
Production of Synthetical Microbial Inoculant for Low-Temperature Daqu Based on Their Core Functional Microflora
*Corresponding Author(s):
Kaizheng ZhangCollege Of Bioengineering, Sichuan University Of Science And Engineering, No. 180 Xueyuan Street, Huixing Road, Zigong City, Sichuan Province, 643000, China
Email:kai7766@126.com
Abstract
By summarizing the core functional microbial flora of low-temperature Daqu, 16 strains belonging to 13 genera were obtained for the preparation of low-temperature Daqu microbial inoculant. In the production of initial microbial inoculants, the core microorganisms were first activated and expanded, and the appropriate centrifugation and concentration conditions were determined by multiple groups of parallel experiments. Then, the single factor experiments with rice husk, bran, and corn straw powder as the carriers and the mixed carrier optimization experiments were conducted, respectively. The protective agents were screened by response surface methodology and artificial neural network combined with genetic algorithm. The results showed that the best centrifugation conditions were 5 min (time), 5000 r/min (speed); The best carrier is bran flour: corn straw flour = 1:1; The theoretical value and actual value of artificial neural combined with genetic algorithm are better than those of response surface method. The optimal ratio of protective agent obtained is: mannitol (3.7%), gelatin (4.0%), trehalose (4.0%), glutamic acid (4.4%). At this time, the actual freeze-drying survival rate is 89.16 %; The mature microbial inoculant meets the corresponding quality requirements, and the resulting Daqu has a good similarity with the traditional low-temperature Daqu in the flavor profile based on electronic nose, indicating that the preparation of synthetical microbial inoculant is successful.
Keywords
Artificial neural network; Carrier; Electronic nose; Low-temperature Daqu; Microbial inoculant; Protective agent
Introduction
Liquor (Chinese Baijiu) is made from sorghum or and other grains through fermentation, distillation and aging. Daqu is the starter for Liquor-making, which plays an important role and provides some materials, most strains and enzymes in this process [1]. Traditonal low-temperature Daqu primarily utilizes barley and peas as raw materials, which are pressed into blocks and subsequently fermented in an open environment to produce mature Daqu.
At present, it is very important for the quality and safety of fermented food to realize the transformation from natural fermentation to controlled fermentation by constructing core microbial groups. Some core microorganisms can drive the fermentation process, not only produce flavor compounds, but also affect the interaction between microorganisms, thus completing the food fermentation process [2,3]. At the same time, the reasonable combination and proportion of these microorganisms, that is, the construction of core microflora, is also of great significance for the controllable production of fermented food [4].
The production of traditional Daqu is easily influenced by environmental conditions, and its strains are complex and diverse, even many harmful strains, which are unfavorable to the quality of Daqu. The controllable production of low-temperature Daqu was gradually realized by preparing microbial inoculant with core function of low-temperature Daqu, which could improve the biological safety of Daqu and lay the foundation for clean production of Fen-flavor liquor. The preparation and quality of core microbial inoculant of Daqu is an important condition and guarantee for clean production of low-temperature Daqu.
In this study, the core microflora of microbial inoculant of low-temperature Daqu was first determined, then the protective agent formula was optimized by response surface method and artificial neural network combined with genetic algorithm, and the initial microbial inoculant was prepared, and its physical and chemical indexes were tested. Finally, the clean Daqu was prepared based on the mature microbial inoculant, and its flavor profile was detected by electronic nose, in order to provide some experimental and research basis for the clean production of low-temperature Daqu.
Materials And Methods
- Materials
The core functional microorganism of low-temperature Daqu was finally determined by consulting relevant literature and screening [5-10].
The bacteria, yeasts and molds used in this experiment are shown in Table 1, all of which are from glycerol preservation strains in our laboratory screened from Daqu or obtained from Southwest Station of China Industrial Microbiology Collection Center (Wenjiang, Sichuan, China).
|
Specific name |
Strain source |
Maximum similarity (%) |
|
Bacillus licheniformis |
Our laboratory |
99.38 |
|
Bacillus subilis subsp.inaquosorum |
Our laboratory |
99.26 |
|
Bacillus amyloliquefaciens |
Our laboratory |
99.45 |
|
Acetobacter cerevisiae LMG 1625(T) |
outsourcing |
99.72 |
|
Lactobacillus pentosus JCM 1558(T) |
Our laboratory |
99.74 |
|
Weissella cibaria KACC 11862(T) |
outsourcing |
99.37 |
|
Leuconostoc pseudomesenteroides |
Our laboratory |
99.64 |
|
Candida glabrata ATCC 90030 (T) |
Our laboratory |
99.91 |
|
Saccharomyces cerevisiae ATCC 9080 (T) |
Our laboratory |
99.45 |
|
Saccharomycopsis fibuligera strain NRRL Y-2388 |
Our laboratory |
99.52 |
|
Thermoascus aurantiacus |
Our laboratory |
99.54 |
|
Pichia kudriavzevii |
Our laboratory |
99.86 |
|
Monascus anka Nakazawa et Sato |
Our laboratory |
99.48 |
|
Aspergillus oryzae |
Our laboratory |
99.78 |
|
Thermomyces lanuginosus |
Our laboratory |
99.85 |
|
Rhizopus oryzae |
Our laboratory |
99.97 |
Table 1: Strains used in the experiment.
Bran, rice husk and corn stalk (all 50 meshes), purchased from local market of Yibin, Sichuan province of China. Barley and peas, purchased in the local agricultural market of Yibin, Sichuan province of China; Traditional low-temperature Daqu, purchased from a Fen-flavor liquor winery in Shanxi province of China.
Vacuum freeze dryer, Beijing Songyuan Huaxing Technology Development Co., Ltd., Electronic nose (INose, equipped with 14 sensors), Shanghai Ruijun International Trade Co., Ltd.
- Methods
Study on centrifugal conditions
The activated and expanded strains were used to study the centrifugal conditions. The centrifugal speed was set at 4000, 5000 and 6000 r/min, and the centrifugal time was set at 3, 5 and 10 min. The centrifugal survival rate was obtained by counting the viable bacteria before and after centrifugation, and the optimal centrifugal concentration process conditions were obtained.
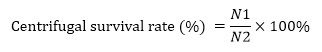 Where: N1, the number of viable bacteria after centrifugation, CFU/mL; N2, the number of viable bacteria before centrifugation, CFU/mL.
Where: N1, the number of viable bacteria after centrifugation, CFU/mL; N2, the number of viable bacteria before centrifugation, CFU/mL.
Carrier performance measurement and single factor experiment
The water absorption and pH value of the crushed and sterilized carrier were measured under aseptic conditions, and the single factor experiment as the immobilization effect of the carrier was carried out. By counting the live bacteria before and after the carrier adsorption, the survival rate of single carrier was calculated, and the data were measured for 5 days, and the maximum value was taken.
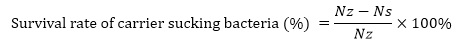 Where: Nz, the total number of viable bacteria before carrier adsorption, CFU/mL; Ns, the total viable count of supernatant after carrier adsorption, CFU/mL.
Where: Nz, the total number of viable bacteria before carrier adsorption, CFU/mL; Ns, the total viable count of supernatant after carrier adsorption, CFU/mL.
Carrier mixing experiment
According to the results of carrier performance measurement and single factor test, the suitability of carrier is analyzed and the mixing ratio of carrier is set. By counting the live microbes before and after carrier adsorption, the survival rate of single carrier was calculated, and the 5-day data were measured, and compared with the survival rate of single carrier, so as to determine the final carrier ratio of microbial inoculant.
Single factor test of microbial inoculant protective agent
There are many protective agents, and the principle and mode of action of each type of protective agent are different. This study was carried out according to the classification of chemical properties, and the best effect in each category was selected by single factor experiment for subsequent compound research, with concentration gradients of 2.8%, 3.9% and 5%. After the bacterial suspension is mixed with the carrier, it is mixed with the protective agent according to the set volume fraction in a sterile beaker, then mixed evenly with a glass rod, and then transferred to a Petri dish with a sterile medicine spoon. After being sealed with plastic wrap, it is immediately placed in the refrigerator at -20°C for pre-freezing. Pre-freezing for 10 h, then vacuum freeze-drying for about 10 h. After vacuum freeze-drying, take out the Petri dish, weigh the total mass, and take out the same mass of freeze-dried powder for dissolution and gradient dilution coating counting.
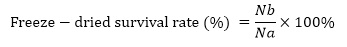 Where: Nb, the viable bacteria quantity before freeze-drying, CFU/mL; Na, viable bacteria after freeze-drying, CFU/mL.
Where: Nb, the viable bacteria quantity before freeze-drying, CFU/mL; Na, viable bacteria after freeze-drying, CFU/mL.
Establishment of RBF artificial neural network model
Artificial neural network technology has its unique features in solving nonlinear problems [11]. The newrbe function in MATLAB R2021a is called to construct an accurate radial basis network. Call format is:
net = newrbe
net = newrbe (P,T, spread)
Where: R×Q-dimensional matrix composed of P and Q input vectors; S×Q dimensional matrix composed of t and q groups of target classification vectors; Spread, the distribution density of radial basis function; Net, return value: a radial basis network.
Setting the dosage of mannitol, gelatin, trehalose and glutamic acid as the input layer and the survival rate as the output layer, 25 test results in the Box-Behnken experimental combination were selected to form the training set of the model, and the remaining four were used as test set samples to evaluate the performance of the network. The factors and levels of Box-Behnken experimental design are shown in Table 2.
|
factor |
-1 |
level 0 |
1 |
|
A mannitol addition |
2.8 |
3.9 |
5.0 |
|
B gelatin addition |
2.8 |
3.9 |
5.0 |
|
C trehalose addition |
2.8 |
3.9 |
5.0 |
|
D glutamic acid addition |
1.67 |
2.8 |
3.9 |
Table 2: The factors and levels of Box-Behnken test.
Genetic algorithm optimization design
The genetic algorithm toolbox of MATLAB is used to optimize the genetic algorithm. An experiment is regarded as an individual, the mapping relationship between input and output is regarded as the fitness function of the genetic algorithm, and the prediction result of the trained neural network is used as the fitness function value of the genetic algorithm, so that the optimal solution and corresponding parameters (mannitol, gelatin, trehalose and glutamic acid) are obtained. Parameters related to GA are set as follows: number of iterations gen max = 50, population size = 100, crossover probability cross = 0.6, mutation probability mutation = 0.015, and individual length = 8.
Preparation of mature microbial inoculant and its physical and chemical indexes
The initial microbial inoculant (freeze-dried powder) was inoculated on the crushed bran culture medium (inoculating according to the ratio of w/w 1:100), then put into a conical flask and cultured at a suitable temperature for about 3 days. During the period, the bottle is shaken every other day to keep its structure loose and facilitate the propagation and growth of microorganisms. After 3 d, it was dried in an oven at 50°C, and vacuum packed when the moisture content was lower than 10 %. The physical and chemical indexes of mature microbial inoculant were tested according to Agricultural Microbial Agents (GB 20287-2006).
- Electronic nose experiment
With reference to the traditional Daqu process, clean low-temperature Daqu was prepared with mature microbial inoculant as the starting flora. The difference from the traditional process was that barley was sterilized with food-grade chlorine dioxide in advance, and after being crushed, 5% mature microbial inoculant was added (the three mature microbial inoculants were evenly mixed according to the ratio of bacteria: mold: yeast = 4:5:1) to make koji embryo, and then it was covered with sterilized cotton cloth in a clean incubator for closed fermentation culture. Experimental method of electronic nose: Weigh 10 g of Daqu powder into a headspace bottle, add 25 mL of saturated sodium chloride solution, balance in a water bath at 60°C for 45 min, and then start detection. Firstly, the sensor was cleaned for 120 s, the sampling time was 60 s, the gas flow rate was 0.3 L/min, the sample preparation time was 10 s, and the operating environment was room temperature.
- Data statistical analysis
SPSS 18.0 was used to analyze the significance of single factor experiment. Design-Expert 8.0.6 design software is used for the design of Box-Behnken experiment and the establishment and data analysis of response surface analysis data set; Matlab R2021a software is used to construct BP-GA neural network, Origin 2021 is used for drawing, and three parallel experiments are set in each group.
Results
- Single-factor experiment
Centrifugation of bacterial liquid can not only ensure the high concentration of bacteria, but also avoid the toxicity of metabolites such as lactate to bacteria [12]. In the centrifugal process, firstly, it is necessary to improve the damage caused by centrifugal force and reduce cell death, and secondly, it is necessary to minimize the residual cells in the supernatant [13]. As can be seen from Figure 1A, the survival rate has been significantly affected under different centrifugal speed and time conditions. When the centrifugation condition was 5 min and 5000 r/min, the survival rate reached the maximum, reaching 88.2%. With the increase of centrifugation time or rotation speed, the survival rate shows a similar trend of increasing first and then decreasing. There are two reasons for this result: on the one hand, when the rotation speed is too low, the centrifugal force is small, the concentration degree is insufficient, and there are more residual bacteria in the supernatant, which leads to the low survival rate, while when the rotation speed is too high, the centrifugal force is enhanced, and the pressure and shear force on the cells are also enhanced, which leads to the cell death more easily; On the other hand, if the centrifugation time is insufficient, it will be difficult to achieve the best separation effect, and if the centrifugation time exceeds a certain limit, the number of bacterial deaths will definitely increase, which will eventually lead to a decrease in survival rate (Figure 1).
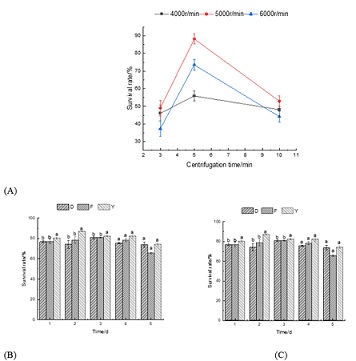 Figure 1: Single factor test results. (A: The survival rate of microbes under different centrifugation times and rotation speeds B: The survival rate of microbes on single carrier C: The survival rate of microbes on mixed carriers).
Figure 1: Single factor test results. (A: The survival rate of microbes under different centrifugation times and rotation speeds B: The survival rate of microbes on single carrier C: The survival rate of microbes on mixed carriers).
Note: D: Rice husk powder; F: Bran powder Y: Corn stalk powder
- Determination of carrier performance
The carrier can immobilize the microbes and prolong the shelf life of microbial inoculant. Solid-state microbial inoculant can better maintain the quantitative advantage of microorganisms in the reaction system, and enhance their tolerance to toxic and harmful substances, thus improving the operation performance of the biological treatment system, and it is more conducive to the preservation and transportation of microbial inoculant because of its small volume and high concentration [14]. Rice husk powder, bran powder and corn stalk powder are selected as carriers. The water absorption rates of the three are 208.95 %, 269.47% and 460.09 % respectively. There is little difference in pH among them, which are 7.20, 6.33 and 6.03, respectively, and they are all pH values at which microbial inoculant can grow normally.
- Single factor test of carrier
Natural organic carriers have the advantages of simple curing operation, easy decomposition by microorganisms and high immobilization density [15]. As can be seen from Figure 1B, the maximum survival rate of microbe of the three carriers and the time to reach the maximum are slightly different. Rice husk powder reached the maximum at the 3rd day: 80.46 %; On the 3rd day, bran powder was 80.75%; On the 2nd day, corn stalk powder was 86.87 %. It is speculated that the reason may be related to the nature of the carrier itself: because of the low density of corn stalk powder and the relatively large surface area under the same quality, the water absorption rate is high, and the water absorption rate is fast, reaching the peak of immobilization at the earliest, and the immobilization effect is also the best.
- Carrier mixing test
Generally speaking, the use of composite carriers is helpful to improve the properties of immobilized particles, so that its comprehensive effect is better than single carrier [16]. Based on the results of single factor experiment, the survival rate of bacteria after mixing the three carriers in the ratio of 1:1 and 1:1:1 is higher than that of single carrier (Figure 1C), and the best effect is F: Y=1:1, at which the survival rate reaches 90.47 %.
- Single factor test of protective agent
Protective agents can reduce the damage of freezing and drying to microbe, protect their physiological activity, and thus improve the survival rate of freeze-dried bacteria [17]. As can be seen from Table 3, all kinds of protective agents have played their roles, with different effects. Among each kind of protectants, the best effects were trehalose 5%, glutamic acid 5%, mannitol 3.9% and gelatin 2.8%, and the freeze-dried survival rates reached 44.24%, 44.70%, 28.22% and 33.83% respectively. Therefore, these four protectants were determined to be used in the compound optimization experiment.
|
Kind |
Agent |
Survival rate/% |
|
Control group
|
__ |
7.51±2.40 |
|
Carbohydrate |
sucrose trehalose lactose |
21.19±3.34 44.24±4.38 32.77±2.47 |
|
Amino acids |
glutamic acid proline |
44.70±3.43 39.01±4.78 |
|
Sugar alcohol |
mannitol |
28.22±3.60 |
|
Complex |
Skim milk gelatin |
24.36±1.73 33.83±3.72 |
Table 3: Single factor test results of protective agent.
- RBF artificial neural network construction
The training and test data sets of the neural network model are shown in Table 4, and the training and prediction effects of the model are shown in Figure 2. The results show that after the training set is trained, R2 reaches 0.99857, which shows that the neural network model has a high degree of fitting, and the predicted survival rate and the expected survival rate are well fitted (Figure 2A). The trained network is used to predict the samples of the test set, and then compared with the experimental values. The results are shown in Figure 2B. It can be seen that the trends are predicted well except for the fourth experiment, which proves that the neural network model has high accuracy and can be used for subsequent simulation.
|
Test number |
A mannitol addition |
B gelatin addition |
C trehalose addition |
D glutamic acid addition |
Freeze-dried survival rate measured value/% |
|
1 |
0 |
0 |
0 |
0 |
88.23±4.31 |
|
2 |
0 |
-1 |
0 |
1 |
85.89±3.48 |
|
3 |
1 |
-1 |
0 |
0 |
73.69±2.67 |
|
4 |
0 |
0 |
0 |
0 |
88.17±5.32 |
|
5 |
0 |
0 |
1 |
1 |
83.67±4.62 |
|
6 |
1 |
1 |
0 |
0 |
80.78±4.91 |
|
7 |
-1 |
0 |
0 |
-1 |
68.45±2.69 |
|
8 |
1 |
0 |
1 |
0 |
67.74±3.67 |
|
9 |
0 |
1 |
-1 |
0 |
78.84±4.81 |
|
10 |
0 |
0 |
-1 |
-1 |
67.18±5.64 |
|
11 |
0 |
-1 |
0 |
-1 |
70.94±2.75 |
|
12 |
1 |
0 |
0 |
-1 |
71.12±3.95 |
|
13 |
0 |
-1 |
-1 |
0 |
74.03±2.76 |
|
14 |
1 |
0 |
-1 |
0 |
75.56±2.16 |
|
15 |
-1 |
0 |
-1 |
0 |
73.94±5.81 |
|
16 |
0 |
1 |
0 |
-1 |
72.67±3.67 |
|
17 |
0 |
0 |
0 |
0 |
86.39±4.28 |
|
18 |
-1 |
-1 |
0 |
0 |
82.31±3.91 |
|
19 |
0 |
0 |
1 |
-1 |
62.56±2.57 |
|
20 |
-1 |
0 |
0 |
1 |
86.45±3.96 |
|
21 |
-1 |
0 |
1 |
0 |
80.78±4.25 |
|
22 |
0 |
0 |
0 |
0 |
87.56±3.16 |
|
23 |
0 |
0 |
-1 |
1 |
79.49±4.82 |
|
24 |
1 |
0 |
0 |
1 |
81.43±3.95 |
|
25 |
0 |
1 |
1 |
0 |
76.27±3.14 |
|
26 |
0 |
1 |
0 |
1 |
84.71±2.85 |
|
27 |
0 |
0 |
0 |
0 |
86.34±1.49 |
|
28 |
0 |
-1 |
1 |
0 |
75.15±2.20 |
|
29 |
-1 |
1 |
0 |
0 |
80.67±2.95 |
Table 4: RBF artificial neural network model training and test sample data set.
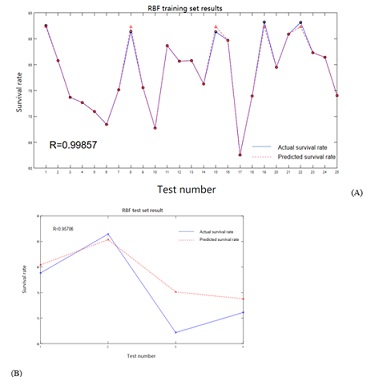 Figure 2: Comparison results of actual and predicted values between training set and test set of artificial neural network model. (A: RBF training set results B: RBF test set result).
Figure 2: Comparison results of actual and predicted values between training set and test set of artificial neural network model. (A: RBF training set results B: RBF test set result).
- Comparison of results between genetic algorithm and response surface method
The genetic optimization process is shown in Figure 3, and the ordinate is the optimal survival rate of each generation. After the iterative operation of crossover-selection-mutation-crossover-selection, the platform time of individual fitness is gradually extended, which shows that the adaptability of individual (experimental operation) is enhanced with the decrease of fitness function. Finally, when the evolutionary algebra is about 40 generations, the fitness curve tends to be stable, and the optimal solution is 91.38%. The corresponding parameter values are mannitol: 3.7%, gelatin: 4.0%, trehalose: 4.0% and glutamic acid: 4.4%. After verification, the actual freeze-drying survival rate was 89.16 %.
The optimum parameters obtained by response surface method were mannitol: 3.2%, gelatin: 3.6%, trehalose: 3.9%, glutamic acid: 3.1%, and the predicted freeze-drying survival rate was 88.45%, which was actually 85.41%. By comparison, it can be seen that the artificial neural network optimization algorithm based on genetic algorithm is better than the response surface method, no matter the predicted value or the actual value.
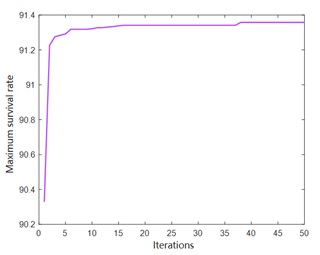 Figure 3: Optimization result of genetic algorithm.
Figure 3: Optimization result of genetic algorithm.
- Physical and chemical indexes of mature microbial inoculant
The microbial inoculant prepared in this study is pure strain. Considering the comprehensive process, it is necessary to detect four indicators: effective viable bacteria number, moisture, pH and shelf life. The results showthat the effective viable count of the three mature microbial inoculants is far higher than the standard of agricultural microbial agents (Table 5). The pH is within the allowable range, belonging to a slightly acidic environment; The moisture content after drying reaches the standard. After drying, vacuum packaging, low-temperature refrigeration and other operations, combined with the "Technical Specification for Quality Assurance of Agricultural Microbial Agents (T/GTM006-2022)"; The dosage form is powder or granule. When the moisture content is in the range of 10%-15%, the shelf life should be 12 months. When the moisture content is in the range of 5%-10%, the shelf life should be 24 months. The stability of the microbial inoculant has been guaranteed to some extent, and the shelf life of the microbial inoculant will be further tested in the future.
|
kind |
Effective viable bacteria/100 million /g |
PH/ acidity /mmol/10g |
Water content/%(after drying) |
|
Bacterial inoculant |
64.24±5.51 |
6.23±0.01 |
4.35±0.24 |
|
Yeast inoculant |
20.35±3.57 |
6.05±0.02 |
2.86±0.37 |
|
Mould inoculant |
47.69±3.86 |
6.56±0.01 |
2.64±0.21 |
|
Agricultural Microbial Agents |
>2 |
5.5-8.5 |
<20 |
Table 5: Physical and chemical indexes of mature microbial inoculant.
- Electronic nose verification experiment
The flavor profiles of clean low-temperature Daqu (XQ1: stored for 1 month, XQ2: stored for 3 months) prepared with mature microbial inoculants and traditional low-temperature Daqu (CQ) were detected and analyzed by electronic nose. The results are shown in Table 6 and the flavor radar map is shown in Figure 4. It can be seen that the flavor profiles of the three kinds of Daqu are similar, and the response values of most aroma types are not different between traditional Daqu and clean Daqu (P >0.05), only the response values of S1(Ammonia, amines) and S13(Sterol, triterpenes) and S14(Lactone, pyrazine) are different (P<0.05). Among them, Hydrogen is a colorless and odorless gas and a clean energy source, while pyrazines have baking and nut flavors and polar flavor thresholds, which can also provide liquor with a special flavor and some medical effects, including dilating blood vessels, inhibiting platelet adhesion and thrombosis, and other beneficial pharmacological effects [18].
|
Sensor number |
Corresponding sensitive volatile gas |
response value |
|
|
|
CQ |
XQ1 |
XQ2 |
||
|
S1 |
Ammonia, amines |
7.3879±0.7614a |
5.9934±0.3428b |
6.0088±0.07801b |
|
S2 |
Hydrogen sulfide, sulfur |
8.0297±0.6952a |
6.9388±0.6852ab |
6.797±0.1292b |
|
S3 |
Hydrogen |
1.2±0.0571a |
1.2392±0.0073a |
1.2386±0.0475a |
|
S4 |
Alcohol, organic solvents |
10.4127±0.3180a |
9.1353±0.9774b |
9.8504±0.0231ab |
|
S5 |
Volatile gases during food cooking |
6.3825±0.4275a |
5.4831±0.4664b |
5.6651±0.0250ab |
|
S6 |
Methane, biogas, hydrocarbons |
1.8792±0.1701a |
1.7738±0.0614a |
1.7743±0.0045a |
|
S7 |
Flammable gas |
3.2501±0.3817a |
2.8264±0.1092a |
3.0874±0.0396a |
|
S8 |
VOC |
5.3662±0.7008a |
4.6419±0.1924a |
4.8878±0.0285a |
|
S9 |
Oxy-hydroxide, gasoline, kerosene |
1.8745±0.1601a |
1.7369±0.0591a |
1.727±0.0024a |
|
S10 |
Alkanes, flammable gas |
2.976±0.1978a |
2.7267±0.0681a |
2.785±0.1615a |
|
S11 |
Aromatic compound |
3.23±0.3682a |
2.8457±0.1537a |
3.1969±0.4011a |
|
S12 |
Sulphide |
4.5275±0.8169a |
4.3516±0.1503a |
4.6774±0.2789a |
|
S13 |
Sterol, triterpenes |
5.1285±0.3660a |
4.3455±0.1915b |
4.5797±0.0382b |
|
S14 |
Lactone, pyrazine |
1.3218±0.0725b |
1.5091±0.0664a |
1.4798±0.0937a |
Table 6: The results of e-nose analysis for XQ and CQ.
Note: Mean values with different lower-case letters in the same row indicate significant differences (p < 0.05)
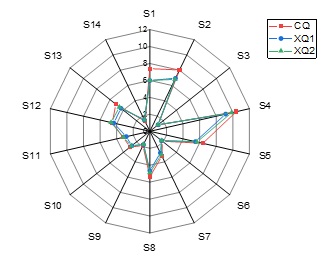 Figure 4: The radar map of e-nose analysis for XQ and CQ.
Figure 4: The radar map of e-nose analysis for XQ and CQ.
Note: CQ: Traditional low-temperature Daqu; XQ: Clean low-temperature Daqu
Discussion
Core microflora is a very important part in the process of liquor fermentation, and many microorganisms work together to produce fermented food with unique aroma and taste. Searching for core microorganisms is helpful to artificially construct a synthetic microbial community and obtain a culturable, repeatable and easy-to-operate fermentation system [19-21].
At present, it is the main way to improve the quality of fermented food by adding some specific endogenous microorganisms [22]. However, at present, enterprises generally adopt the method of gradually expanding the cultivation of strains and then adding them, which may lead to the degradation and pollution of strains, and the application of microbial agents can effectively avoid the above problems [23].
The concentration and collection of bacteria is an important link in the preparation of microbial inoculant. Centrifugal concentration is the most widely used method, and centrifugal conditions have great influence on the final centrifugal concentration effect [24].
Microbial immobilization technology can attach microorganisms to carriers. Compared with traditional technology, it has the advantages of high biological concentration, simple solid-liquid separation, strong stress resistance, good stability and strong mechanical properties [25]. The natural organic carrier has the advantages of innocuity and good mass transfer performance. For the sake of food safety, this study selected three natural organic carriers for single factor and compound experiments.
There are many reasons for the damage to cells during vacuum freeze-drying, such as the mechanical force caused by the growth of ice crystals during pre-freezing, the damage caused by the change of electrolyte content during freezing, and the damage caused by dehydration during drying [26,27]. They can damage cells by changing the structure and function of cell membranes, or by denaturing and inactivating various active protein in bacterial cells, or even by damaging bacterial genetic material [28-30]. Different types of protective agents have different working principles and modes of action. For example, trehalose has a relatively higher glass transition temperature and is less reducible, which can increase the free energy of protein, thus inhibiting the degradation of protein. In the drying process, sugar will replace the hydrogen bond between protein and water molecules, thus stabilizing protein [31]; Mannitol is often used as cryoprotectant for freeze-dried drugs because of its low temperature protection, easy molding and high melting temperature of eutectic [32]. Amino acids are often used as freeze-dried fillers, which can prevent the effective components from escaping with the sublimation of water vapor, in addition, they also have the function of adjusting pH and preventing the buffer salt in the drug from crystallizing due to the change of pH value, thus keeping the protein stable [33]. For this reason, in actual production, a variety of protective agents are generally used in a proper proportion to achieve better protection effect.
Response surface method and artificial neural network are two commonly used compound optimization methods. Response surface method uses multivariate quadratic regression equation to fit the functional relationship between factors and response values, and obtains the optimal process parameters through the analysis of regression equation. However, response surface method mainly fits multivariate quadratic nonlinear model, which has great limitations for other models. Artificial neural network technology has its uniqueness in solving nonlinear problems. In the 1990s, some scholars combined neural network with improved genetic algorithm [34-37]. Now, some scholars have used this tool to achieve good results in process optimization research [38-44]. This is mainly because neural network has strong advantages in learning and automatic pattern recognition, but its global search ability is weak, while genetic algorithm has advantages in solving complex global optimal problems, and its overall optimization is remarkable. If the neural network optimized by genetic algorithm is used to deal with complex nonlinear problems, we can learn from each other's strengths and complement each other's strengths [45].
Electronic nose is a device that uses electronic sensors to identify food aroma. It can directly identify volatile aroma components without separating and identifying specific volatile compounds. It has the advantages of simple operation, rapid detection and no special sample pretreatment [46], and it is a powerful tool for analyzing and comparing the flavor profiles of food and drugs.
Conclusion
In this study, a variety of methods were used, including single factor experiment, compound research, and response surface method coupled with artificial neural network genetic algorithm (BP-GA). From centrifugation conditions to carrier selection to protectant selection, several groups of parallel experiments were carried out, and the complete preparation process of low-temperature Daqu starter was optimized many times. Finally, the best centrifugation conditions were determined as time 5 min, rotation speed 5000 r/min, and the survival rate was 88.2% The best carrier is bran powder: corn stalk powder 1:1, and the survival rate of microbe is 90.47%. The best ratio of protective agent is: mannitol (3.7%), gelatin (4.0%), trehalose (4.0%), glutamic acid (4.4%), and the survival rate is 89.16%. The prepared mature microbial inoculant meets the corresponding quality requirements, and the resulting clean Daqu has good similarity with the traditional low-temperature Daqu in electronic nose inspection results. This study shows that the microbial inoculant of low-temperature Daqu can be prepared based on artificially synthesizing microbial flora, which provides an experimental basis for the clean production of Fen-flavor Daqu.
Acknowledgement
This study was supported by the Key R&D Programme of the Sichuan Province of China (2023YFS0484)
References
- Fu JQ (1983) Classification and application of Chinese distiller's yeast. China brewing 7-10.
- Rolle RS, Holzapfel WH (2002) Small-scale fermentations in developing countries. Journal of the Science of Food & Agriculture 75.
- Benjamin E, Wolfe R (2015) Fermented Foods as Experimentally Tractable Microbial Ecosystems. Cell: 49-55.
- Giraffa G (2004) Studying the dynamics of microbial populations during food fermentation. FEMS microbiology reviews 28.
- Wang ZM, Lu ZM, Shi JS, Xu ZH (2016) Exploring flavour-producing core microbiota in multispecies solid-state fermentation of traditional Chinese vinegar. Scientific reports 6.
- Yh A, Xh A, Bo YB (2021) Contrasting the microbial community and metabolic profile of three types of light-flavor Daqu 13: 73-76.
- Li JY (2017) Biochemical characterisation and dominance of different hydrolases in different types of Daqu - a Chinese industrial fermentation starter. Journal of the Science of Food & Agriculture 2.
- Wang CH (2014) Study on microbial community structure of different flavor Daqu based on clone library method. Sichuan Institute of Technology 15: 58-60.
- Du AM, Li L, Li J (2021) Research and application of core functional microorganisms in light flavor Baijiu brewing. Brewing in China 3: 26-28.
- Zhou S, Hu JY, Cui Y (2019) Analysis of microbial diversity of Qingxiang Daqu by high-throughput sequencing technology. Chinese Journal of Food 19: 7.
- Huang L (2010) Research on the Improvement and Application of BP Neural Network Algorithm. Chongqing Normal University 12: 16-17.
- Ren XY, He ZG, Li WX (2017) Effect of the preparation process of direct throw starter on the survival rate of lactic acid bacteria. Journal of Food Science and Technology 35: 6.
- Lei XY (2013) Study on the preparation process of lactic acid bacteria active dry bacterial agent. Southwest University 18: 14-15.
- Santos VL, Heilbuth NM, Linardi VR (2010) Degradation of phenol by Trichosporon sp. LE3 cells immobilized in alginate. Journal of Basic Microbiology 41: 171.
- Li HL, Chen LH, Xiao CH (2020) Research progress of carrier materials for microorganism immobilization. Modern Chemical Industry 40: 5.
- Peng CY, Liu TX, Gao YH (2021) The latest research progress of carrier materials for microbial immobilization. Modern chemical industry 12: 3.
- Zhao ZH, Yue TL, Wang YN (2021) The biological mechanism of trehalose and its application in active dry yeast (AADY). Food Research and Development 27: 5.
- Qun W, Yan X (2012) Transcriptome profiling of heat-resistant strain Bacillus licheniformis CGMCC3962 producing Maotai flavor. Journal of agricultural and food chemistry 60.
- Rolle RS, Holzapfel WH (2002) Small-scale fermentations in developing countries 24: 5.
- Zmwa E, Zmla B, Yjy D (2015) Batch-to-batch uniformity of bacterial community succession and flavor formation in the fermentation of Zhenjiang aromatic vinegar. Food Microbiology 50: 64-69.
- Peng MY, Lu ZM, Zhang XJ (2021) Distinct cooccurrence patterns and driving forces of abundant and rare bacterial communities in the multispecies solidstate fermentation process of cereal vinega. Systems Micro Bio (002-002).
- Wang P, Wu Q, Xu Y (2018) Core microbiota in the fermentation process of Chinese Baijiu and its relationship with environmental factors. J of Micro 58: 12.
- Owusu-Kwarteng J, Tano-Debrah K, Akabanda F, Jespersen L (2018) Technological properties and probiotic potential of Lactobacillus fermentum strains isolated from West African fermented millet dough. BMC microbiology 15: 1.
- Tan H (2007) Exploration of conditions for efficient growth of lactic acid bacteria and development of direct throw starter. Hunan Agricultural University 46: 7.
- Wan Q, Zhao ZX, Ju K, Zhang XY, Lei R, et al. (2019) Research and application of immobilized nitrifying bacteria technology. Water Treatment Technology 45: 24-29.
- Morgan CA, Herman N, White PA (2006) Preservation of micro- organisms by drying: Areview. J Microbiol Methods 66: 183-193.
- Van de Guchte M, Serror P, Chervaux C (2002) Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82: 187-216.
- Wang B, Tian FW, Li JR, Chen W, Zhang H (2009) Effect of freeze-drying on the permeability of lactic acid bacteria cell membrane. Micro Bulletin 36: 684-688.
- Martos GI, Minahk CJ, De V, Morero R (2007) Effects of protective agents on membrane fluidity of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. Letters in applied microbiology 45: 3.
- Santivarangkna C, Wenning M, Foerst P, Kulozik U (2007) Damage of cell envelope of Lactobacillus helveticus during vacuum drying. J of appl micro 102: 3.
- Jones LS, Randolph TW, Kohnert U (2001) The effects of Tween 20 and sucrose on the stability of anti-L-selectin during lyophilization and reconstitution. J Pharm Sci 90: 1466-1477.
- Tian Y, Wu MY (2018) Research progress of freeze-drying protection methods for biological products. China Medical Biotechnology 13: 73-76.
- Kim AI, Akers MJ, Nail SL (1998) The physical state of mannitol after freeze-drying: effects of mannitol concentration, freezing rate, and a noncrystallizing cosolute. J Pharm Sci 87: 931-935.
- M D (1992) A practical investigation of parallel genetic algorithms and their application to the structuring of artificial neural networks. University of Buckingham 23: 6.
- Chen ZJ (2002) Optimal Design of Feedforward Neural Network Based on Improved Genetic Algorithm. Computer Engineering 4: 120-129.
- Palmes PP, Hayasaka T (2005) Mutation-based genetic neural network. IEEE Trans on Neural Networks 16: 3.
- Chen JH, Shi HM, Chen RY (1992) Genetic algorithm for training of artificial neural networks. J of Huazhong University of Sci and Tech 1: 215-222.
- Pan HB, Qin LQ, Huang YT, Liang XL, Huang GH, et al. (2021) Artificial neural network combined with genetic algorithm to optimize the protectant to improve the frost resistance of Lactobacillus royi. Food Sci 42: 70-77.
- Zhang XY, Li RD, Mo MG, Mei LH, Liu YP, et al. (2022) Optimization of Lactobacillus casei LTL1361 lyophilized protectant formula based on artificial neural network coupled genetic algorithm (BP-GA) [J/OL]. Food Ind Sci and Tech 1-16.
- Wei ZS, Zhan P, Tian HL, Ma XP, Wang P, et al. (2019) Directed preparation of chickpea maillard peptide based on GA-BP neural network. Chinese Journal of Food 19: 147-153.
- Fang BS, Chen HW, Xie XL, Wan N, Mei YX, et al. (2000) Optimization of xylitol fermentation medium based on neural network and genetic algorithm. Journal of Biological Engineering 648-650.
- Luo JF, Lin W (2009) Fermentation Economics Based on Neural Network and Genetic Algorithm Medium Optimization. Journal of Food and Biotechnology 28: 424-428.
- Zhang SC, Zeng XY, Dong YS, Hu B, Hu C (2014) Optimization of bacterial cellulose culture parameters by neural network and genetic algorithm. Journal of Sichuan University (Natural Science Edition) 51: 371-377.
- Zhao WQ, Yin YG, Qiu A (2007) Research on optimization of preparation parameters of salidroside sustained-release microcapsules by genetic algorithm. Food Science 70-72.
- Nie Q (2012) On the Combination of Genetic Algorithm and Artificial Neural Network. Textile Industry and Technology 41: 35-37.
- Karakaya D, Ulucan O, Turkan M (2018) Electronic Nose and Its Applications: A Survey. International Journal of Automation and Computing 17: 179-209.
Citation: Liu Q, Tang H, Huang H, Zhang J, Hou Y, et al. (2023) Production of Synthetical Microbial Inoculant for Low-Temperature Daqu Based on Their Core Functional Microflora. J Food Sci Nutr 9: 169.
Copyright: © 2023 Qingsong Liu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

