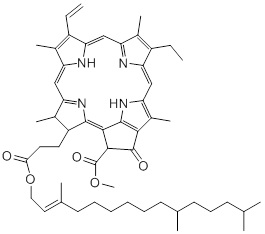
Figure 5: Pheophytinisation causes loss of the central magnesium-atom of chlorophyll a, yielding the photodegradation product pheophytin a, of which the structure is given.
When chlorophyll degrades under the influence of light, it can degrade into red-coloured intermediates, after which its colour can fade completely [32]. This phenomenon is widely being observed during fall, where the leaves transform from green into yellow, orange, red and, ultimately, grey leaves. This is because the oxidation of chlorophyll elongates the conjugated system of chlorophyll, thereby forming phyllochromobilins [36]. This formation of red intermediates was also supported by the study by Llewellyn et al., in which was pointed out that the amount of chlorophyll decreased more rapidly than the formation of the colourless end-products [31].
Chlorophyll A
Already in the 1970s, scientists were trying to determine the photo-degradation products of chlorophylls. A study pointed out that photo-oxidation causes the chlorophyll to degrade and that chlorophyll A is degraded via an oxidation at the methine-bridge, since methyl ethyl maleimide was found as a degradation product [32]. At the end of the century, Suzuki et al. found hydrophilic non-coloured compounds and small organic acids as degradation products of chlorophyll A [37]. The proposed reaction was the formation of a monopyrrole intermediate, followed by further degradation via hydrolytic reactions to form hydrolytic monopyrroles and organic acids. Monopyrroles have the risk of acute toxicity so the photodegradation of chlorophyll A might pose health risks, although its concentration in food is probably too low to be harmful [38]. The group of Llewellyn et al. measured no presence of conjugated degradation products, which corresponds with previous results from Suzuki et al. [39]. Similarly, organic acids were found to be the photodegradation products of chlorophyll A. These acids included citric, malonic, succinic and lactic acid and alanine and glycerol. However, it should be noted that both studies were performed in an aqueous environment. In real food samples, the matrix can also be hydrophobic, which could possibly result in different degradation products. A recent study by Petroví et al. from 2017 took into consideration the influence of different types of light on the photodegradation of chlorophyll [35]. Irradiation with white light and UV-B light was compared and it was found that continuous exposure to UV-B light formed the degradation products in a shorter time period than white light. A logical explanation is the higher energy of UV-B light in comparison with that of white light. As described earlier, one of the causes of photodegradation was pheophytinisation. However, it should be mentioned that this process is not directly caused by light itself, rather than by the biological aging of the chlorophyll. An older research by Heaton et al. in 1996 already showed that other aging processes first degrades chlorophyll into pheoboride molecules, after which these can degrade further under the influence of light and oxygen [40]. This means that the direct degradation by light for chlorophyll is not the only mechanism, but its degradation products formed by different processes could also be sensitive to light. After UV-B irradiation, Petroví et al. detected OH-Pheophytin A (OH-PheoA) and OH-PheoA’ as the degradation products of chlorophyll A [35]. Irradiation with visible light showed, in addition to the aforementioned products, formation of OH-lactone-PheoA. Furthermore, studies showed that the irradiation of chlorophyll can lead to the formation of reactive oxygen species, of which the exact mechanisms are still under debate [7,35].
Chlorophyll B
The study by Maunders et al. also showed that, even though chlorophyll B is more light-sensitive than chlorophyll A, the photodegradation of chlorophyll B is slower than that of A [41]. The highly-conjugated structure of chlorophyll makes it a photosensitiser, leading to high reactivity with oxygen species [29,34]. The latter can induce invasive oxidations, which can lead to damaging or even killing cells. When chlorophyll B and lipids are present in the same matrix, the photodegradation of chlorophyll is slowed down [42]. This is probably due to a competition between the two compounds to react with singlet oxygen, of which lipids are more sensitive than chlorophyll B. On the other hand, when chlorophyll is present in a lipid product (vegetable oil), the quality of the product decreased significantly, due to an increased rate of photodegradation of the lipid [43].
Flavonoids
Flavonoids are a broad group of light-sensitive compounds commonly present in fruits and its structure is composed of a diphenyl propane carbon-skeleton [44]. Due to its strong absorbance between 200 and 380nm, it protects important cellular compounds from ultraviolet radiation, making flavonoids anti-mutagenic, anti-oxidative, and anti-carcinogenic [45,46]. Besides being a healthy food component, flavonoids can also be used as natural food colorants [47]. Considering the molecular structure of these compounds, the presence of highly conjugated systems makes this class very light-sensitive. In fact, photo-oxidation is found to be the main degradation mechanism of flavonoids [7,48]. Flavonoids function as photosensitisers, which means that these compounds can absorb a photon themselves in order to protect other compounds that are present in food [6]. The photo-oxidation can occur either via a type-I or type-II mechanism. For the type-I mechanism, the main degradation products are hydroxyl-peroxyl- and hydroxyl-radicals [6]. For the type-II mechanism, the final degradation products are oxides, hydroperoxides and dioxetanes. The type-I photo-oxidation is caused by H-abstraction, whereas the type-II is initiated by reaction with singlet oxygen [8].
Riboflavin (vitamin B)
Riboflavin (vitamin B2) is a flavonoid that is commonly present in food products, including milk, meat, energy drinks, cheese, white wine and beer [6]. Riboflavin is also known as the hydrophilic vitamin B2, that is involved in vital processes in the body, for instance by acting as a cofactor in enzymatic reactions and the transfer of electrons and hydrogen-atoms [49,50]. Besides, the light-absorbing properties of riboflavin were also found to prevent the colour loss of beverages [51]. Already in the 1980s it was known that riboflavin can act as a photosensitiser that degrades due to light [52]. Even earlier, in 1960, Holmström and Oster showed that riboflavin degrades under the influence of visible light and does not necessarily need a photosensitiser to degrade [53]. In the 1990s a study by Bekbölet showed the wavelength-dependent photodegradation of riboflavin, i.e. 450 nm was the most damaging wavelength to riboflavin [24]. Exposed to light, riboflavin can be promoted into the excited triplet state. The excited triplet sensitiser can degrade via either type-I or type-II photosensitisation. Due to the type-I degradation, radicals can be formed that can react with riboflavin to form the degradation products lumichrome, lumiflavin and hydroxyl radicals [7]. With the type-II mechanism, singlet oxygen reacts with riboflavin to form degradation products. This breakdown of riboflavin reduces the positive health effects of the flavonoid. The photodegradation mechanism has been explored quite thoroughly in earlier studies and Sheraz et al. proposed a general scheme of the photodegradation pathway of riboflavin [49] (Figure 6).
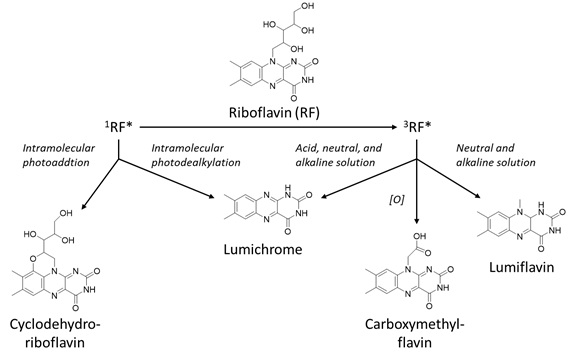
Figure 6: Photosensitised oxidation of riboflavin, based on Sheraz et al. [49].
Photoreduction, -addition and -dealkylation were described as the main degradation mechanisms for both inter- and intramolecular reactions of riboflavin [49]. The photodegradation products are formylmethylflavin, lumichrome, lumiflavine, carboxymethylflavin, 2,3-butanedione, and cyclodehydroriboflavin. Furthermore, the pH affected the final degradation products. At a higher pH, also diketo-compounds and beta-keto acids were formed via alkaline hydrolysis [54]. Riboflavin was proven to be less stable at low pH [55]. This could be the cause of better scavenging of radical species at a high pH, as demonstrated by a study by Barua et al., in which the anti-oxidising properties of the flavan-3-ol (+)-catechin were more efficient at low pH [56]. Of all the detected degradation products, the only one with health risks is 2,3-butanedione as it can cause acute toxicity [57]. Besides pH-dependence, the presence of oxygen also determines the degradation pathway, as aerobic conditions favour type-I mechanisms. Therefore, the intramolecular photo reduction occurs under anaerobic conditions and leads to fading of the colour [53,58]. This process is not necessarily irreversible and thus could be reversed after addition of oxygen. Besides colour loss, riboflavin also loses its dietary benefits while gaining off-flavours [55]. Nonetheless, food products are composed of a great number of different compounds. Thus, it is not unthinkable that other compounds present in the food matrix could prevent certain degradation mechanisms from occurring. For example, when both riboflavin and carotenoids are present in a food product, the carotenoid can prevent riboflavin-sensitised oxidation with the inner-filter effect [9]. In this way, no excited triplet state riboflavin can be formed. Furthermore, another important parameter to consider is the wavelength at which the product is exposed. Sheraz et al. showed that the degradation of riboflavin was faster under ultraviolet than visible light [49]. The same trend was also observed in the degradation of chlorophylls [35].
Flavonols, flavanones, flavanols and flavones
Flavonols find their origins in fruits and vegetables and vivid green leaves are rich in flavonols [59]. Flavonols have anti-oxidising properties and can protect against ultraviolet radiation. Studies investigating the flavonol quercetin have shown that the absorbance of UV-A and UV-C light prevent the formation of reactive oxygen species, thereby preventing DNA-damage. The absorbance of these types of light can also prevent liposome peroxidation by UV-C light and reduce skin damage by UV-B light. Excited-state intramolecular hydrogen transfer induces photodegradation of flavon-3-ols and subsequent relaxation releases energy in the form of heat [6]. Known photodegradation products of quercetin under the influence of UV-A and UV-B light are listed in table 1 [59]. Flavanols, compounds commonly present in tea, are capable of quenching singlet oxygen [8]. 4-hydroxy benzoic acid was found to be one of the final photodegradation product of flavanols and flavanones. The quenching properties of flavanols have also proven to be pH-dependent. For instance, the flavan-3-ol (+)-catechin was a more efficient scavenger at high pH [56]. This means that the anti-oxidising properties can be less powerful in acidic foodstuffs, such as sodas and dairy-products, causing these products to be more light-sensitive. The most efficient anti-oxidisers within the group of flavonoids are the flavones. They originate from flowers, leaves and fruits [46]. The flavone apigenin is proven to protect against UV-A and UV-B light and thereby preventing the reactions with reactive oxygen species [59]. Remarkably, studies showed that photodegradation of flavones is faster in polar solvents than in non-polar solvents [45,60]. This suggests that a type-I photosensitised degradation mechanism is favoured, which is more probable in a hydrophilic environment. A recent study by Chaaban et al. detected four degradation products for the flavone eridictyol [61]. Unfortunately, the structure of these degradation products was not characterised and the exact reaction mechanism is still unknown.
|
Compound |
Photodegradation Reaction |
Photodegradation Product |
Reference |
|
Carotenoids β-carotene |
Photo-isomerisation H-abstraction Singlet oxygen quenching, inner filter effect
|
Cis-isomers (9-cis main isomer) β-carotene radical, β-carotene radical cations β-carotene-5,8-endoperoxide, β -carotene 5,6-epoxide, β-apo-14’-carotenal, β-apo-10’-carotenal, β-apo-8’-carotenal, β-ionone |
[18-20,26] [62] [8,17,24] |
|
|
|||
|
Lycopene |
Photo-isomerisation Photosensitized oxidation |
Cis-isomers Apo-6’-lycopenal and oxygenated species |
[26] [17] |
|
Chlorophylls Chlorophyll A |
Photo-oxidation Hydrolytic reactions |
Methyl ethyl maleimide, hydrolytic monopyrroles, organic acids Organic acids (lactic, citric, succinic and malonic acid), alanine and glycerol OH-Pheoa and OH-Pheoa’ and OH-lactone-Pheoa (only in visible light) Organic acids, hydrolytic monopyrroles |
[32] [31,37] [35] [7,32,35] |
|
Flavonoids Riboflavin (vitamin B2) |
Photo-oxidation
Alkaline hydrolysis |
Lumichrome, lumiflavin and hydroxyl radicals, formylmethylflavin, lumichrome, lumiflavine, carboxymethylflavin, 2,3-butanedione and cyclodehydroriboflavin Diketo compounds and β-keto acid |
[7,49] [54] |
|
Quercetin |
Hydrogen transfer |
2,4,6-trihydroxybenzaldehyde, 2-(3’-4’-dihydroxybenzoyloxy)-4,6-dihydroxybenzoic acid and 3,4-dihydroxyphenyl-ethanol |
[59] |
|
Flavanone and flavanol |
Photo-oxidation |
4-hydroxy benzoic acid |
[8] |
|
Isoflavonoids |
Photo-oxidation |
3’-hydroxydaidzein |
[63] |
|
Anthocyanidins |
Photo-oxidation |
Chalcone |
[64] |
|
Lipids Linoleic acid |
H-abstraction |
9-10E 12Z-LAOOH, 9-10E 12E-LAOOH, 10-8E 12Z-LAOOH, 12-9Z 13E-LAOOH, 13-9Z 11E-LAOOH and 13-9E 11E-LAOOH |
[15,65]
|
|
Linoleic acid ethyl ester |
H-abstraction |
9-10E 12Z-ELAOOH, 9-10E 12E-ELAOOH, 10-8E 12Z-ELAOOH, 12-9Z 13E-ELAOOH, 13-9Z 11E-ELAOOH and 13-9E 11E-ELAOOH |
[15,65]
|
|
Phytosterols Ergosterol |
Photo-oxidation, photosensitised oxidation (accelerated degradation) Photo-oxidation |
7β-hydroxy (main), 7α-hydroxy, 5β,6β-epoxy, 7-keto, 5α,6α epoxy and 6β-hydroxy Lumisterols, tachysterols and previtamin D2 |
[8,66] [67] |
Table 1: Overview of the photodegradation reactions and corresponding degradation products per compound class.
Isoflavones
The main source of isoflavones is soy [6]. Examples of isoflavones are genistein, daidzein and glycitein [8]. Riboflavin activity can increase the activity of anti-oxidants in these isoflavones-compounds. Hydrogen abstraction and reactions with singlet oxygen are the causes of photodegradation of daidzein. 3’-hydroxydaidzein and two dimers were found to be the main degradation products [63]. The other common isoflavone, genistein, functions as an anti-oxidant by aiding anti-oxidant enzymes, scavenging free radicals and by blocking UV-A and UV-B radiation [59].
Anthocyanins and anthocyanidins
The final types of flavonoids that will be considered in this section are the anthocyanins and anthocyanidins. These group of flavonoids cause the vivid colours in brightly-coloured fruits, such as strawberries, blackberries, blueberries, grapes and avocado’s as well as in eggplant [6]. Anthocyanins are unstable compounds that are sensitive towards many factors, including the presence of other compounds, enzymes, pH, temperature, as well as light and oxygen [68]. The colour is pH-dependent and can range from red at low pH-values and purple and blue colour at pH-values above 6 [30]. Since food products usually have a mild pH, the anthocyanins are mostly red-coloured or even colourless. Anthocyanins and -cyanidins can protect plant structures that play a role in photosynthesis against light irradiation by absorbing ultraviolet light and blue and green light [6,69]. However, for this protection, anthocyanins need to bind or interact to another compound to form a stable complex [8]. Instead of absorbing light, anthocyanins can also transfer light into heat by deactivating the excited triplet state [6]. Besides functioning as reactive oxygen species scavengers, anthocyanins can also chelate with metals to prevent toxic metal concentrations leading to complications in plants [70]. A recent study by Chen et al. reported that temperature had a higher impact on the degradation of anthocyanins than light [71]. Anthocyanidins can also protect plants by converting the excited states back to the ground state while releasing the excess energy as harmless heat [8]. Due to their instability, other compounds, such as peptides, amino acids or phenolic compounds, must be present in the matrix to prevent anthocyanidins from degrading. Chalcone was found to be the photodegradation product, as well as the thermal degradation product of the anthocyanide malvidin [64]. This compound is toxic, so the photodegradation of anthocyanidins not only leads to colour fading or colour loss, but it could also pose health risks to the consumer, although that might be negligible at the low concentrations present in foodstuffs. Furthermore, in real life, anthocyanins are not the only compound present in the food matrix. In many foodstuffs, ascorbic acid (vitamin C) is present in the product. It was already proven that ascorbic acid influences the photodegradation of other compounds, including anthocyanins [5,72]. A recent publication by Gérard et al. demonstrated the effect of ascorbic acid on the colour loss of anthocyanins in different plant extracts [73]. This study showed that anthocyanins alone were less stable in air than in a nitrogen atmosphere, whereas they became more stable in air than in nitrogen atmosphere when ascorbic acid was present. An explanation for this is that when anthocyanins are exposed to light, peroxy-radicals can be formed under aerobic conditions. However, addition of ascorbic acid could prevent peroxidation from happening via hydrogen transfer, explaining the protecting properties of ascorbic acid under aerobic conditions.
Lipids
Another class of compounds to be considered are lipids. Lipids can degrade in numerous ways under the influence of light via lipid oxidation and the rate of oxidation is increased due to microbial spoilage and the presence of ultraviolet light [11,74]. When lipids are exposed to light, the volatile degradation products lead to off-flavours and off-odours [7]. Moreover, the formation of harmful degradation products poses a danger regarding human health [16]. Therefore, it is utterly important that the degradation products of lipids are discussed, especially regarding the fact that lipids are abundant in food. The photodegradation of lipids occurs via the process of photo-oxidation, which is a type of photosensitised degradation. Fatty acids, the main components of vegetable oils, can separately not absorb light when the wavelength is lower than 220nm [16]. Moreover, the sensitivity of lipids towards light can be explained via the nature of photodegradation itself. Oxygen is more soluble in hydrophobic matrices, making the light-sensitive properties of lipids a very logical phenomenon. When hydrogen peroxide is formed, it can react further into hydroxy radicals, which are reactive species that can initiate a cascade of radical reactions [62]. The peroxide radical is particularly reactive towards electrophiles, meaning that double-bond containing compounds, such as unsaturated fatty acids, are very sensitive for this radical-based degradation. The resulting oxidation reactions would lead to the formation of peroxides, which could cause rancidity in food products such as vegetable oil and meat [6,75]. However, real lipid products are not only composed of fatty acids, but other compounds as well. These compounds could influence the degradation. Likewise, anti-oxidants can prevent lipids from degrading by:
- • Reacting with free radicals; or
- • By decreasing the formation of hydroperoxides [75].
Fatty acids
As mentioned before, one of the components of fats are fatty acids. Vegetable oils are mainly composed of triglycerides, which are triacylglycerols that are made from three fatty acids. Oils can be degraded into hydroperoxides under the influence of multiple factors, including the presence of catalysts, heat, oxygen and light, The instability of these hydroperoxides leads to further degradation into a large variety of degradation products, such as lactones, carboxylic acids, ketones, alcohols and acids [16,76]. Spatari et al. compared different types of vegetable oil (i.e. soybean, olive, corn, linseed, sunflower and corn oil) on light-sensitivity [76]. As expected, the fatty acid (linoleic and oleic acid) contents decreased when the vegetable oils were exposed to light. Unfortunately, the degradation products of these oils were not discussed, but remarkable is the difference between the extent of photodegradation between the different oils. For instance, olive oil was proven to be the most light-stable oil, whereas soybean and sunflower oil were demonstrated to be very light-sensitive. Possibly the presence of many polyunsaturated fatty acids in soybean makes it more reactive towards light in comparison with the other oils. As corn oil also contains a considerable amount of fatty acids, the same trend as for soybean oil would have been expected. However, corn oil was clearly more stable towards light than sunflower oil. Peanut oil and sunflower oil were both rich in vitamin E that should function as an anti-oxidant [7], so the expectation would be that these oils would be less light-sensitive than the other oils that lack vitamin E. However, no further explanation for this explanation has been given by Spatari et al., hence this must be studied more thoroughly. This example illustrates that the composition of food, which is very complex, influences the photodegradation. Linoleic Acid (LA) and its Ethyl Ester (ELA) are two compounds that are commonly present in food products and cosmetics [65]. In 2019, Ito et al. performed a detailed study on the photodegradation of these compounds and a large variety of hydroperoxide isomers were found to be the photo-oxidation products, including linoleic acid and ethyl ester hydroperoxides (LAOOH and ELAOOH, respectively). Choe and Min already described the reaction mechanism of this degradation of linoleic acid in 2005 [62]. The oxidation occurs via the reaction between a hydroxy radical and linoleic acid via hydrogen abstraction (Figure 7). This radical can either abstract a hydrogen-atom from the lipid to form lipid radicals or a hydrogen-atom from lipid hydroperoxides to form peroxy radicals.
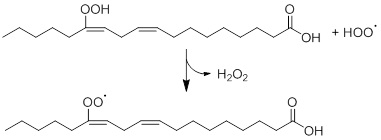
Figure 7: Photodegradation of linoleic acid hydroperoxide via hydrogen abstraction, based on Choe and Min [62].
As displayed in table 1, photodegradation of linoleic acid yielded six isomers and the light-induced degradation of linoleic acid ethyl ester yielded six other isomers [65]. The results by Ito et al. showed how sensitive the fatty acids are towards light and oxygen, as these degradation products were already being formed during the processing of the product. Moreover, the formation of the hydroperoxides induces formation of off-flavours and off-odours, decreasing the appearance, flavour, hence the quality of the product.
Sterols
Besides fatty acids, other lipids can be present in foodstuffs as well. For instance, phytosterols are sterol molecules that are chemically similar to cholesterol [77]. Phytosterols are being used as additives in food products and because of its structural similarities with cholesterol, these could be beneficial for individuals that have high levels of cholesterol [78]. Phytosterols are sensitive to many factors, such as oxygen, heat, lipid matrix, the presence of transition metals and light [66]. The photo-degradation was demonstrated to occur faster when photosensitisers were present in the matrix. Similar results were obtained by Li et al. in 2019 [43]. Just as other lipids, phytosterols are degraded via photo-oxidation under the presence of light. For a long time, the exact reaction mechanism was not known yet, until Zhao et al. proposed the photo-oxidation reactions in detail in 2019 [66]. The corresponding photodegradation products included 7β-hydroxy, 7α-hydroxy, 5β,6β-epoxy, 7-keto, 5α,6α epoxy and 6β-hydroxy, of which 7β-hydroxy was the main product. In 2017, similar degradation products under the influence of sunlight and UV-light were described [8]. Moreover, it is noteworthy to mention that the thermal degradation products that Lin et al. have described were the same as the photodegradation products, suggesting that thermal and photodegradation might yield the same products [79]. Safety risks of these compounds have not been reported, however the decomposition of the phytosterols into smaller degradation products leads to functionality loss of the sterols.
Ergosterol is a type of phytosterol that poses positive health effects when it is degraded by light [8]. Under the influence of UV-light, ergosterol degraded into multiple compounds, such as lumisterols, tachysterols and previtamin D2, of which all could be transformed into vitamins D [67]. Therefore, it can be used as a supplement for vitamin D. This example shows that the photodegradation of food compounds does not always negatively influence human health. Especially regarding the fact that the majority of the population has a vitamin D-deficiency, the photodegradation of ergosterol could be beneficial to restrain this problem [80]. Unfortunately, another degradation product, tachysterol, was reported as toxic, hence the overall health benefit was compromised [81].
The analysis of photodegradation products
Now that the general photodegradation reactions of the compound classes are understood, methods for the analysis of photodegradation species will be critically discussed. An overview of all analytical methods that were described in the literature for the analysis of photodegradation products in food are given in Table 2.
|
Compound Class |
Product |
Sample Preparation |
Separation Technique |
Detection Technique |
Year |
Reference |
|
Carotenoids |
||||||
|
α-and β-carotene and lycopene |
Vegetable juice
|
Extraction, filtration (cheesecloth)
|
RPLC C18, (4.5 x 250mm, 5mm) |
UV (variable wavelength) |
1987 |
[27] |
|
β-carotene |
Standards |
Dissolvation, filtration (membrane filter)
|
RPLC C18 (4.6 x 250mm, 5mm) |
PDA (450nm for β-carotene, 600 nm for chlorophyll a) |
1998 |
[19] |
|
Lycopene |
Tomatoes |
Homogenisation (blender), extraction |
RPLC polymeric C30 (4.6 x 250mm, 3mm) |
DAD (472nm) |
2003 |
[26] |
|
Chlorophylls |
||||||
|
Chlorophyll A/B |
|
[82] |
TLC GC |
MS, NMR |
1970 |
[32] |
|
Chlorophyll A/B |
Plant material |
Homogenisation, filtration (cotton), centrifugation
|
RPLC with ion pair C18 (25cm, 5mm) IEC divinylbenzene resin (30cm)
|
UV (210nm) |
1974 1990 |
[31,83] |
|
Chlorophyll |
Plant material |
Dissolvation, centrifugation, extraction |
TLC |
Spectrophotometer |
1983 |
[41]
|
|
Chlorophyllin |
Standards |
- Dissolvation, extraction Acidification, extraction |
- - RPLC C18 (4.6 x 250mm, 10mm) |
IR Cyclic voltammetry MS (quadrupole ion trap) |
1999 |
[33] |
|
Chlorophyll A |
Radish seedlings, barley |
Extraction
|
RPLC ODP-50 (4.6 x 250mm) |
Spectrophotometer (280nm) |
1999 |
[37] |
|
Chlorophyll |
Standards in sunflower oil |
Headspace
|
- GC |
DAD FID |
2001 |
[34] |
|
Chlorophyll B |
Standards in paraffin oil and triglycerides |
Dissolvation |
RPLC C18 (4.6 x 250mm, 5mm) |
UV/Vis (438nm) |
2014 |
[42] |
|
Chlorophyll B |
Spinach |
Homogenisation (grinding), extraction, centrifugation, filtration Extraction (open column chromatography) |
RPLC (UHPLC), C18 (2.1 x 50mm, 1.9mm) |
DAD (380, 407, 430, 660nm MS (ion trap) |
2017 |
[35,84] |
|
Chlorophyll |
Chlorophyll-spiked rapeseed oil |
- |
- |
UV (620-720 nm) |
2019 |
(Li et al. 2019) [43] |
|
Flavonoids |
||||||
|
Flavones |
Standards |
Dissolvation |
RPLC C18 (4 x 125mm, 5mm)
|
UV (190-400nm) |
1993 |
[45] |
|
Anthocyanidin |
Standards |
- |
RPLC C8 (4.6 x 250mm, 5mm) |
UV (280nm) |
1993 |
[64] |
|
Riboflavin (vitamin B2) |
Standards |
- |
TLC
|
UV (365nm) Spectrophotometer (240nm) |
2004 |
[54] |
|
Riboflavin (vitamin B2) |
Standards |
Dissolvation
|
RPLC C18 (3.9 x 150mm) RPLC C18 (3.9 x 150mm) |
UV/Vis (260nm) MS (Quadrupole) |
2010 |
[63] |
|
Riboflavin (vitamin B2) |
Milk |
Centrifugation, ultrafiltration |
RPLC ODS (3 x 100mm, 3mm) RPLC (UHPLC) HSS T3 (2.1 x 150mm, 1.8mm) |
Fluorescence (420nm excitation, 530nm emission) MS (HR) |
2018 |
[55] |
|
Anthocyanin |
Sweet potato (purple) |
Homogenization (freeze drying and milling), extraction pH alternation Dissolvation |
-
- - |
UV/Vis (525, 700nm) Colorimeter Spectrophotometer (517nm) |
2019 |
[71] |
|
Anthocyanin |
Carrot (black)/grape juice/sweet potato (purple) extracts in beverages |
Buffering Dissolvation (and degassing)
Dissolvation |
- -
- |
UV/Vis (200-700nm) Cyclic voltammetry ESR Fluorimeter |
2019 |
[73] |
|
Lipids |
||||||
|
General |
Vegetable oils |
Dissolvation, extraction, acidification |
RPLC C18 (4.6 x 250mm) |
DAD |
2016 |
[76] |
|
Linoleic acid and linoleic ethyl ester |
Standards Liquor
|
Dissolvation |
- RPLC ODS-3 (2.1 x 150mm, 5mm) (LAOOH) RPLC C18 (2.1 x 150mm, 5mm) (ELAOOH) |
1H-NMR (400 MHz) MS (Quadrupole ion trap) MS (Quadrupole ion trap) |
2019 |
[65] |
|
Phytosterol |
Standards |
Dissolvation, saponification, incubation, SPE |
GC |
MS (Not specified) |
2019 |
[66] |
Table 2: Overview of the analytical methods used to analyse photodegradation products of the compounds of interest.
Sample preparation
Sample preparation is key for accurate characterisation of photodegradation products. During the pre-treatment step, the analytes of interest must be extracted from the matrix in a correct manner. Insufficient extraction of the analytes could lead to underestimation of the levels of certain compounds in food or inability to detect certain species and improper treatment of the sample could lead to degradation of the sample. In the latter case, a sample would not only be degraded by light but by the stress of the extraction method as well. When the aim is to study the photodegradation of food compounds, it is important that other factors that could induce degradation are excluded. For instance, besides light-degradation, compounds can also degrade via pH-differences and heat. Especially regarding the fact that some studies suggest that thermal and photodegradation yield the same products, it is important to carefully perform the sample preparation [64,79]. Since the matrix of the product determines the type of sample treatment, this section will be divided into different subsections in which suitable preparation steps will be described per type of food product.
Beverages
Generally, minimal sample preparation is needed when analysing beverages. Common pre-treatments include (liquid-liquid) extraction, filtration and occasionally homogenisation. Another type of sample preparation that has been found for the analysis of beverages is buffering to change the pH [73]. However, the operator must be careful when changing the pH as it can degrade food components such as the food dyes carminic acid and gardenia blue [7,85]. Moreover, the pH can also influence what type of degradation products will be formed, as shown by the study of Ahmad et al. [54]. As the pH in various food products can be different, it might be interesting to determine the degradation products under different pH-conditions. In this way, the type of photodegradation products that are present in different food matrices with specific pH-values could be predicted.
Fruits and vegetables
In most fruit and vegetable samples, the first step is homogenisation of the product. Traditionally, the first step is performed using a blender. However, during blending heat is being formed that can induce thermal degradation. In that way, not solely the photodegradation products cannot be separated from the thermal degradation products. Therefore, nowadays, freeze-drying is a more common method to homogenise the sample. In that way, no heat is being formed, which reduces the risk of degrading the product by other factors than light. Following homogenisation, the sample can be filtered and/or centrifuged to remove solids. During these steps, part of the analyte can get trapped onto the filter/precipitate resulting in a lower recovery of the analyte. When the sample is spiked with a representative standard, the analyte loss can be estimated. This can be used to correct the detected values when quantitative analysis is desired. Besides filtration and centrifugation, changing the pH can be another step, of which the disadvantages have already been described. The final step in the sample preparation of plant material is extraction of the analyte. Liquid-Liquid Extraction (LLE) was the most common type of extraction that was found in the studied literature. Another type of extraction that can be used is open-column chromatography, which has been used by Petroví et al. in 2017 [35]. During their analysis, the open column contained silica particles, meaning that the principle is similar to Solid-Phase Extraction (SPE). Both open-column chromatography and SPE could be useful as a sample clean-up step as it can retain the interfering compounds on the silica, rather than the analyte. In contrast to LLE, SPE can be performed on-line. When (part of) the sample preparation can be performed on-line along with the analysis, the overall method becomes more efficient: it saves time, automation is possible, it reduces errors by the operator, and it might even minimise the risk of degradation during the off-line sample preparation. However, suitable sorbents must be available to realise this.
Oils and fats
In line with beverages, sample preparation of oils is less difficult than for solid samples as the analyte is already present in a liquid matrix. As table 2 shows, not all samples needed extensive sample preparation before analysis. For instance, when only UV-detection was performed, the product could be analysed directly. Similarly, when High-Performance Liquid Chromatography (HPLC) was used as a separation technique, sample preparation was not necessarily needed as HPLC can also separate the analyte from the other components. When Gas Chromatography (GC) is desired, head-space extraction could be performed to extract volatile analytes while transferring it into the gaseous phase. These given examples all have the advantage that these are compatible with an on-line system. However, more extensive sample preparation might be needed as well. For instance, Spatari et al. used saponification to hydrolyse the triglycerides to separate the different kind of fatty acids [76]. This procedure is not easy to automate and therefore time-consuming. Another type of food product with high levels of fat is milk, which is an emulsion of fat and water. The sample preparation for milk is also more difficult than for vegetable oils. Fracassetti et al. prepared milk samples by first centrifugation and subsequent ultra filtration of the bottom layer, followed by filtration [55]. In contrast to the saponification procedure, the method by Fracassetti et al. is less labour-intensive. These examples illustrate that the sample preparation of oils is not necessarily difficult, but the type of treatment depends on the product and the type of analysis.
It can be concluded that the sample preparation is an important step in the analysis of food products and one should take care to develop a method that is non-destructive towards the analyte. Different circumstances, (e.g. pH, solvent and external factors such as heat formation) can be tested during the degradation studies of standards to understand how compounds degrade in different matrices. It is suggested to first start analysing the standard on its own, after which combinations of two or more food components can be tested. This can be used to predict the type of degradation products that will be formed after light-illumination of real food products, which are mixtures of different compounds. Depending on the analyte and the type of matrix, even on-line coupling of sample preparation steps, such as headspace and SPE, can be possible, making the degradation studies less time-consuming and easy to automate. Such an on-line system would be especially useful for routine analysis in industry.
Separation
The techniques used for the separation of the photodegradation products from carotenoids, chlorophylls, flavonoids and lipids are enlisted in table 2. Since this table already gives an overview of all techniques found in the cited literature, only the trends and critical remarks will be given in the next section to show which techniques are useful for the analysis of photodegradation products.
Carotenoids
HPLC was used as a separation technique in all studies that have been investigated in this review. Either a silica C18-column or a polymeric C30-column was used for the separation for the degradation species of carotenoids. Comparison of a C30-column with C18-columns showed that the latter are suitable for the analysis of the more hydrophobic carotenoids, flavonoids, phenolic acids and isomers [86]. The C30-columns are suited for distinguishing the cis-isomer degradation products from carotenoids. However, Turcsi et al. emphasised that this isomeric separation power comes with the cost of poor separation of polar carotene-species [87]. Thus, it is advised to use C18-columns for the general analysis of photodegradation products of carotenoids and to use polymeric C30-columns for the specific analysis of long-chain hydrophobic photo-isomerisation products.
Chlorophylls
Chlorophyll has been analysed for over several decades and in earlier days Thin Layer Chromatography (TLC) and GC have been used as separation techniques [34,41,82]. N
ACKNOWLEDGEMENT
A big thanks to Denice van Herwerden, Ruben Kranenburg, Chris Lukken and Sharene Veelders for reviewing and helpful feedback. Iris Groeneveld is acknowledged for her useful discussions during the meetings.This study was performed in the MSc+-program of COAST. This work is part of the TooCold project (Toolbox for studying the Chemistry Of Light-induced Degradation) carried out within the TTW Open Technology Programme with project number 15506 which is (partly) financed by the Netherlands Organisation for Scientific Research (NWO).
DECLARATION OF INTEREST STATEMENTS
The authors did not receive financial support that could have influenced the conclusions of this review and state no known conflicts of interest.
REFERENCES
- Singh RP, Anderson BA (2004) Understanding and Measuring the Shelf-Life of Food. In: Steele R (eds.). Understanding and Measuring the Shelf-Life of Food (1stedn).Woodhead Publishing Limited, Cambridge, England. Pg no: 3-17.
- Petruzzi L, Corbo MR, Sinigaglia M, Bevilacqua A (2016) Microbial Spoilage of Foods: Fundamentals. Elsevier Ltd.
- Kong F, Singh RP (2010) Chemical deterioration and physical instability of foods and beverages. Elsevier Ltd.
- Wyrwa J, Barska A (2017) Innovations in the food packaging market: Active packaging. Eur Food Res Technol. 243: 1681-1692.
- Galaffu N, Bortlik K, Michel M (2015) An industry perspective on natural food colour stability. Elsevier Ltd.
- Huvaere K, Skibsted LH (2015) Flavonoids protecting food and beverages against light. J Sci Food Agric. 95: 20-35.
- Duncan SE, Chang HH (2012) Implications of light energy on food quality and packaging selection. Advances in Food and Nutrition Research 67: 25-73.
- Lu B, Zhao Y (2017) Photooxidation of phytochemicals in food and control: A review. Ann NY Acad Sci1398: 72-82.
- Cardoso DR, Libardi SH, Skibsted LH (2012) Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct 3: 487-502.
- Koutchma T, Orlowska M, Zhu Y (2018) Fruit preservation. Novel and Conventional Technologies.
- Koutchma T, Forney LJ, Moraru, Carmen I (2009) Ultraviolet Light in Food Technology: Principles and Applications. In: Ultraviolet Light in Food Technology: Principles and Applications (1stedn). CRC Press. Pg no: 296.
- Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, et al. (2006) A genetically encoded photosensitizer. Nat Biotechnol 24: 95-99.
- Min DB, Boff JM (2002) Chemistry and reaction of singlet oxygen in foods. Compr Rev Food Sci Food Saf 1: 58-72.
- Bradley DG, Min DB (1992) Singlet oxygen oxidation of foods. Crit Rev Food SciNutr31: 211-236.
- Choe E, Huang R, Min DB (2005) R?: Concise Reviews / Hypotheses in Food Science Chemical Reactions and. J Food Sci 70: 28-36.
- Ahmed M, Pickova J, Ahmad T, Liaquat M, Farid A, et al. (2016) Oxidation of Lipids in Foods.Sarhad J Agric 32: 230-238.
- Boon CS, McClements DJ, Weiss J, Decker EA (2010) Factors influencing the chemical stability of carotenoids in foods. Crit Rev Food SciNutr 50: 515-532.
- Pesek CA, Warthesen JJ (1990) Kinetic Model for Photoisomerization and Concomitant Photodegradation of β-Carotenes. J Agric Food Chem 38: 1313-1315.
- Chen BH, Huang JH (1998) Degradation and isomerization of chlorophyll a and β-carotene as affected by various heating and illumination treatments. Food Chem 62: 299-307.
- Schieber A, Carle R (2005) Occurrence of carotenoid cis-isomers in food: Technological, analytical, and nutritional implications. Trends Food Sci Technol 16: 416-422.
- Levin G, Mokady S (1994) Antioxidant activity of 9-cis compared to all-trans β-carotene in vitro. Free Radic Biol Med 17: 77-82.
- Liu Q, Suzuki K, Nakaji S, Sugawara K (2000) Antioxidant activities of natural 9-CIS and synthetic all-trans β-carotene assessed by human neutrophil chemiluminescence. Nutr Res 20: 5-14.
- Relevy NZ, Ruhl R, Harari A, Grosskopf I, Barshack I, et al. (2015) 9-cis beta-carotene Inhibits Atherosclerosis Development in Female LDLR-/- Mice. Funct Foods Heal Dis 5: 67-79.
- Bekbölet M (1990) Light Effects on Food. J Food Prot 53: 430-440.
- Hansen E, Skibsted LH (2000) Light-induced oxidative changes in a model dairy spread. Wavelength dependence of quantum yields. J Agric Food Chem 48: 3090-3094.
- Shi J, Le Maguer M, Bryan M, Kakuda Y (2003) Kinetics of lycopene degradation in tomato puree by heat and light irradiation. J Food Process Eng 25: 485-498.
- Pesek CA, Warthesen JJ (1987) Photodegradation of Carotenoids in a Vegetable Juice System. J Food Sci 52: 744-746.
- Sato R, Ito H, Tanaka A (2015) Chlorophyll b degradation by chlorophyll b reductase under high-light conditions. Photosynth Res 126: 249-259.
- Tanaka A, Tanaka R (2006) Chlorophyll metabolism. Curr Opin Plant Biol 9: 248-255.
- Sigurdson GT, Tang P, Giusti MM (2017) Natural Colorants: Food colorants from natural sources. Annu Rev Food SciTechnol 8: 261-280.
- Llewellyn CA, Mantoura RFC, Brereton RG (1990) Products of chlorophyll photodegradation-1. detection and separation. PhotochemPhotobiol 52: 1037-1041.
- Jen JJ, Mackinney G (1970) On the Photodecomposition of Chlorophyll in vitro - II. PhotochemPhotobiol 11: 303-308.
- Salin ML, Alvarez LM, Lynn BC, Habulihaz B, Fountain AWI (1999) Photooxidative Bleaching of Chlorophyllin. Free Radic Res 31:97-105.
- Thron M, Eichner K, Ziegleder G (2001) The influence of light of different wavelengths on chlorophyll-containing foods. LWT - Food SciTechnol34: 542-548.
- Petrovi S, Zvezdanovi J, Markovi D (2017) Chlorophyll degradation in aqueous mediums induced by light and UV-B irradiation?: An UHPLC-ESI-MS study. Radiation Physics and Chemistry 141: 8-16.
- Hörtensteiner S, Hauenstein M, Kräutler B (2019) Chlorophyll breakdown-Regulation, biochemistry and phyllobilins as its products. Adv Bot Res 90: 213-259.
- Suzuki Y, Shioi Y (1999) Detection of Chlorophyll Breakdown Products in the Senescent Leaves of Higher Plants. Plant Cell Physiol 40: 909-915.
- Sigma-Aldrich/Merck (2019) Alanine.Sigma-Aldrich/Merck. Pg no: 1-7.
- Llewellyn CA, Mantoura RFC, Brereton RG (1990) Products of chlorophyll photodegradation-2. structural identification. PhotochemPhotobiol 52: 1043-1047.
- Heaton JW, Marangoni AG (1996) Chlorophyll degradation in processed foods and senescent plant tissues. Trends Food SciTechnol 7: 8-15.
- Maunders MJ, Brown SB (1983) The effect of light on chlorophyll loss in senescing leaves of sycamore (Acer pseudoplatanus L.). Planta. 158: 309-311.
- Lee E, Ahn H, Choe E (2014) Effects of light and lipids on chlorophyll degradation. Food Science and Biotechnology 23: 1061-1065.
- Li X, Yang R, Lv C, Chen L, Zhang L, et al. (2019) Effect of chlorophyll on lipid oxidation of rapeseed oil. Eur J Lipid Sci Technol 121: 1-4.
- Cook NC, Samman S (1996) Flavonoids---Chemistry, metabolism, cardioprotective effects, and dietary. Nutr Biochem 7: 66-76.
- Monici M, Mulinacci N, Baglioni P, Vincieri FF (1993) Flavone micellar systems UV-induced reactions organic solvents. J Photochem Photobiol B Biol 20: 67-172.
- Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: An overview. J Nutr Sci 5: 47.
- Vinha AF, Rodrigues F, Nunes MA, Oliveira MBPP (2018) 11 - Natural pigments and colorants in foods and beverages. Elsevier Inc.
- Islam MS, Patras A, Pokharel B, Wu Y, Vergne MJ, et al. (2016) UV-C irradiation as an alternative disinfection technique: Study of its effect on polyphenols and antioxidant activity of apple juice. Innov Food Sci Emerg Technol 34: 344-351.
- Sheraz MA, Kazi SH, Ahmed S, Anwar Z, Ahmad I (2014) Photo, thermal and chemical degradation of riboflavin. Beilstein J Org Chem 10: 1999-2012.
- Thakur K, Tomar SK, Singh AK, Mandal S, Arora S (2017) Riboflavin and health?: A review of recent human research. Crit Rev Food Sci Nutr 57: 3650-3660.
- Gosetti F, Chiuminatto U, Mazzucco E, Calabrese G, Gennaro MC, et al. (2013) Non-target screening of Allura Red AC photodegradation products in a beverage through ultra high performance liquid chromatography coupled with hybrid triple quadrupole/linear ion trap mass spectrometry. Food Chem 136: 617-623.
- Sattar A, deMan JM, Alexander JC (1977) Light-induced degradation of vitamins i. kinetic studies on riboflavin decomposition in solution. Can Inst Food Sci Technol J 10: 61-64.
- Holmström B, Oster G (1961) Riboflavin as an electron donor in photochemical reactions. I(11): 1957-1961.
- Ahmad I, Fasihullah Q, Noor A, Ansari IA, Ali QNM (2004) Photolysis of riboflavin in aqueous solution?: A kinetic study. Int J Pharm 280: 199-208.
- Fracassetti D, Limbo S, Incecco PD, Tirelli A, Pellegrino L (2018) Development of a HPLC method for the simultaneous analysis of riboflavin and other flavin compounds in liquid milk and milk products. Eur Food Res Technol 244: 1545-1554.
- Barua MG, Escalada JP, Bregliani M, Pajares A, Criado S (2017) Antioxidant capacity of (+) -catechin visible-light photoirradiated in the presence of vitamin B2. Redox Rep 22: 282-289.
- Sigma-Aldrich/Merck (2019) Sigma Aldrich.
- Smith BEC, Metzler DE, Smith EC, Metzler DE (1963) The photochemical degradation of riboflavin.
- Saewan N, Jimtaisong A (2013) Photoprotection of natural flavonoids. J Appl Pharm Sci 3: 129-141.
- Tommasini S, Calabrò ML, Donato P, Raneri D, Guglielmo G, et al. (2004) Comparative photodegradation studies on 3-hydroxyflavone: Influence of different media, pH and light sources. J Pharm Biomed Anal 35:389-397.
- Chaaban H, Ioannou I, Paris C, Charbonnel C, Ghoul M (2017) The photostability of fl avanones , fl avonols and fl avones and evolution of their antioxidant activity. Journal Photochem Photobiol A Chem 336: 131-139.
- Choe E, Min DB (2005) Chemistry and reactions of reactive oxygen species in food. J Food Sci 70: 28-36.
- Park C, Yeo J, Park M, Park J, Lee J (2010) Effects of riboflavin photosensitization on daidzein and its photosensitized derivatives 75: 659-666.
- Furtado P, Figueiredo P, Chaves das Neves H, Pina F (1993) Photochemical and thermal degradation of anthocyanidins. J Photochem Photobiol A Chem 75: 113-118.
- Ito J, Komuro M, Parida IS, Shimizu N, Kato S, et al. (2019) Evaluation of lipid oxidation mechanisms in beverages and cosmetics via analysis of lipid hydroperoxide isomers. Sci Rep 7387: 1-13.
- Zhao Y, Yang B, Xu T, Wang M, Lu B (2019) Photooxidation of phytosterols in oil matrix?: E ff ects of the light , photosensitizers and unsaturation degree of the lipids 288: 162-169.
- Mattila P, Lampi AM, Ronkainen R, Toivo J, Piironen V (2002) Sterol and vitamin D2 contents in some wild and cultivated mushrooms. Food Chem 76: 293-298.
- Sun J, Bai W, Zhang Y, Liao X, Hu X (2011) Identification of degradation pathways and products of cyanidin-3- sophoroside exposed to pulsed electric field. Food Chem 126: 1203-1210.
- Zhu H, Zhang TJ, Zhang P, Peng CL (2016) Pigment patterns and photoprotection of anthocyanins in the young leaves of four dominant subtropical forest tree species in two successional stages under contrasting light conditions. Tree Physiol 36: 1092-1104.
- Landi M, Tattini M, Gould KS (2015) Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot 119: 4-17.
- Chen CC, Lin C, Chen MH, Chiang PY (2019) Potato Extracts. Foods 393: 1-13.
- Gosetti F, Bolfi B, Mazzucco E, Manfredi M, Robotti E, et al. (2018) LC-MS/MS approach for the identification of unknown degradation products of dyes in beverages. Natural and Artificial Flavoring Agents and Food Dyes Handbook of Food Bioengineering 229-260.
- Gérard V, Ay E, Morlet-Savary F, Graff B, Galopin C, et al. (2019) Thermal and photochemical stability of anthocyanins from black carrot, grape juice, and purple sweet potato in model beverages in the presence of ascorbic acid. J Agric Food Chem. 67: 5647-5660.
- Moreira D, Gullón B, Gullón P, Gomes A, Tavaria F (2016) Bioactive packaging using antioxidant extracts for the prevention of microbial food-spoilage. Food Funct 7: 3273-3282.
- Králová M (2015) The effect of lipid oxidation on the quality of meat and meat products. Maso Int 2: 125-132.
- Spatari C, Luca M De, Ioele G, Ragno G (2017) A critical evaluation of the analytical techniques in the photodegradation monitoring of edible oils. LWT-Food Sci Technol 76: 147-155.
- Scholz B, Guth S, Engel KH, Steinberg P (2015) Phytosterol oxidation products in enriched foods: Occurrence, exposure, and biological effects. Mol Nutr Food Res 59: 1339-1352.
- Semeniuc CA, Cardenia V, Mandrioli M, Muste S, Borsari A, et al. (2016) Stability of flavoured phytosterol-enriched drinking yogurts during storage as affected by different packaging materials. J Sci Food Agric 96: 2782-2787.
- Lin Y, Knol D, Trautwein EA (2016) Phytosterol Oxidation Products (POP) in foods with added phytosterols and estimation of their daily intake: A literature review. Eur J Lipid Sci Technol 118: 1423-1438.
- Galesanu C, Mocanu V (2015) Vitamin D deficiency and the clinical consequences. Rev Med Chir Soc Med Nat Iasi 119: 310-318.
- Santa Cruz Biotechnology I (2019) Tachysterol St Cruz Biotechnol.
- Jen JJ, Mackinney G (1970) On the photodecomposition of chlorophyll in vitro I. Reaction rates. Photochem Photobiol 11: 297-302.
- Iriyama K, Ogura N, Takamiya A (1974) Chlorophyll method from for plant extraction material and using partial dioxane purification of. J Biochem 76: 901-904.
- Wright SW, Jeffrey SW, Mantoura RFC, Llewellyn CA, Bjørnland T, et al. (1991) Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Mar Ecol Prog Ser 77: 183-196.
- Jespersen L, Strømdahl LD, Olsen K, Skibsted LH (2005) Heat and light stability of three natural blue colorants for use in confectionery and beverages. Eur Food Res Technol 220: 261-266.
- Vy?uchalová K, Jandera P (2015) Comparison of a C30 bonded silica column and columns with shorter bonded ligands in reversed?phase LC. Chromatographia 78: 861-871.
- Turcsi E, Nagy V, Deli J (2016) Study on the elution order of carotenoids on end capped C18 and C30 reverse silica stationary phases. A review of the database. J Food Compos Anal 47: 101-112.
- Franco MS, Padovan RN, Fumes BH, Lanças FM (2016) An overview of multidimensional liquid phase separations in food analysis. Electrophoresis 37: 1768-1783.
- Cacciola F, Dugo P, Mondello L (2017) Multidimensional liquid chromatography in food analysis. TrAC Trends Anal Chem. 96: 116-123.
- Waters (2019) HSS (High Strength Silica) Technology.
- Kissinger PT, Heineman WR (1983) Cyclic voltammetry. J Chem Educ 60: 702-706.
- Kunzelmann M, Winter M, Åberg M, Hellenäs KE, Rosén J (2018) Non-targeted analysis of unexpected food contaminants using LC-HRMS. Anal Bioanal Chem 410: 5593-5602.
- Laganà A, Cavaliere C (2015) High-resolution mass spectrometry in food and environmental analysis. Anal Bioanal Chem 407: 6235-6236.
- Agüera A, Martínez-Piernas AB, Campos-Mañas MC (2017) Analytical strategies used in HRMS. Appl High Resolut Mass Spectrom Food Saf Pestic Residue Anal 59-82.
- Elipe MVS (2003) Advantages and disadvantages of nuclear magnetic resonance spectroscopy as a hyphenated technique. Anal Chim Acta 497: 1-25.
- Kohno M (2010) Applications of electron spin resonance spectrometry for reactive oxygen species and reactive nitrogen species research. J Clin Biochem Nutr 47: 1-11.
- Zang S, Tian S, Jiang J, Han D, Yu X, et al. (2017) Determination of antioxidant capacity of diverse fruits by Electron Spin Resonance (ESR) and UV-vis spectrometries. Food Chem 221: 1221-1225.
- Chen Q, Xie Y, Xi J, Guo Y, Qian H, et al. (2018) Characterization of lipid oxidation process of beef during repeated freeze-thaw by electron spin resonance technology and Raman spectroscopy. Food Chem 243: 58-64.
- Schug KA, Sawicki I, Carlton DD, Fan H, McNair HM, et al. (2014) Vacuum ultraviolet detector for gas chromatography. Anal Chem 86: 8329-8335.
- García-Cicourel AR, van de Velde B, Verduin J, Janssen HG (2019) Comprehensive off-line silver phase liquid chromatography × gas chromatography with flame ionization and vacuum ultraviolet detection for the detailed characterization of mineral oil aromatic hydrocarbons. J Chromatogr A 1607: 460391.
- Lelevic A, Souchon V, Moreaud M, Lorentz C, Geantet C (2019) Gas chromatography vacuum ultraviolet spectroscopy: A review. J Sep Sci 43: 150-173.
Citation: Verduin J, den Uijl MJ, Peters RJB, van Bommel MR (2020) Photodegradation Products and their Analysis in Food. J Food Sci Nutr 6: 067.
Copyright: © 2020 Joshka Verduin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

