
Pre-Adaptation of Saccharomyces Cerevisiae to Low Temperatures Affects the Resistance of Yeast Cells to Subsequent Autolysis, High Temperature and Overpressure
*Corresponding Author(s):
Anatoly BozhkovBiology Research Institute, Karazin Kharkiv National University, Kharkiv, Ukraine
Email:niibio@karazin.ua
Abstract
Obtaining Water-soluble Components (WC) from Sac cells. Cerevisiae containing amino acids, peptides, proteins, vitamins and microelements is an urgent task of biotechnology. In order to maximize the extraction of VA from yeast cells, various methods of processing an aqueous suspension of cells were used; incubation at 6°C, (pre-adaptation); carrying out autolysis at 55°C; the action of high temperature (121°C) and excess pressure (1.5atm.) - autoclaving. In samples that were obtained after separation of cells and cell walls by centrifugation at 3000g for 15min. the content of VA was determined from the dry residue, the spectral characteristic in the UV region of the spectrum, and the content of trace elements (Zn, Fe, Mn, Co, Ni, Cu, Pu, Cd). It was shown that pre-incubation of an aqueous suspension of yeast at 6°C for 96 hours (pre-adaptation) increases the yield of VA after autolysis and even more after autoclaving. Sequential processing of yeast suspension; pre-adaptation, autolysis, autoclaving provides almost complete removal of VA (up to 70% of the total yeast biomass) from cell walls. It was found that such sequential treatment of the yeast cell suspension enriches the composition of VC with peptides and proteins compared to the composition obtained after autolysis. It was found that the composition of autolysates does not contain toxic lead and cadmium, and it contains trace elements in a successful combination from the standpoint of nutritional value (Zn>Fe>Mn>Co>Ni>Cu). A sequential three-stage procedure for obtaining Saccharomyces cerevisiae VC cells is proposed.
Keywords
Autoclaving; Autolysis; Pre-adaptation to low temperatures; Trace elements; Water-soluble components; Yeast
Introduction
As is known, Saccharomyces cerevisiae is one of the most promising eukaryotic microbiological objects in modern biotechnology [1-3]. They are characterized by high growth ability, resistance to foreign microflora, the ability to absorb various food sources, are easily separated from the culture medium, and do not pollute the environment with spores [4]. In food technologies, Saccharomyces cerevisiae is most often used; their biology is well studied, which makes it possible to obtain new strains not only by classical breeding methods, but also with the help of modern recombinant technologies [5].
Proteins and vitamins are of the greatest value to the yeast biomass of Saccharomyces serevisiae, and a set of microelements is of no less interest. The amino acid composition of yeast is superior to the protein of cereals and slightly inferior in nutritional value to the protein of milk and fishmeal [6]. In terms of vitamin content, the yeast Saccharomyces cerevisiae surpasses all protein feeds; ergosterol, a precursor of vitamin D, is of great interest in this regard [7].
Along with this, enzymes, polysaccharides, flavors, sorbents and other products are obtained from the biomass of the yeast Saccharomyces cerevisiae [8,9]. Despite the great biotechnological potential of Saccharomyces cerevisiae is not yet fully used, this is due to the fact that the yeast cell wall, which accounts for up to 30-35 percent of the biological mass of the cell and includes chitin, mannoproteins and other components are extremely resistant to various influences [10].
Such a cellular organization of yeast provides them with high mechanical and chemical strength and, as a result, poor digestibility in the digestive tract, and, in addition, can induce allergic reactions. In this regard, there is a need to separate the water-soluble components of the cell with high nutritional and biological activity from the cell walls. Currently, various options for obtaining biologically active substances from yeast have been proposed, which are based on the mechanical destruction of cells, followed by the separation of water-soluble components from cell walls and cell fragments; enzymatic hydrolysis using exoenzymes; chemical degradation of cell components by alkaline and acidic solutions; and the use of autolysis, that is, the activation of endogenous lysosomal enzymes of yeast cells.
The most widely used in the production of products from yeast, at present, is autolysis [11,12]. This is due to the technological simplicity and low cost of its implementation. However, this method has a number of disadvantages. First, the relatively low yield of target products; secondly, the unstable composition of the obtained substances and the duration of the autolysis procedure.
We believe that the process of autolysis can be influenced not only by the conditions of its implementation (such as temperature, incubation time, characteristics of the medium), but also by the functional state of the yeast cells that undergo autolysis.
From the standpoint of the structural organization, the Saccharomyces serevisiae cell can be represented as follows: 1. Water-soluble components this is part of the proteins, peptides, amino acids, sugars, nucleic acids, microelements; 2 Cell organelles and supramolecular complexes that are poorly soluble in aqueous solutions, they account for most of the cell and 3. Cell wall, which are insoluble in water and poorly hydrolyzed, they account for 25 to 35% of the cell biomass [13].
The production of water-soluble components of yeast cells can be reduced to changing the characteristics of intermolecular and molecular interactions between cell components and ensuring the “accessibility” of hydrolytic enzymes to the corresponding substrates and, as a consequence, increasing the yield of water-soluble cell components.
It is known that the functional state and composition of cell components can change significantly in the process of adaptation to extreme environmental conditions. So, with an increase or decrease in incubation temperature, the synthesis of stress proteins is induced in the cell and the membranes of the cell and other supramolecular complexes, as well as the physicochemical characteristics of the cell, can be significantly rearranged [14-16].
It can be assumed that pre-incubation of Saccharomyces cerevisiae cells, in particular at a low temperature (+6°C), may affect the structural and functional organization of cells, which may affect the efficiency - the qualitative and quantitative yield of water-soluble compounds in the aquatic environment.
It is known that the combination of high temperature and pressure induces energy rearrangements of cellular structures and disruption of intermolecular and even molecular interactions in the cell [17], which can increase the release of water-soluble components of yeast cells into the medium.
In this regard, the hypothesis was tested according to which preliminary adaptation (pre-adaptation) of the yeast culture, followed by autolysis and auto cloning, can ensure the destruction of intermolecular and partially covalent bonds in the cell and obtain the maximum possible input of water-soluble components. To do this, we studied the complex effects of pre-adaptation of Saccharomyces cerevisiae cells to a temperature of +6°C, autolysis at 55°C and autoclaving (the combined effect of high temperature (121°C) and excess pressure (1.5atm.) for 10min on quantitative yield of water-soluble components and qualitative composition in terms of spectral characteristics and composition of essential microelements.
The objective of our study was to determine the effect of preconditions for yeast processing, which can provide the maximum yield of yeast components, and the effect of these conditions on the ionic composition of the resulting product.
Material And Methods
Baker's yeast S. cerevisiae was used in the work. A 33% yeast cell suspension was prepared in distilled water (based on the dry residue). Various variants of samples were prepared from the resulting suspension for further processing (Figure 1).
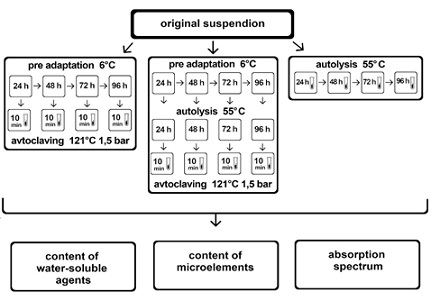 Figure 1: Scheme of experimental variants of yeast processing in order to obtain water-soluble components, which shows different modes of yeast suspension processing and indicators that were determined every 24 hours for 4 days, for this, 10ml of suspension were taken from the samples.
Figure 1: Scheme of experimental variants of yeast processing in order to obtain water-soluble components, which shows different modes of yeast suspension processing and indicators that were determined every 24 hours for 4 days, for this, 10ml of suspension were taken from the samples.
After various modes of yeast treatment, Water-soluble Components (WC) were separated from cells and cell organelles by centrifugation at 3000g for 15min at 20°C. The supernatant was separated from the precipitate and divided into 2 parts. In one part, the amount of dry matter was determined. To do this, an aliquot was dried at 55°C to constant weight. The amount of dry, water-soluble substances was weighed on an analytical balance. The dry residue of VA was used to determine the composition of microelements. The second part of the obtained VCs was used for the spectral analysis of amino acids, proteins, and nucleic acids.
In order to pre-adapt yeast to low temperature, one of the variants of the yeast suspension was incubated at a temperature of 6°C. After 24, 48, 72, and 96 hours, aliquots were taken and the amount of VA, the content of trace elements, and spectral characteristics were determined in them (Figure 1).
Autolysis of an aqueous suspension of yeast was carried out at a temperature of 55C with stirring for 24, 48, 72 and 96 hours. Every 24 hours, 10ml of suspension were taken according to the established scheme (Figure 1).
When studying the effect of high temperature and overpressure, the samples were autoclaved at 121°C, 1.5atm for 10min. All subsequent operations were performed as for the previous options (Figure 1).
To determine the integral composition of the water-soluble components of yeast, spectral analysis was performed in the UV region of the spectrum. To do this, the spectral characteristics were recorded in the range from 200 to 340nm on a UV-2600 Shimadzu spectrophotometer (Japan).
The content of the ionic composition was determined in the initial (control) yeast suspension, in the samples after 96 h of autolysis, and in the samples after 96h of autolysis followed by autoclaving. To do this, the obtained samples were dried to a constant weight at a temperature of 55°C, one gram of dry matter was taken, and sample preparation for atomic absorption spectrometry was carried out as described in [18], Concentrated nitric acid was added to the obtained samples, stirred and evaporated to a wet residue. After cooling, nitric acid of 1.5% concentration was added to the samples, and the resulting mixture was sonicated for 25 minutes. Later, Triton X-100 and nitric acid at a concentration of 1.5% were added to the samples, and after mixing, the ion concentrations were determined on an S-115 M-1 atomic absorption spectrometer (Ukraine).
All experiments were repeated at least 3 times. Data analysis was performed using Excel 2013 (Microsoft Corporation, USA) and STATISTICA 8 (Stetsoft, USA). Data are presented as group means and Standard Error (x SE), which were subjected to statistical processing using the non-parametric Mann-Whitney U test [19]. Differences were considered significant at P < 0.05.
Results
Evaluation of the efficiency of autolysis and the subsequent effect of temperature and pressure on the yield of water-soluble components from the biomass of Saccharomyces cerevisiae
As is known, a yeast cell suspension always contains a small amount of destroyed cells and a certain amount of cellular exometabolites. It turned out that if an aqueous suspension of yeast is prepared and the cells are removed by centrifugation within 24 hours, then from 0.6 to 1g / l of water-soluble components were detected in it, which is no more than one percent of the total mass of cells. This initial yeast suspension was taken as a control (Figure 2, zero point on the axis).
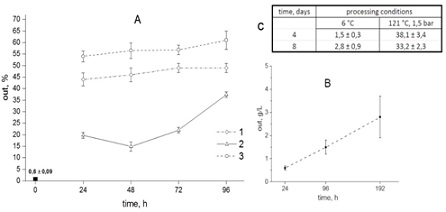 Figure 2: A. the content of water-soluble yeast components as a percentage of the initial biomass in the control sample (zero point on the axis) after incubation of an aqueous suspension of yeast at a temperature of 55°C for 24, 48, 72 and 96 hours - autolysis (1), after incubation of yeast suspension at 6°C for 24, 48, 72 and 96 hours, followed by autoclaving of these yeast suspensions at 121°C (1.5atm.) for 10minutes. (2) And after autolysis followed by autoclaving (3). B. the content of water-soluble yeast components after their incubation for 4 and 8 days at 6°C and after subsequent autoclaving. ? is the content of water-soluble yeast components after their incubation at 6°? after 24, 96 and 192 hours. * - variants for which P < 0.05 compared with 24 hours are marked, ** - variants are marked compared to the control (initial suspension).
Figure 2: A. the content of water-soluble yeast components as a percentage of the initial biomass in the control sample (zero point on the axis) after incubation of an aqueous suspension of yeast at a temperature of 55°C for 24, 48, 72 and 96 hours - autolysis (1), after incubation of yeast suspension at 6°C for 24, 48, 72 and 96 hours, followed by autoclaving of these yeast suspensions at 121°C (1.5atm.) for 10minutes. (2) And after autolysis followed by autoclaving (3). B. the content of water-soluble yeast components after their incubation for 4 and 8 days at 6°C and after subsequent autoclaving. ? is the content of water-soluble yeast components after their incubation at 6°? after 24, 96 and 192 hours. * - variants for which P < 0.05 compared with 24 hours are marked, ** - variants are marked compared to the control (initial suspension).
In the event that the yeast suspension is incubated for 24 hours at a temperature of +55°C, i.e. ensured the “optimal” mode of autolysis, the content of water-soluble components (WC) was about 45 g in terms of dry residue, which was more than 40% of the total yeast biomass (Figure 2A, curve 1).
An increase in the autolysis time to 48 hours was accompanied by a slight increase in the yield of water-soluble components, and by 72 hours this indicator reached a stationary level (Figure 2A curve-1).
Therefore, if we assume that an increase in the release of VA from yeast cells is provided by endogenous enzymes of lysosomes of yeast cells, which are activated by a temperature of 55°C, then the hydrolysis of cellular components occurs on the first day of incubation.
In order to change the structural and functional organization of yeast cells, pre-adaptation of the culture to a low temperature (6°C) was carried out. It is known that when a culture is transferred under conditions that are not optimal for them (stress), there is a decrease in the intensity of general metabolism and induction of the synthesis of a group of stress proteins, structural and functional reorganization of cell structures, including membranes [20]. It has been shown that when the temperature drops below 10°C, the activity of enzymes in the cell decreases by 80-90% of the initial value [21]. Under such conditions, the destruction of weak intermolecular interactions, the death of some part of the cells and, as a consequence, the transition of water-soluble components into the aquatic environment can occur.
It was found that if the yeast suspension was kept at 6°C for 24 hours, then 0.6g/l passed into the medium, while after 96 hours the amount of substances released from the cells increased by 2 times (Figure 2C). A further increase in the yeast holding time under such conditions up to 8 days increased slightly compared to 96 hours (differences are not significant) (Figure 2c).
The results obtained confirm the assumption made about the change in the structural and functional organization of yeast cells during their cultivation at 6°C. “Optimal” from the standpoint of the VC release on Wednesday may be an exposure of 96 hours. If these assumptions are correct, then it can be expected that additional actions aimed at increasing the release of VA from cells into the medium will depend on the preliminary time of pre-adaptation of the yeast suspension to extreme conditions for them.
It is known that high temperature leads to the destruction of intermolecular interactions, and primarily hydrogen bonds [20], which may be accompanied by the disintegration of supramolecular structures, and as a result, a possible increase in the yield of VC cells in the aqueous medium.
In the next series of experiments, the yeast cell suspension was exposed to a temperature of 121°C, at an overpressure of 1.5atm for 10 minutes, i. e. autoclaving after yeast pre-adaptation at 6°C for 24, 48, 72 and 96 hours.
It turned out that autoclaving of the yeast suspension, which was carried out after pre-adaptation for 24, 48 and 72 hours, provided a yield of 18 to 22% VA in terms of dry residue from the total yeast mass, which was 2 times less compared to the yield of water-soluble components at autolysis (Figure 2A curve -2).
However, if the yeast suspension was subjected to pre-adaptation at 6°C for 96 hours and then subjected to autoclaving, then the VA yield increased to 38% of the yeast biomass, i.e. almost twice as compared with 24-72 hours of storage at 6°C (Figure 2A curve -2). At the same time, an increase in pre-adaptation of yeast to 8 days followed by autoclaving did not affect the increase in the release of VA into the aquatic environment (Figure 2C).
The obtained results confirm the assumption that pre-adaptation of yeast at 6°C for exactly 96 hours did provide an increase in the yield of VA after additional exposure to high temperature and pressure. It can be assumed that autolysis and autoclaving are accompanied by two different mechanisms for increasing the yield of water-soluble components into the aqueous medium. If the first can be attributed to the hydrolytic destruction of yeast cell molecules, then the second can be attributed to the “mechanical” disassembly of molecular complexes into separate components. In this regard, it was of interest to combine these two methods for obtaining water-soluble yeast components in order to increase their yield. It turned out that pre-adaptation followed by autolysis and additional autoclaving was accompanied by an increase in the yield of water-soluble components, so their amount after 24 hours of autolysis followed by autoclaving was 54%, did not change after 48 and 72 hours of autolysis and increased to 61% after 96 hours (Figure 2A curve -3).
It is important to note that it was after 96 hours of pre-adaptation of the yeast suspension that the greatest release of VA into the medium took place and after further successive action of autolysis and autoclaving. If the yeast cell sediment after pre-adaptation with subsequent autolysis and autoclaving was washed with an additional portion of water, then up to 6-10% of the yeast components were transferred into the aqueous solution, which in total amounted to about 70% of the cell components of the initial biomass, i.e. only cell walls remained in the sediment after centrifugation.
It can be assumed that the conditions for obtaining VC will affect not only the quantitative, but also the qualitative characteristics of the products obtained.
Evaluation of the integral composition of water-soluble components of Saccharomyces cerevisiae obtained by different methods
Of greatest interest among yeast cellular components are amino acids, peptides, and nucleic acids, the integral composition of which can be determined from absorption spectra in the ultraviolet region. It was found that after yeast autolysis, which was carried out at 55°C for 24, 48, 72 and 96 hours, the components were represented by two main fractions, protein (which is represented by a mixture of free amino acids and peptides - the absorption region is 200-230nm) and nucleotide (absorption region with a clear peak at 260nm) (Figure 3).
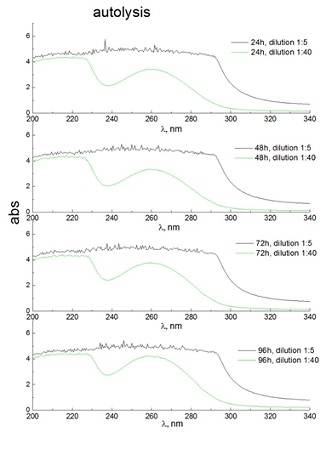 Figure 3: Typical absorption spectra of water-soluble components of yeast in the ultraviolet region, which were obtained after 24, 48, 72 and 96 hours of autolysis (55°C). Typical absorption spectra are shown when the initial solution of VA is diluted by 5 (1:5) and 40 times (1:40).
Figure 3: Typical absorption spectra of water-soluble components of yeast in the ultraviolet region, which were obtained after 24, 48, 72 and 96 hours of autolysis (55°C). Typical absorption spectra are shown when the initial solution of VA is diluted by 5 (1:5) and 40 times (1:40).
It should be noted that the ratio of these two fractions (peptide/nucleotide) was the same for samples obtained at different time exposures from 24 to 96 hours (Figure 3). In the event that the amount of water-soluble components in the sample increased by 8-9 times due to an increase in their concentration, then in this case substances were recorded that absorbed in a wider range of the spectrum, from 200 to 290nm (Figure 3). At the same time, a wide range of individual peptides and proteins, which also include aromatic amino acids, was revealed. It should be noted that their composition was unstable (i.e., it depended on the time of autolysis (Figure 3).
At the next stage of the work, we determined the spectral composition of substances that were present in water-soluble components after pre-adaptation at 6°C for 24 and 96 hours of incubation, followed by autolysis for 4 days and additional autoclaving after autolysis at 121°C, 1.5atm, 10 minutes. It turned out that after incubation of the yeast suspension at 6°C for 24 hours, a slight absorption was detected in the region of 200-205nm and it linearly decreased to 240nm. A slight absorption was recorded in the region of 260-270 nm (Figure 4).
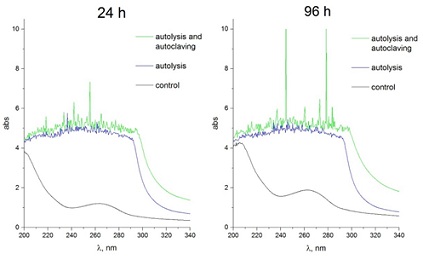 Figure 4: Typical absorption spectra of water-soluble yeast components for samples that were obtained after pre-adapting the yeast suspension at 6°C for 24 and 96 hours, and after autolysis, which was carried out at 55°C for 24 and 96 hours and after - adaptation, autolysis followed by autoclaving at 121°C (1.5atm.) for 10min.
Figure 4: Typical absorption spectra of water-soluble yeast components for samples that were obtained after pre-adapting the yeast suspension at 6°C for 24 and 96 hours, and after autolysis, which was carried out at 55°C for 24 and 96 hours and after - adaptation, autolysis followed by autoclaving at 121°C (1.5atm.) for 10min.
In the event that an aqueous suspension of yeast was incubated at 6°C for 96 hours, the amount of water-soluble amino acids and peptides (absorption area 200-210nm) increased compared to a 24-hour exposure (Figure 4). These results indicate that after 96 hours of pre-adaptation, structural and functional rearrangements occurred in yeast cells, which could lead to an increase in the yield of VA after autolysis and autoclaving.
Carrying out yeast autolysis for 24 hours at a temperature of 55°C after their pre-adaptation at 6°C for 24 hours was accompanied by the production of VC containing a fairly wide range of amino acids, proteins and nucleic acids (Figure 4). However, of greatest interest is the composition of VC, which was obtained after successive procedures of pre-adaptation, autolysis, and subsequent autoclaving. Thus, the amount of substances that were absorbed in a wide range from 200 to 300nm in comparison with autolysis increased, and this was especially pronounced after 96 hours of pre-incubation and 96 hours of autolysis, followed by autoclaving at 121°C (1.5atm.) for 10 min. (Figure 4).
It should be noted that the components obtained under such conditions differed from the autolysate components in organoleptic properties as well. Therefore, a sequential procedure for processing yeast cell suspension, which consists of three stages: pre-adaptation, autolysis and autoclaving, allows transferring up to 70% of yeast cell components, which contain free amino acids, peptides, proteins and nucleic acids, into the aqueous phase. Further complete analysis of the composition of the water-soluble components of yeast will allow us to determine the areas of their most effective application.
Composition of microelements in water-soluble components of Saccharomyces cerevisiae obtained by different methods
As is known, along with proteins, vitamins and other macromolecules, mineral components also take an active part in the regulation of metabolism. They perform extremely diverse functions in the body and therefore they are conditionally divided into essential and toxic [22]. Deficiency of essential, as well as the presence of toxic trace elements in the body, leads to a variety of pathologies [23,24]. In this regard, it is of great interest to determine the composition of microelements in the obtained yeast VA.
Determination of the composition of microelements in yeast biomass and autolysates showed an unexpected and important result. It turned out that highly toxic heavy metals - cadmium and lead were absent, both in yeast and in autolysates (Table 1). It was found that the yeast biomass contains a fairly large amount of such essential elements as zinc and iron, manganese, nickel, and copper ions, which were contained in small amounts (Table 1).
|
Groups |
Cu |
Zn |
Fe |
Mn |
Ni |
Cd |
Pb |
|
|
Control |
X |
0,0097 |
0,6267 |
0,4267 |
0,0500 |
0,0335 |
n/d |
n/d |
|
± |
0,0012 |
0,0491 |
0,1994 |
0,0064 |
0,0061 |
|||
|
Autolysis |
X |
0,0273* |
1,7000* |
0,5300 |
0,1503* |
0,0307 |
n/d |
n/d |
|
± |
0,0034 |
0,0100 |
0,1762 |
0,0047 |
0,0044 |
|||
|
Autolysis and Autoclave |
X |
0,0183* |
1,7100* |
0,4800 |
0,1423* |
0,0400 |
n/d |
n/d |
|
± |
0,0027 |
0,0173 |
0,0115 |
0,0017 |
0,0038 |
|||
Table 1. The content of some metal ions (g/l) in the original yeast suspension (X±SE), in water-soluble yeast components, which were obtained after 96 hours of pre-adaptation followed by 96 hours of autolysis (55°C) and subsequent autoclaving at 121°C (1.5atm.) within 10min. Averages from three independent experiments and standard errors are presented; * Marked variants for which P < 0.05 compared to the original biomass; n/d – ion variants that were not detected in the samples, i.e. they are less than 10-4 g/l.
If the autolysate was subjected to additional autoclaving, the microelement composition remained unchanged compared to the autolysate (Table 1).
Therefore, Yeast VA obtained by their pre-adaptation at 6°C followed by autolysis and autoclaving allows obtaining a water-soluble product rich not only in proteins and amino acids, but successfully balanced in essential microelements and does not contain toxic heavy metals.
Discussion
Yeast Saccharomyces cerevisiae contains various biologically active compounds that can be used in pharmacy, food industry, biotechnology (enzymes, vitamins) and agriculture (growth stimulants). However, their production or “extraction” from cells is associated with great difficulties and low efficiency due to the peculiarities of the structural organization of yeast cells [25]. Several different approaches have been proposed for extracting biologically active compounds from Saccharomyces cerevisiae cells [26-29]. In the available arsenal of methods, the autolysis method still attracts with its low cost and ease of execution. The main disadvantage of this method is the relatively low yield of the target product and the relative duration of the procedures.
As is known, autolysis is the postmortem decomposition of yeast cell components as a result of natural activation of endogenous hydrolytic enzymes [30]. The process of cell death is ensured by the launch of a cascade of enzymatic processes. This process begins with a violation of the barrier function of cell membranes, which leads to a drop in the pH value in the cell to 5.0 and below, and this is accompanied by the activation of lysosomal hydrolytic enzymes, followed by hydrolysis of macromolecules, partial violations of cell walls and the release of some of the water-soluble components into the aquatic environment [31]. Under such conditions, a relatively large part of the macromolecules does not undergo hydrolysis and does not pass into the aqueous phase. This can be explained by several features: most of the proteins, sugars and lipids of the cell are part of supramolecular complexes and are poorly soluble and/or not inaccessible to the action of enzymes; autolysis does not create optimal physical-chemical conditions for the activity of hydrolytic enzymes; enzyme activity is maintained for a relatively short period of time. Under natural conditions, the complete breakdown of yeast cells into molecules is provided by various groups of microorganisms. Another feature of the autolysis process is that different authors point to different yields of water-soluble components, i.e. on its different efficiency, even when using similar or identical autolysis conditions [12,32].
We believe that such differences are due to the fact that the yeast that underwent the process of autolysis were in different structural and functional states, and this will affect the rate and efficiency of the decay of cellular structures during autolysis. It is known that the functional response of biological systems to external influences, including the process of disintegration of cellular structures, depends on the state of the system at the time of impact [32]. Water-soluble yeast β glucan fractions with different molecular weights: Extraction and separation by acidolysis assisted-size exclusion chromatography and their association with proliferative activity. International Journal of Biological Macromolecules.
We believe that when performing autolysis, it is necessary to take into account the structural and functional state of yeast cells before the onset of autolysis and the role of weak intermolecular interactions in the structural and functional organization of the cell.
When evaluating the functional state of Saccharomyces cerevisiae cells, it is necessary to proceed from the fact that yeast cells, like all biological systems, have a high adaptive capacity. It is well known that when the cultivation temperature is changed both downward and upward from the optimum for a given object, the synthesis of a large group of stress proteins is induced and the synthesis of the main cell proteins is inhibited [33,34], along with this, pronounced conformational changes in the structure of cell membranes [35] and other supramolecular complexes, which is accompanied by a change in the activity of enzymes, the intensity of respiration and the entire metabolism. As an example of the effect of cultivation temperature on the functional characteristics of Saccharomyces cerevisiae cells, it is sufficient to note the fact that the optimal temperature for the growth of Saccharomyces cerevisiae is 29°C, and for fermentation 35°C, and lowering the temperature of the medium to 6°C reduces the intensity of cell respiration by 5 times their optimum temperature [36].
The results of this work showed that the cultivation of Saccharomyces cerevisiae cells at a temperature of 6°C for 4 days was accompanied by three important effects: 1- an increase in the transfer of water-soluble components to the medium from 0.6 to 3g/l from 24 hours to 4 days subsequent autoclaving of such cells provided an increase in the yield of water-soluble components by at least 50% compared with cells that did not undergo such pre-adaptation, 3-absorption in the region of 200-210nm after 96 hours of pre-adaptation was increased compared to 24 hours of incubation at 6°?, i.e. there is a change in the composition of the VC (Figure 4). Given that at temperatures below 10°C, the activity of hydrolytic enzymes is very low, the overall metabolic activity is also reduced, against this background, stress protein synthesis and protein conformational changes [37], occur. Lowering the temperature in the cell greatly reduces the thermal motion of molecules, which ensures the maintenance and dynamics of weak intermolecular interactions [38], which may be accompanied by conformational changes in supramolecular complexes.
It is known that it is weak intermolecular interactions that form molecular complexes that determine all the vital processes of the cell and the organism as a whole. As the patriarch of molecular biology J. Watson once noted, life is intermolecular interactions [39,40].
Intermolecular interactions are represented by specific (hydrogen bond, hydrophobic interactions, etc.) and nonspecific intermolecular interactions [41]. If specific intermolecular interactions are relatively well studied and ensure the maintenance of the dynamics of molecular and supramolecular structures, nucleic interactions, enzyme-substrate interactions, the formation of an antibody-antigen complex, etc., then nonspecific intermolecular interactions in biological systems have not been studied fully enough. At the same time, they can make a significant contribution to the structural and functional organization of the cell.
Nonspecific intermolecular interactions include induction, orientational and dispersion interactions. These interactions are due to instantaneous dipole moments arising from the continuous movement of electrons [42]. Since the force of attraction during dispersion interactions is proportional to the length of the molecules, their role in the formation of supramolecular complexes consisting of large macromolecules can be significant [15]. When the temperature of the medium is lowered to 6°C, these nonspecific interactions can be weakened and affect the destruction of weak intermolecular interactions, which can play an important role in the partial decomposition of high-molecular complexes and their transition to the aqueous phase, and an increase in the efficiency of hydrolysis after subsequent autolysis is likely, which we and observed in the experiment (Figure 5).
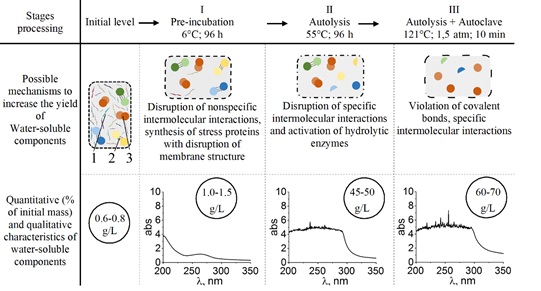 Figure 5: Scheme that demonstrates the possible mechanisms for the destruction of intermolecular (1 - non-specific, 2 - specific) and covalent bonds (3) in yeast cells with different methods of yeast treatment, as well as the quantitative yield of water-soluble components and their qualitative (spectral) characteristics.
Figure 5: Scheme that demonstrates the possible mechanisms for the destruction of intermolecular (1 - non-specific, 2 - specific) and covalent bonds (3) in yeast cells with different methods of yeast treatment, as well as the quantitative yield of water-soluble components and their qualitative (spectral) characteristics.
The available data and the results obtained in this work allow us to state the following explanations for the increase in the yield of VA during sequential treatment of yeast cells. The transfer of a Saccharomyces cerevisiae cell suspension from a temperature of 20-22°C at 6°C induces a stress response, which manifests itself in the violation of nonspecific intermolecular interactions, conformational changes in a number of macromolecules and membrane structures, the synthesis of stress proteins, and a change in the ratios between cell components (Figure 5). After such a pre-adaptation of cells, the start of autolysis provides a more complete release of water-soluble components of yeast cells, both as a result of a violation of intermolecular interactions and an increase in the availability of enzymes to the substrate. (Figure 5). In the event that the cell suspension after autolysis was subjected to additional action of high temperature and excess pressure, the yield of water-soluble components increased significantly and, moreover, the composition of the obtained components changed (Figure 5).
This effect can be explained by the fact that under such conditions the action of a complex of factors is ensured. First of all, the destruction of specific intermolecular interactions and the loss of specific structures of macromolecules, the violation of some covalent interactions and the systemic change in physicochemical characteristics in the cell. The best known of which is the formation of free radicals (OH and H), which will contribute to the partial oxidation of proteins and nucleic acids [43]. It can be assumed that eventually these processes will lead to additional qualitative and quantitative changes in the water-soluble components of yeast cells (Figure 5).
It should be noted that this process of destruction of yeast cells is stochastic, which can explain the fact that many authors obtained a diverse composition of auto lysates.
However, the most significant and somewhat unexpected were the data on the ionic composition of the autolysates compared to the yeast biomass (Table 1). These results may be due to several reasons. As is known, in the process of adaptation and death of cells, significant changes in the ionic composition occur, of course, to the greatest extent and primarily in sodium, potassium, magnesium, and chlorine [44]. However, numerous data indicate that there are complex mutual influences between different ions in the cell, and a change in some ions leads to a change in other ions [45,46]. Along with this, changes in the ionic composition of autolysates can be associated with a difference in the ionic composition of the cell cytoplasm and cell walls [47] and the removal of yeast cell walls will lead to a relative change in the content of ions in the water-soluble fraction of cells. And, finally, certain ions can be methodically detected in the auto lysate.
Regardless of the contribution of one or another mechanism to the formation of the ionic composition of yeast autolysate, it can be argued that it lacks highly toxic lead and cadmium and contains the most important essential microelements (Zn, Fe, Mn) and ultramicroelement (Cu) in a well-balanced ratio, which makes such a product extremely promising as a functional food additive. The results of, he works allow us to propose a complex scheme for obtaining water-soluble yeast components, which consist of three successive stages: pre-adaptation; autolysis; autoclaving (Figure 5). We believe that a detailed study of the mechanisms of cell destruction requires additional research, which is important not only for the practical application of target products, but also for understanding the fundamental principles of yeast cell organization.
Conclusion
- Pre-adaptation of yeast to a relatively low temperature (6°C) for 96 hours contributes to the restructuring of the structural and functional states of cells, which was accompanied by an increase in the release of part of the yeast components into the medium.
- Consecutive: pre-adaptation; autolysis and autoclaving of the yeast suspension allows almost complete separation of the water-soluble components of the yeast cells from the cell walls.
- Optimal for obtaining water-soluble components of yeast cells is 96-hour pre-adaptation of cells at 6°C followed by autolysis for 96 hours and autoclaving for 10 minutes at 1.5atm.
- Sequential pre-adaptation autolysis and autoclaving allows not only to increase the yield of water-soluble yeast components, but also to obtain a product with a different qualitative composition of amino acids, peptides, proteins and nucleic acids, but also well balanced in essential microelements.
References
- Cakar ZP, Turanl?-Y?ld?z B, Alk?m C, Y?lmaz Ü (2012) Evolutionary engineering of Saccharomyces cerevisiae for improved industrially important properties. FEMS yeast research 12: 171-182.
- Cui B, Lin L, Wang B, Liu W, Sun C (2022) Therapeutic potential of Saccharomyces boulardii in liver diseases: from passive bystander to protective performer? Pharmacological Research 175: 106022.
- Schlabitz C, Lehn DN, de Souza CFV (2022) A review of Saccharomyces cerevisiae and the applications of its byproducts in dairy cattle feed: Trends in the use of residual brewer's yeast. Journal of Cleaner Production 332:
- Çelik E, Çal?k P (2012) Production of recombinant proteins by yeast cells. Biotechnology advances 30: 1108-1118.
- Baghban R, Farajnia S, Rajabibazl M, Ghasemi Y, Mafi A, et al. (2019) Yeast expression systems: Overview and recent advances. Molecular Biotechnology 61: 365-384.
- Jach ME, Serefko A, Ziaja M, Kieliszek M (2022) Yeast protein as an easily accessible food source. Metabolites 12: 63.
- Náhlík J, Hrn?i?ík P, Mareš J, Rychtera M, Kent CA (2017) Towards the design of an optimal strategy for the production of ergosterol from Saccharomyces cerevisiae Biotechnology progress 33: 838-848.
- Demirgül F, ?im?ek Ö, Bozkurt F, Dertli E, Sa?d?ç O (2021) Production and characterization of yeast extracts produced by Saccharomyces cerevisiae, Saccharomyces boulardii and Kluyveromyces marxianus. Preparative Biochemistry & Biotechnology 52: 657-667.
- Lazo?Velez MA, Serna?Saldívar SO, Rosales?Medina MF, Tinoco?Alvear M, Briones?García M (2018) Application of Saccharomyces cerevisiae var. boulardii in food processing: A review. Journal of Applied Microbiology 125: 943-951.
- Klis FM, Boorsma A, De Groot PW (2006) Cell wall construction in Saccharomyces cerevisiae. Yeast 23: 185-202.
- Mirzaeia M, Mirdamadi S, Ehsani MR, Aminlari M, Hoseini SE (2015) Characterization of yeast protein enzymatic hydrolysis and autolysis in Saccharomyces cerevisiae and Kluyveromyces marxianus. Journal of Food Biosciences and Technology 5: 19-30.
- Takalloo Z, Nikkhah M, Nemati R, Jalilian N, Sajedi RH (2020) Autolysis, plasmolysis and enzymatic hydrolysis of baker's yeast (Saccharomyces cerevisiae): A comparative study. World Journal of Microbiology and Biotechnology 36: 1-14.
- Orlean P (2012) Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 192: 775-818.
- Tanguler H, Erten H (2008) Utilisation of spent brewer's yeast for yeast extract production by autolysis: The effect of temperature. Food and bioproducts processing 86: 317-321.
- Calbo J, López?Moreno A, de Juan A, Comer J, Ortí E, et al. (2017) Understanding noncovalent interactions of small molecules with carbon nanotubes. Chemistry-A European Journal 23: 12909-12916.
- Dimopoulos G, Limnaios A, Aerakis E, Andreou V, Taoukis P (2021) Effect of high pressure on the proteolytic activity and autolysis of yeast Saccharomyces cerevisiae. Innovative Food Science & Emerging Technologies 74: 102865.
- Guyot S, Ferret E, Gervais P (2005) Responses of Saccharomyces cerevisiae to thermal stress. Biotechnology and bioengineering 92: 403-409.
- Yurchenko OI, Chernozhuk TV, Baklanov ?N, Kravchenko OA (2021) Analysis of water and bottom sediments of the tiger river (Iraq) using ultrasonic treatment, nonionic surface active substances and β-diketonates of metals as standard samples. Journal of Chemistry and Technologies 29: 173-178.
- Gilbertson DD, Kent M, Pyatt FB (1985) Data analysis and Interpretation I: Introduction and the Mann-Whitney U test. In Practical Ecology for Geography and Biology Springer, Boston, USA.
- Dougherty RC (1998) Temperature and pressure dependence of hydrogen bond strength: A perturbation molecular orbital approach. The Journal of chemical physics 109: 7372-7378.
- Hossain MA, Rana MM, Kimura Y, Roslan HA (2014) Changes in biochemical characteristics and activities of ripening associated enzymes in mango fruit during the storage at different temperatures. BioMed Research International 2014: 1-11.
- Eide DJ, Clark S, Nair TM, Gehl M, Gribskov M, et al. (2005) Characterization of the yeast ionome: A genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae. Genome biology 6: 1-13.
- Wu CY, Bird AJ, Chung LM, Newton MA, Winge DR, et al. (2008) Differential control of Zap1-regulated genes in response to zinc deficiency in Saccharomyces cerevisiae. BMC Genomics
- Ohnuki T, Sakamoto F, Yamasaki S, Kozai N, Shiotsu H, et al. (2015) Effect of minerals on accumulation of Cs by fungus Saccaromyces cerevisiae. Journal of Environmental Radioactivity 144: 127-133.
- Nandy, S. K., & Srivastava, R. K. (2018). A review on sustainable yeast biotechnological processes and applications. Microbiological research, 207, 83-90. https://doi.org/10.1016/j.micres.2017.11.013
- Shynkaryk, M. V., Lebovka, N. I., Lanoisellé, J. L., Nonus, M., Bedel-Clotour, C., & Vorobiev, E. (2009). Electrically-assisted extraction of bio-products using high pressure disruption of yeast cells (Saccharomyces cerevisiae). Journal of food engineering, 92(2), 189-195. https://doi.org/10.1016/j.jfoodeng.2008.10.0 41
- Wu T, Yu X, Hu A, Zhang L, Jin Y, et al. (2015) Ultrasonic disruption of yeast cells: Underlying mechanism and effects of processing parameters. Innovative Food Science & Emerging Technologies 28: 59-65.
- Liu D, Ding L, Sun J, Boussetta N, Vorobiev E (2016) Yeast cell disruption strategies for recovery of intracellular bio-active compounds-A review. Innovative food science & emerging technologies 36: 181-192.
- Thakkar A, Barbera E, Sforza E, Bertucco A, Davis R, et al. (2021) Flash hydrolysis of yeast (Saccharomyces cerevisiae) for protein recovery. The Journal of Supercritical Fluids 173: 105240.
- Forbes SL, Perrault KA, Comstock JL (2017). Microscopic post-mortem changes: The chemistry of decomposition. Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment 26-38.
- Babayan TL, Bezrukov MG (1985) Autolysis in yeasts. Acta Biotechnologica 5: 129-136.
- Zheng Z, Huang Q, Ling C (2019) Water-soluble yeast β?glucan fractions with different molecular weights: Extraction and separation by acidolysis assisted-size exclusion chromatography and their association with proliferative activity. International journal of biological macromolecules 123: 269-279.
- Giannelli L, Yamada H, Katsuda T, Yamaji H (2015) Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. Journal of Bioscience and Bioengineering 119: 345-350.
- Karemore A, Yuan Y, Porubsky W, Chance R (2020) Biomass and pigment production for Arthrospira platensis via semi?continuous cultivation in photobioreactors: Temperature effects. Biotechnology and Bioengineering 117: 3081-3093.
- Dawaliby R, Trubbia C, Delporte C, Noyon C, Ruysschaert JM, et al. (2015) Phosphatidylethanolamine is a key regulator of membrane fluidity in eukaryotic cells. Journal of Biological Chemistry 291: 3658-3667.
- Rozhko IS, Shtoiko RI (2022) Miracle plants in amateur gardening: expectations and realities. Forecasts and prospects of scientific discoveries in agricultural sciences and food 71-75.
- Anandakrishnan R, Drozdetski A, Walker RC, Onufriev AV (2015) Speed of conformational change: Comparing explicit and implicit solvent molecular dynamics simulations. Biophysical Journal 108: 1153-1164.
- Jerabek-Willemsen M, Wienken CJ, Braun D, Baaske P, Duhr S (2011) Molecular interaction studies using microscale thermophoresis. ASSAY and Drug Development Technologies 9: 342-353.
- Domínguez CM, Ramos D, Mendieta-Moreno JI, Fierro JL, Mendieta J, et al. (2017) Effect of water-DNA interactions on elastic properties of DNA self-assembled monolayers. Scientific reports 7: 1-8.
- Camiruaga A, Usabiaga I, Calabrese C, Lamas I, Basterretxea FJ, et al. (2021) Exploring the influence of intermolecular interactions in prebiotic chemistry using laser spectroscopy and calculations. Chemistry-A European Journal
- Ai M, Zhou Q, Guo S, Fan H, Cao Y, et al. (2020) Characteristics of intermolecular forces, physicochemical, textural and microstructural properties of preserved egg white with Ca(OH)2 addition. Food Chemistry 314: 126206.
- Baiz CR, B?asiak B, Bredenbeck J, Cho M, Choi JH, et al. (2020) Vibrational spectroscopic map, vibrational spectroscopy, and intermolecular interaction. Chemical Reviews 120: 7152-7218.
- Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, et al. (2017) Targeting free radicals in oxidative stress-related human diseases. Trends in pharmacological sciences 38: 592-607.
- D’Arcy MS (2019) Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biology International 43: 582-592.
- Wang J, Sun R, Li J (2016) Influence of K+, Na+ or Ca2+ ions on the interaction between AmB and saturated phospholipids by Langmuir technique. Chemical Research in Chinese Universities 32: 242-247.
- Breygina M, Klimenko E (2020) ROS and Ions in Cell Signaling during Sexual Plant Reproduction. International Journal of Molecular Sciences 21: 9476.
- Bakhos E, Skaff W, Esvan J, Monnier A, Sieczkowski N, et al. (2021) Use of FT?NIR and XPS techniques to distinguish cell hull fractions prepared by autolysis or HPH from Saccharomyces cerevisiae and Brettanomyces bruxellensis International Journal of Food Science Technology 56: 5062-5070.
Citation: Bozhkov A, Belous A, Bozhkov A, Ganin V, Ivanov E, et al. (2023) Pre-Adaptation of Saccharomyces Cerevisiae to Low Temperatures Affects the Resistance of Yeast Cells to Subsequent Autolysis, High Temperature and Overpressure. J Food Sci Nutr 9: 172.
Copyright: © 2023 Andrey Bozhkov, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

