
Polylactic Acid (PLA) as a Bioplastic and its Possible Applications in the Food Industry
*Corresponding Author(s):
Vega-Baudrit JIndustrial Chemistry, Chemistry School, Director Of The National Nanotechnology Laboratory LANOTEC At National Center Of High Technology CeNAT, National University Of Costa Rica, Costa Rica
Tel:+506 25195835,
Email:jvegab@gmail.com
Abstract
The bioplastics are generating a growing interest not only in the plastics industry but in society in general. The objective of this paper is to analyze Polylactic Acid (PLA) as a bio-based and biodegradable bioplastic, specifically made from renewable resources. Its properties, both mechanical and thermal, will be analyzed, as well as its how it can be degraded, conditions and characteristics through the mention of different studies carried out and finally its potential applications in different industrial areas like medicine, textile and food packaging.
From this review, it is evident that Polylactic acid has many applications in the field of medicine, however, for us the greatest focus should be given to the commercial applications that this bioplastic could have specifically in packaging, since the plastics are currently used a lot for packaging and this is one of the largest sources of existing pollution.
Keywords
Amylopectin; Amylose; Biodegradability; Bioplastics; Cellulose; Polylactic acid, Starch
INTRODUCTION
Traditional plastics such as polyethylene and polypropylene are economical, lightweight and versatile, however, they last for many years after being discarded, due to their properties of high resistance to corrosion, water and bacterial decomposition. It becomes waste that is difficult to eliminate and, consequently, a serious environmental problem due to its petrochemical origin [1].
The annual production of plastics exceeded 300 million tons in 2015, which generated 34 million tons of plastic waste throughout the world and 93% of them are disposed of in landfills and oceans [2].
Because of this, there is a huge need to promote the production of plastics from raw materials based on renewable sources, such as bioplastics, which are characterized by being made from organic materials and which are mostly biodegradable [3]. Thanks to the use of bioplastics, the remains of containers and wrappings that end up in sinks or oceans will be naturally degraded by contact with water and climatic agents, destroying and emitting organic molecules that do not have a harmful impact on the environment [3].
According to the International Standard Organization (ISO), bioplastics are defined as polymeric structures that use vegetable raw materials that are essentially derived from renewable resources [4].
Bioplastics can be classified according to their source, which can be divided into three subgroups, the first of which are the bioplastics generated directly from the biomass, that is, from the extracted starch, such as Polylactic Acid (PLA), the second are those generated from vegetable oils and the last, those generated from Polyhydroxyalkanoates (PHA) [4].
The focus has been on the use of starch as a raw material, due to its availability and to the fact that it is economically competitive with oil. Corn starch is generally used, although other sources are being investigated, such as potatoes, barley, oats, cassava, beet, among others.
Starch is a natural polymer whose granules consist of macromolecular structures arranged in layers and whose characteristics in terms of composition, quantity and form vary according to the type of source from which it comes. The starch granules are composed of external layers of amylopectin and inner layers of amylose, the proportion of which varies depending on the source of the starch. Its chemical composition is that of a polysaccharide formed only by glycosidic units, that is, it is a macromolecule formed by many repeating glucose molecules (Figure 1 and 2).
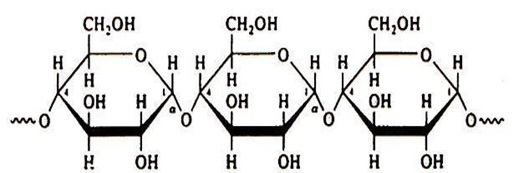 Figure 1: Molecular structure of amylose [5].
Figure 1: Molecular structure of amylose [5].
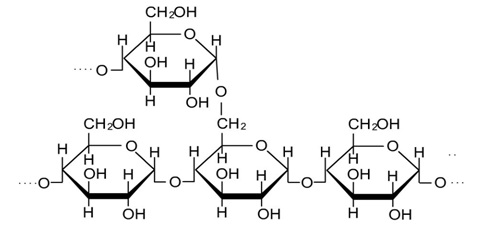 Figure 2: Molecular structure of amylopectin [5].
Figure 2: Molecular structure of amylopectin [5].
The process of forming a bioplastic from starch begins with its proper extraction of sugars (mainly dextrose, but also glucose and sucrose) from the vegetable starch, then the microorganisms transform it into a smaller molecule of a substance called lactic acid that becomes the dimer or lactide that is purified and polymerized to Polylactic acid without the need for chemical solvents [6].
Lactic Acid (LA), also named 2-hydroxy propionic acid, is the basic monomer of PLA. It has an asymmetric carbon that generates two enantiomeric forms, L-LA and D-LA, that produces PLLA and PDLA respectively, and the combination of the two optical isomers creates PDLLA as shown in the figure 3 [7,8].
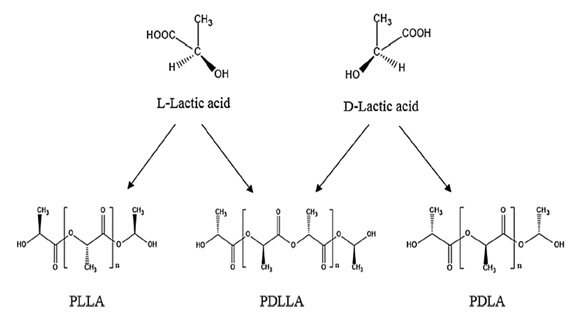 Figure 3: Representation of the enantiomers for the LA and the possible outcomes for PLA [8].
Figure 3: Representation of the enantiomers for the LA and the possible outcomes for PLA [8].
The proportion of D- and L-enantiomers lead to different stereoblocks of PDLLA. The ratio of said enantiomers, which is the stereochemical composition of PLA whether it is PLLA or PDLA, as well as the structure of the PLA stereoblock, could affect important properties, such as the extent of crystallization and thermal properties. Having said that, PLA can be manufactured with a wide range of properties by changing the molecular weight, composition of the polymer, and distribution of stereoisomers of the polymer chains [7,8].
With low molecular weight (<50,000 Da) PLA has a melting point of 130-150ºC and a glass transition temperature of 48ºC which is the temperature when the polymer changes from a hard or brittle state to a molten or viscous state; nevertheless, with high molecular weight (>50,000 Da), PLA has a higher melting point being 170-180ºC and the glass transition temperature is also increased to 58ºC; a higher transition temperature and melting point are indicators of better thermal stability of the polymer [8].
PLA possesses attractive mechanical properties such as a low haze or turbidity, which is the cloudiness of a fluid and is measured by the percentage of light that it deflects, a high tensile strength that represents the maximum stress that a polymer can withstand before breaking under pulling, expressed in MPa. Elongation at break, also known as fracture strain, is the ratio between changed length and initial length after breakage of the polymer, reported as percentage, and tear resistance is used to determine how well a material behaves the effect of tearing in g/mm [8].
As displayed in table 1, PLA possesses properties that compares to PET, PP and nylon. This indicates the potential of PLA as an alternative material to conventional plastics [7,8].
|
Property |
PLA |
PP |
PET |
Nylon |
|
Density (g/cc) |
1.25 |
0.9 |
1.4 |
1.2 |
|
Haze (%) |
2.1 |
1-4 |
2-5 |
2-3 |
|
Tensile strength (MPa) |
109.97 |
189.95 |
204.95 |
249.94 |
|
Tensile modulus (MPa) |
3299.26 |
2399.46 |
3799.15 |
1824.59 |
|
Ultimate elongation (%) |
160 |
110 |
140 |
125 |
|
Tear resistance (g/mm) |
0.3810 |
0.1316 |
0.4572 |
0.3302 |
Table 1: Mechanical properties of PLA and petroleum-based polymers [8].
Note: PET (Polyethylene Terephthalate), PP (Polypropylene).
The two main methods to produce LA are by bacterial fermentation of basic carbohydrates or by chemical synthesis. Chemical synthesis has many limitations, including limited production capacity, inability to produce only the desired isomer, and high manufacturing costs. The bacterial fermentation process to produce LA is preferred and can be classified as homofermentative or heterofermentative, depending on the bacteria used in the process. In the heterofermentative method, a lower quantity of LA is produced along with significant levels of other metabolites highlighting acetic acid, ethanol, glycerol and carbon dioxide; whereas in the homofermentative method a higher quantity of LA is produced with minor levels of other metabolites, which means a better yield. For the reasons mentioned, the homofermentative method is more frequently used by industry [7].
In the homofermentative method, species of the Lactobacillus genus, such as Lactobacillus delbrueckii, L. amylophilus, L. bulgaricus and L. leichmannii to indicate a few, are used under lightly acid conditions of a pH range from 5.4 to 6.4, a temperature range from 38 to 42ºC and an anaerobic condition. The nutrients used are usually simple sugars such as glucose and maltose from corn or potato or other sources containing a rich source of starch [7].
The polymerization of lactic acid can be given by the L-lactide ring-opening method or by direct condensation. Since condensation is an equilibrium reaction, there are difficulties in removing a certain amount of water during the later stages of polymerization, which limits the molecular weight of the polymer obtained by this method, consequently most of the research has focused on the method of ring-opening, as shown in figure 4, [6].
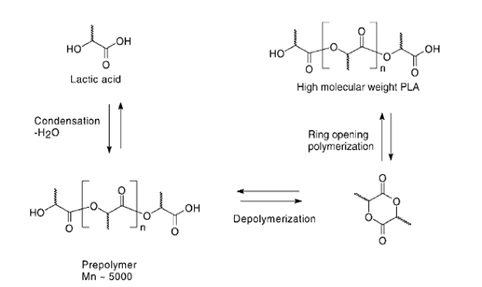 Figure 4: Reactions of the polymerization of lactic acid to obtain PLA by the ring-opening method [9].
Figure 4: Reactions of the polymerization of lactic acid to obtain PLA by the ring-opening method [9].
This method uses two molecules of lactic acid that undergo simple esterification and then catalytically cyclizes to make a cyclic dilactate ester. Although dimerization also generates water, it can be separated prior to polymerization due to a significant drop in polarity. High molecular weight PLA is produced from lactide by ring-opening polymerization generally using a catalyst. In this mechanism, a wide range of polymers with high molecular weights is obtained [9].
Polylactic Acid (PLA), finally, is a thermoplastic biopolymer (Figure 5). Its cross-linking of chains gives rise to biodegradable plastic sheets that serve as the basis for the production of numerous non-polluting plastic products [6].
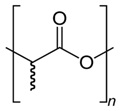 Figure 5: Structure of polylactic acid [6].
Figure 5: Structure of polylactic acid [6].
PLA is one of the most common bioplastics used today, however, the process for this material to be degraded is very specific and has to occur in the appropriate facilities, that is, if it ends up in a landfill, it will remain there for thousands of years like a normal plastic [10].
There are three terms that are common when it comes to plastics, so it is important to clarify how they relate to each other and how they differ, these terms are: Degradable, Biodegradable and Compostable.
Degradability is chemical or biological decomposition, regardless of the time it is achieved, that is, any plastic is degradable. On the other hand, biodegradable plastic can be digested, so that the carbon atoms in the chains of the polymer are broken and become simpler and more stable. However, only bioplastics are biodegradable within a reasonable period. Finally, compostability indicates the biodegradation of plastic in a certain amount of time, maintaining specific conditions to achieve it [2].
BIODEGRADABILITY
A biodegradable plastic is a plastic which degradation results from the action of naturally occurring microorganisms such as bacteria, fungi, and algae. It Is considered biodegradable if a significant change in the chemical structure occurs in the exposed material, resulting in carbon dioxide, water, inorganic compounds, and biomass production [11]. In some cases, biodegradability is considered synonymous with compostability if there is no visible or toxic residues under this conditions. Is important to understand that the term “biodegradable” by itself is not useful because biodegradation is not always ensured. If the environment is not favorable for degradation, the material will not degrade in a short time, even in the presence of enzymes that could speed up the rate of chemical bond breakage. This is why it is important to specify the particular environment in which biodegradation of the polymer is expected to happen [12].
Polylactic Acid (PLA) is a bio-based polymer that is also biodegradable in some specific conditions that are going to be commented below; and when it biodegrades does not pollute the environment [13]. The degradation of the polymer is divided into heterogeneous and homogeneous degradation, which are also called surface and intramolecular degradation of polymers and it can exist in three different ways from chemical angle: (a) scission of the main chains, (b) scission of the side chains and (c) scission of the intersectional chain [13]. In the case of the PLA, degradation occurs mainly through scission of ester bonds, this is how the long polymeric chains are broken down into shorter oligomers, dimers or monomers. Specifically, the ester bonds of PLA fragment into carboxylic acid and alcohol by chemical hydrolysis due to hydrion [11-13]. These shorter units are small enough to pass through the cell walls of microorganisms and be used as substrates for their biochemical processes and thus can be degraded by microbial enzymes [11].
Besides these ways of degradation, there are some factors that influence directly into the biodegradability of the polymers like the mechanical properties: Factors associated with the higher order structure (glass Transition temperature (Tg), melting Temperature (Tm), crystallinity, crystal structure and modulus of elasticity), chemical characteristics of the polymer like factors associated with the first-order structure (chemical structure, molecular weight and molecular weight distribution) and factors related to surface conditions (surface area, hydrophilic, or hydrophobic properties) [11,14]. In the case of the high molecular weight PLA, this is degraded at a slower rate than those with low molecular weights. In general, the higher the melting point is, the lower the degradability tends to be and in the case of the PLA this melting temperature (Tm) has a great effect on enzymatic degradability [14]. Another important characteristic about the PLA is that amorphous chains are far more susceptible to hydrolysis and crystalline regions are more resistant to degradation and by contrast hydrolysis occurs at a much slower rate [15]. In one study, there was considerably higher degradation of fully amorphous PLA than of semi crystalline PLA under similar conditions of hydrolytic degradation (phosphate-buffered solution at pH 4.0 and 37ºC). After 18 weeks, amorphous PLLA showed a weight decrease of about 14% with respect to its initial mass, whereas similar weight losses for semi crystalline PLA by hydrolysis (phosphate buffered saline at pH 3.4 and 37ºC) were only evident after 20 months [12].
The process of biodegradation can be aerobic or anaerobic [12]. The degradation behavior of PLA under anaerobic conditions has been less studied than under aerobic conditions [11], but a study reported that an anaerobic biodegradation is accelerated when the degrading temperature is greater than the PLA glass transition Temperature (Tg). This may be due to the fact that above Tg, the amorphous part of the PLA becomes more susceptible to microorganisms. However, the anaerobic biodegradation is apparently slowed when the degrading temperature is below PLA Tg [14]. Degradation under aerobic conditions, which takes place during composting, has been more deeply investigated [12]. It starts with a sequential mechanism beginning with a simple chemical hydrolysis occurring in the presence of water at elevated temperatures, to decrease the molecular weight of the PLA. And then, biotic degradation in which microorganisms utilize the lactic acid oligomers as an energy source and to generate carbon dioxide, water and biomass [14,15].
Aerobic biodegradation generally means composting. Composting is a natural process in which organic materials are decomposed to produce humus, a soil-like substance. The major groups of thermophilic and mesophilic microorganisms involved are fungi, bacteria, and actinomycetes [12]. The studies mostly talk about industrial composting because the conditions difference between home and industrial composting are very different. For example, studying the biodegradation of PLA bioplastic under home composting conditions during 11 months showed a very slow biodegradation [11], another study showed that amorphous PLA is easily degradable under anaerobic conditions but they estimated that under these anaerobic environment conditions (20ºC approximately) the degradation will take 100+ years [16]. At industrial scale the conditions are very different, the environment must have a high oxygen content (not less than 6%), be warm (approximately 60-70ºC), humid (approximately 60%) and controlled (pH 8.5) [11] and for these characteristics, compost has proved to be the most suitable environment for biodegradation of PLA [12]. This polymer can be degraded in a composting environment after 45-60 days at 50-60ºC by microorganisms in the compost [15]. Commercially available PLA bottles degraded visibly in 30 days under composting conditions with PLA bottles, having a lower degradation rate due to a higher degree of crystallinity [17]. Therefore, while compostable PLA cannot be regarded as hydrolysable or biodegradable under normal environmental conditions [15].
As we mentioned before, PLA is susceptible to hydrolysis due to the hydrolysable functional groups in its backbone and the degradation under aerobic conditions occurs in two steps, first is the hydrolytic degradation, this one occurs in stages: First is the diffusion of water into the material, then the hydrolysis of chains in the amorphous part, which can occur through an autocatalytic mechanism because of the carboxylic acid end groups of PLA, its oligomers can catalyze the ester linkages since the pKa of PLA’s carboxylic acid end group and its oligomers is lower (~3) than most carboxylic acid groups (4.5 to 5) leading to faster rate of degradation [12,15].
The second step of the aerobic degradation is the enzymatic degradation, this one occurs when the microorganisms degrade PLA only after high molecular weight PLA goes under hydrolysis and the molecular weight of PLA falls 10000 Da or less [15]. Typically, biodegradable polymers are degraded by microbial attack in a single step but in the case of the PLA it has been demonstrated that microorganisms are only able to assimilate oligomers or low molecular weight products that have been released from the polymer matrix as a consequence of prior a biotic hydrolysis of PLA [12].
Two main types of enzymes are involved in microbial depolymerization processes: extracellular and intracellular depolymerases. During microbial degradation, PLA-degrading microorganisms first excrete extracellular depolymerase of PLA in order to break the longer units down into shorter molecules, preparing them for further degradation by intracellular enzymes [11]. This extracellular depolymerase need usually to be stimulated by some inducers such as silk fibroin, elastin, gelatin, and some peptides and amino acids. After the extracellular depolymerization occurs, the low molecular weight compounds enter in microbial membranes and decompose into carbon dioxide, water or methane by intracellular enzymes [13]. Currently, multiple types of microbes can degrade PLA, but they mostly are actinomycetes, a fraction of them belong to bacteria and fungus. Some studies show that PLA-degrading bacteria are not distributed widely in the natural environment. The first reported PLA-degrading bacteria was Bacillus brevis, it was isolated from 144 soil samples using an enrichment culture medium [13]. In another study they used the actinomycete Streptomyces, they obtained that 26 of the 40 soil samples showed PLA-degradation and also that a 36,7 % of the samples of PLA were degraded by Amycolatopsis sp. after 7 days of cultivation at 30ºC in culture broth [18]. These types of microorganisms are very important to degrade the PLA, for example, a study showed that the PLA biodegradation in the soil incubated with Pseudonocardia sp. RM423 was higher than that in uninoculated soil but also is important to mention that they did the study under mesophilic and thermophilic conditions (58ºC) [19]. As is shown, there are many options to degrade PLA using microorganisms but is necessary to keep looking forward for new methods to do it.
MEDICAL AND FOOD APPLICATIONS
The concern caused by the unforgiving environmental problems caused by the residues of polymers of petrochemical origin, especially those used in packaging, have motivated a growing interest in the use of alternatives of renewable origin [20]. Biopolymers, both natural and synthetic, have an important potential to be applied as food packaging [21]. In spite of it, at present there does not exist a biopolymer that expires with all the physical requests to be used industrially on a large scale.
That is why studies have been conducted to evaluate the efficiency of the PLA as a substitute for the original containers petrochemical, therefore [22], indicates that the PLA is a polymer versatile that presents different possibilities in the industrial applications of textiles, medicine and mainly in the packaging of food and single-use products.
The Technological University of Denmark carried out a study in PLA packages for yogurt, butter, margarine and cheeses, where it was observed that it has a very good mechanical protection, moisture barrier, protection to light, fats and gases. In addition, it was studied if during the process of biodegradation presents the migration of lactic acid to the products, proving that the migration was so little that it was classified as null and it was concluded that it is ideal for the packaging of foods with high breathing or life Short, bakery, fruits and vegetables.
In addition to the studies that have been carried out in the use of PLA in packaging, it was also thought in these for medical use, specifically in the use of bioabsorvibles materials that allow for less invasive surgical processes, in addition their physical characteristics make It is possible to design parts that replace the metal parts.
Therefore [23], conducted a study with the purpose of evaluating the complications observed with the use of implants created from the polymer PLLA, for which we observed the results obtained in a period of 28 months after the effectuated Implant with the biopolymer. Five patients presented signs and symptoms inherent in the implant, in a period of 24, 2 months, on the other hand, two patients answered to the medical treatment while three remaining ones must be submitted to an extraction process since the implant was not bio absorbed. Because of the small number of complications observed, bioabsorbable implants seem safe and effective. Their use in arthroscopic surgery would be a breakthrough because it would simplify postoperative follow-up and surgeries review.
The implants are only a few of the applications studied for the polymers derived from lactic acid, since tissue engineering has been a field in which you have worked to find a solution for medical problems critics through the development and implementation of the chemical knowledge [7].
Showed [24], that the polymer presents no reactions or products harmful to health since the degradation of PLA in the body occurs through a process of hydrolysis, which produces lactic acid which is produced naturally by the body. Therefore, this allows for a drain in a natural way, generating only inflammation in the nearby tissues to the application.
There are techniques that allow form threads from the PLA, these yarns are used in the manufacture of textiles with features that allow for different applications, a noteworthy feature of this polymer is its polarity, that to be quite polar gives it the characteristics necessary for the manufacture of towels absorbent, So based on the study by [25], the company Biovation developed a handkerchief antimicrobial that facilitated the cleanliness and increased the effectiveness thanks to its characteristics polar.
CONCLUSION
Bioplastics made of Polylactic acid are a positive alternative that can decrease the excessive consume of non-biodegradable plastics created from petroleum.
Compared to plastics made from petroleum, Polylactic acid is biodegradable, but it is significant to know that this is true under certain conditions and with the presence of specific microorganisms, so it is important to inform consumers so these biodegradables bioplastics can be properly disposed.
It is evident that Polylactic acid has many applications in the field of medicine, however, for us the greatest focus should be given to the commercial applications that this bioplastic could have specifically in packaging, since the plastics are currently used a lot for packaging and this is one of the largest sources of existing pollution.
REFERENCES
- Valero-Valdivieso MF, Ortegón Y, Uscategui Y (2015) Bioplastics: Advances and perspec Dyna 80: 171-180.
- Emadian SM, Onay TT, Demirel B (2017) Biodegradation of bioplastics in natural environments. Waste Management 59: 526-536.
- Calabrò PS, Grosso M (2018) Bioplastics and waste management. Waste management 78: 800-801.
- Endres HJ (2017) In Advances in Biochemical Engineering/Biotechnology 166: 427-468.
- Sagnelli D, Hebelstrup KH, Leroy E, Rolland-Sabaté A, Guilois S, et al. (2016) Plant-crafted starches for bioplastics production. Carbohydrate Polymers 152: 398-408.
- Garlotta D (2001) A literature review of Polylactic acid. Journal of Polymers and the Environment 9: 63-84.
- Castro-Aguirre E, Iñiguez-Franco F, Samsudin H, Fang X, Auras R (2016) Poly(lactic acid)-Mass production, processing, industrial applications, and end of life. Adv Drug Deliv Rev 107: 333-366.
- Rivero CP, Hu Y, Kwan TH, Webb C, Theodoropoulos C, et al. (2016) 1-Bioplastics from solid waste. Curr Dev Biotechnol Bioeng Solid Waste Manag 1-26.
- Langer R, Basu A, Domb AJ (2016) Special issue: Polylactide (PLA) Based Biopolymers. Advanced Drug Delivery Reviews 107: 1-2.
- Maitz MF (2015) Applications of synthetic polymers in clinical medicine. Biosurface and Biotribology 1: 161-176.
- Bátori V, Åkesson D, Zamani A, Taherzadeh MJ, Sárvári Horváth I (2018) Anaerobic degradation of bioplastics: A review. Waste Manag 80: 406-413.
- Gorrasi G, Pantani R (2017) Hydrolysis and biodegradation of poly(lactic acid). Advances in polymer science 279: 119-151.
- Qi X, Ren Y, Wang X (2017) New advances in the biodegradation of Poly(lactic) acid. Int Biodeterior Biodegrad 117: 215-223.
- Nurul Fazita MR, Jayaraman K, Bhattacharyya D, Mohamad Haafiz MK, Saurabh CK, et al. (2016) Green composites made of bamboo fabric and poly (lactic) acid for packaging applications-a review. Materials (Basel) 9.
- Karamanlioglu M, Preziosi R, Robson GD (2017) Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym Degrad Stab 137: 122-130.
- Kolstad JJ, Vink ETH, De Wilde B, Debeer L (2012) Assessment of anaerobic degradation of IngeoTM polylactides under accelerated landfill conditions. Polym Degrad Stab 97: 1131-1141.
- Kale G, Auras R, Singh SP, Narayan R (2007) Biodegradability of polylactide bottles in real and simulated composting conditions. Polym Test 26: 1049-1061.
- Penkhrue W, Khanongnuch C, Masaki K, Pathom-aree W, Punyodom W, et al. (2015) Isolation and screening of biopolymer-degrading microorganisms from northern Thailand. World J Microbiol Biotechnol 31: 1431-1442.
- Apinya T, Sombatsompop N, Prapagdee B (2015) Selection of a Pseudonocardia RM423 that accelerates the biodegradation of poly(lactic) acid in submerged cultures and in soil microcosms. Int Biodeterior Biodegrad 99: 23-30.
- Cruz-Morfin R, Martínez-Tenorio Y, López-Malo Vigil A (2013) Biopolímeros y su integración con polímeros convencionales como alternativa de empaque de alimentos. Temas Sel Ing Aliment 7: 42-52.
- García O, Pinzón M, Sánchez S (2013) Extracción y propiedades funcionales del almidón de yuca, Manihot esculenta, variedad ICA, como materia prima para la elaboración de películas comestibles. @limentech Cienc y Tecnol Aliment 11: 13-21.
- Serna CL, Rodríguez de SA, Albán AF (2017) Ácido Poliláctico (PLA): Propiedades y Aplicaciones. Ing y Compet.
- Arce G, Lacroze P, Barclay F, Pereira E, Previgliano J (2003) Implantes bioabsorbibles de ácido poliláctico en cirugía artroscóp Rev. Argentina Artrosc 10: 23-29.
- Hamad K, Kaseem M, Yang HW, Deri F, Ko YG (2015) Properties and medical applications of polylactic acid: A review. Express Polym Lett 9: 435-455.
- Sierra AR, Madariaga FJG, Gomez J, Franco-Urquiza E (2016) Diseño y desarrollo de productos a base de compuestos formados por residuos de fibra de agave y bioplástico. Conference Paper.
Citation: Jiménez L, Mena MJ, Prendiz J, Salas L, Vega-Baudrit J (2019) Polylactic Acid (PLA) as a Bioplastic and its Possible Applications in the Food Industry. J Food Sci Nut 5: 048.
Copyright: © 2019 Jiménez L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

