
Review on Synthesis and Applications of Silver Nanoparticles, their Characterization and Toxicity Effects: Future Outlook
*Corresponding Author(s):
Srinivasulu Reddy MotireddyDepartment Of Zoology, Faculty Of Natural Sciences, Sri Venkateswara University, Tirupati, Andhra Pradesh, India
Email:pvr9490641036@gmail.com / profmsrsvu@gmail.com
Abstract
Silver Nanoparticles (AgNPs) have been targeted by researchers because of their unique properties. Recent advances in Nanoscience have revolutionized the prevention, diagnosis and treatment of many diseases. Metal NPs, especially AgNPs, are widely used in biological sciences. More importantly, AgNPs should not only be large NPs, but the synthesis of NPs should be easy and cost-effective. Chemical and physical methods are the main methods for the synthesis of AgNPs, but they are very expensive and absorb toxic substances. In this review, we focus on how to use cost-effective and potentially more selective plants for the biosynthesis of AgNPs. In recent years, many methods have been studied for the synthesis of AgNPs. This review provides an overview of the preparation of AgNPs from physical, chemical and biosynthetic applications. Therefore, the purpose of this review article is to show the current status and future prospects of the above technologies in the industry, especially these capabilities and limitations of the technology. This review also explores the mechanisms of action, synthesis methods and properties of AgNPsto examine their role in therapy and disease. The reason for its toxicity is also explained. The problem with medicine and the body is that it is very expensive to bind and absorb toxins. Chemicals can be used to solve this problem. We also discuss the toxicity of AgNPs and their impact on the environment and human health.
Keywords
AgNPs; Classification; Chemical synthesis; Physical synthesis; Biological synthesis; Characterisation; Applications; Silver nanotoxicity
Graphical Abstract
Introduction
There are still a few years left for truly revolutionary Nanotechnology products, materials and applications. "Nanotechnology" is a new phenomenon in research centers around the world. Today's Nanotechnology products are mostly products that continue to be created using some type of Nanotechnology process. It is used to kill parasites. As we advance and improve existing products by producing smaller products and better materials at a lower cost, organizations that develop Nanomaterials will grow rapidly and will now represent the majority of multinational companies.
Today's science includes the creation, synthesis and use of structures on the order of 1-100nm. There are many applications of Nanoparticles (NPs) in the pharmaceutical industry, cosmetics, food and feed, environmental health, technology, and more. The applications include electronic transistors, light emitters, and photoelectrochemical applications. There is a rapidly developing field of technology. The synthesis and controlled degradation of NPs is an important area of research. The fields of chemistry, engineering, physics, and medicine are all related to the study of particles in biological systems. It is important to create clean, non-toxic and environmentally friendly ways to synthesis and collect metal particles that can reduce metal through some metabolism pathways [1]. In the scientific and chemical community, nanomaterials have attracted attention due to their unique properties and widespread use in removing pollutants and pollutants from the environment. New materials are created by combining science, chemistry, engineering and biology [2].
Many products in human life, such as fabric, laundry, food, and medicine, have been studied in many with silver particles [3]. Many techniques have been applied to obtain AgNPs due to the diversity of Silver metal and Silver-based compounds [4-6]. There are biosynthetic processes [7]. The simplicity and safety of the process, the low financial impact, and the reproducibility of test results make most AgNPs, sodium citrate or sodium borohydride ready for processing [8-9]. AgNPs stability is of particular interest because of the limitation of antibiotic use due to their reduced antibacterial activity or lack of antibacterial activity as they are unstable in many organisms. Various materials have been used to produce AgNPs [10-12]. Means stability. Many researchers have used a variety of methods to synthesise metal particles [13]. Chemicals and expensive materials that are difficult to use on a large scale make it difficult to make small particles. Scientists have recently been using products derived frombacteria and other plant parts in a less toxic, cost-effective and eco-friendly way. A group of arthropods called the metabolic arthropods [14].
Has been known for a long time. Since the 19th century, silver-based compounds have been used in antibiotics. There are a variety of physical, biological and chemical applications for particles. In China, where it is said to be effective against bacteria, it is used as a disinfectant in train stations and elevators. There are many ways of making silver particles. Physical, chemical and biological processes are included. One of the main things that makes physical and chemical methods less expensive than synthetic methods is the fact that many of them are expensive or chemical. The biological method is a good way to make silver particles [15]. There is a need to develop methods that do not use chemicals in the synthesis process. Green methods include complex salts, polysaccharides, Tollens, biological methods and irradiation, which are superior to methods containing reagents associated with environmental toxicity. In the green synthesis of NPs, it is important to consider the choice of heavy medium and the choice of non-toxic reducing agents and stabilizers. AgNPs have attracted attention for their unique properties and can be used in a wide range of applications. Some of the key NPs are shown in table 1. Physical and chemical methods have been used to make AgNPs [16].
|
Important Applications of Silver NPs |
|
Treatment of ulcerative colitis & acne Treatment of dermatitis Inhibition of HIV-1 replication Enhanced Raman Scattering Spectroscopy (SERS) Detection of viral structures (SERS & silver nanorods) Antimicrobial effects against infectious organisms Remote laser light-induced opening of microcapsules Silver/dendrimer nanocomposite for cell labeling Molecular imaging of cancer cells Coating of hospital textile (e.g., surgical gowns & face mask) Coating of catheter for cerebrospinal fluid drainage Coating of surgical mesh for pelvic reconstruction Coating of breathing mask patent Coating of endotracheal tube for mechanical ventilatory support Coating of driveline for ventricular assist devices Coating of central venous catheter for monitoring Coating of intramedullary nail for long bone fractures Coating of implant for joint replacement Orthopedic stockings/ Additive in bone cement Implantable material using clay-layers with starch-stabilized silver NPs Superabsorbent hydrogel for incontinence material/ Hydrogel for wound dressing Additive in polymerizable dental materials patent Silver-loaded SiO2 nanocomposite resin filler (Dental resin composite) Polyethylene tubes filled with fibrin sponge embedded with silver NPs dispersion |
Table 1: Important applications of silver nanoparticles.
Chemical reduction using various organic and reducing inorganic agents is one of the most common chemical applications for the synthesis of silver particles. Many of the methods are still under development, and the challenges are stability and aggregation of particles. It is still difficult to extract and purify the particles for further use [17]. Concerns have been raised about the safety of workers, consumers and the environment because of the rapid development and use of nanomaterials. The study of the effects of NPs on organisms was the subject of a 2004 study by Donaldson and colleagues [18]. This new subcategory of toxicology is based on the idea that smaller particles behave differently than larger ones. Oberdrster et al. To show the concept of toxicology. "Nanotoxicology:" is the title. "An Approach to the Study of Ultra Small Particles" suggests that the first study to examine the extent of toxicological effects started from an aerosol test [19]. Conventional particle toxicology studies natural and man-made particles on a large scale, while Nanotoxicology research specifically produces particles. Special conditions lead to new applications when the size of 1-100nm is accepted [20]. It is an important question whether the fate of Nanomaterial evaluation methods will be viable or incomplete, as the specific toxicity of materials created using certain Nanoscale materials is still unclear (Table 2).
|
Method |
Silver Precursor |
Reducing Agent |
Stabilizing Agent |
Size (nm) |
|
Chemical reduction |
AgNO3 |
DMF |
- |
<25 |
|
Chemical reduction |
AgNO3 |
NaHB4 |
Surfactin (a lipopeptide biosurfactant) |
3-28 |
|
Chemical reduction |
AgNO3 |
Trisodium citrate (initial)+SFS (secondary) |
Trisodium citrate |
<50 |
|
Chemical reduction |
AgNO3 |
Trisodium citrate |
Trisodium citrate |
30-60 |
|
Chemical reduction |
AgNO3 |
Ascorbic acid |
- |
200-650 |
|
Chemical reduction |
AgNO3 |
NaHB4 |
DDA |
~7 |
|
Chemical reduction |
AgNO3 |
Paraffin |
Oleylamine |
10-14 |
|
Chemical reduction (thermal) |
AgNO3 |
Dextrose |
PVP |
22±4.7 |
|
Chemical reduction (thermal) |
AgNO3 |
Hydrazine |
- |
2-10 |
|
Chemical reduction (oxidation of glucose) |
AgNO3 |
Glucose |
Gluconic acid |
40-80 |
|
Chemical reduction (polyol process) |
AgNO3 |
Ethylene glycol |
PVP |
5–25 |
|
Chemical reduction (polyol process) |
AgNO3 |
Ethylene glycol |
PVP |
50-115 |
|
Electrochemical (polyol process) |
AgNO3 |
Electrolysis cathode: titanium anode: Pt |
PVP |
~11 |
|
Chemical reduction (Tollen)
|
AgNO3 |
m-Hydroxy benzaldehyde |
SDS |
15-260 |
|
Physical synthesis |
Ag wires |
Electrical arc discharge, water |
- |
~10 |
|
Physical synthesis |
AgNO3 |
Electrical arc discharge |
Sodium citrate |
14-27 |
|
Chemical reduction (microemulsion) |
AgNO3 |
Hydrazine hydrate |
AOT |
2-5 |
|
Chemical reduction (microemulsion) |
AgNO3 |
Hydrazine hydrate |
AOT |
<1.6 |
|
Photochemical reduction (pulse radiolysis) |
AgClO4 |
Ethylene glycol |
- |
17-70 |
|
Photochemical reduction (microwave radiation) |
AgNO3 |
Ethylene glycol |
PVP |
5-10 |
|
Photochemical Reduction (photoreduction) |
AgNO3 |
UV light |
- |
4-10 |
|
Photochemical reduction (X-ray radiolysis) |
Ag2SO4 |
X-Ray |
- |
~28 |
|
Photochemical reduction (X-ray radiolysis)
|
AgNO3 |
CMCTS, UV |
CMCTS |
2-8 |
Table 2: Some important physical, chemical and photochemical methods for synthesizing and stabilizing silver NPs.
Note: DMF: N,N’-dimethylformamide; NaHB4: Sodium borohydrate; SFS: Sodium formaldehyde sulphoxylate; DDA: Dodecanoic acid; PVP: Polyvinyl pyrrolidone; SDS: Sodium dodecyl sulphate; AOT: Bis (2-ethylhexyl) sulfosuccinate; CMCTS: Carboxymethylated chitosan.
The preparation of AgNPs through physical, chemical and synthetic applications is covered in this review.
Materials And Methods
All AgNPs studies are presented in this review. The negotiation process is not included. The methods, materials, uses and adverse effects of AgNPs are summarized in table 3 [21].
|
Synthesis |
Properties |
Applications |
Toxicity |
|
Chemical Chemical reduction Photochemical Electrochemical Micro emulsion/reverse micelle |
Antifungal effect, destroy fungi membrane integrity against deserving in dental anddeod orant applications. |
In medical field used for surgical instruments, prostheses, catheters, medical wounds. |
Exposure metal nanoparticles to human lung epithelial cells could increase ROS, which can lead to oxidative stress and cellular damage. |
|
Physical Thermal decomposition Electrical arc discharge Laser ablation Ionization Microwave Irradiation Evaporation/ condensation. Ultrasonic |
Antibacterial properties used in medical, food and textile fields. In addition, these are used as antiviral to prevent HIV-1 and inhibit the virus entry. |
Also in water treatments and filtration to eliminate microorganisms. |
Cell morphology changes, ytotoxicity, and immunological responses may affect fertility. |
|
Biological Biological reduction (Green chemistry) using plants, fungi, and bacteria Via reducing or capping agent such as polysaccharides, polyphenols, polyoxometalate, or tollens |
High electromagnetic interaction, electrical capacitance, electrochemical stability, catalytic activity, and non linear optical behavior. |
In addition, these are used for textiles/ clothing, home appliances, food preservation and packaging, paints, cosmetics, and electronics. |
Reduce mitochondrial function and lactate dehydrogenase (LDH) leakage. |
Table 3: Summary of AgNP mechanisms of synthesis, properties, applications, and potential toxic effects.
Syntheses of AgNPsUsing Various Medicinal Plant Extracts
Environmental protection, fast, non-toxic and economical are some of the advantages of the synthesis of AgNPs. The reduction and stability of Silver ion is due to the combination of plant extracts of biomolecules. Plants remove and reduce AgNO3 and can be identified with a UV-Vis spectrophotometer. Plants are listed in table 4, that are capable of producing silver particles [22].
|
Plants |
Size in nm |
Plant Part |
|
Alternanthera dentate |
50-100 |
Leaves |
|
Abutilon indicum |
7-17 |
Leaves |
|
Acorus calamus |
31.83 |
Rhizome |
|
Argyreia nervosa |
20-50 |
Seeds |
|
Acalypha indica |
20-30 |
Leaves |
|
Brassica rapa |
16.4 |
Leaves |
|
Carica papaya |
25-50 |
Leaves |
|
Cymbopogan citratus |
32 |
Leaves |
|
Centella asiatica |
30-50 |
Leaves |
|
Coccinia indica |
10-20 |
Leaves |
|
Citrus sinensis |
10-35 |
Peel |
|
Calotropis procera |
19-45 |
Plant |
|
Datura metel |
16-40 |
Leaves |
|
Eucalyptus hybrid |
50-150 |
Peel |
|
Eclipta prostrate |
35-60 |
Leaves |
|
Ficus carica |
13 |
Leaves |
|
Musa paradisiacal |
20 |
Peel |
|
Moringa oleifera |
57 |
Leaves |
|
Melia dubia |
35 |
Leaves |
|
Memecylon edule |
20-50 |
Leaves |
|
Nelumbo nucifera |
25-80 |
Leaves |
|
Plumbago zeylanica |
60 |
Leaves |
|
Premna herbacea |
10-30 |
Leaves |
|
Psoralea corylifolia |
100-110 |
Seeds |
|
Thevetia peruviana |
10-30 |
Latex |
|
Vitex negundo |
5 and 10-30 |
Leaves |
|
Vitis vinifera |
30-40 |
Fruit |
|
Ziziphora tenuior |
8-40 |
Leaves |
Table 4: Synthesis of silver nanoparticles from different medicinal plants.
Synthetic Methods for AgNPs
Silver particles are used in many ways. There are advantages and disadvantages to each method. bacteria reduce Ag to AgO, acting as a protective, reducing or stabilizing agent [23]. In recent years, biomethods based on natural products have gained popularity due to their low cost, high efficiency and non-toxicity [24]. There are various methods for making silver particles (Figure 1). Chemical methods. There are physical methods. There are biological methods.
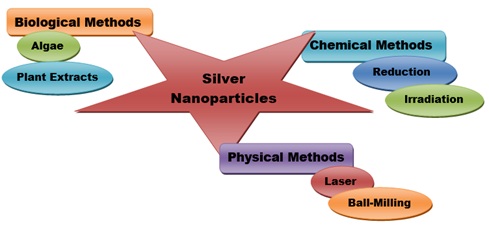 Figure 1: The schematic diagram for the synthesis of silver nanoparticles.
Figure 1: The schematic diagram for the synthesis of silver nanoparticles.
Chemical Methods
There are many ways in which silver particles can be combined. Chemical methods are more convenient than biological methods. It has been reported that Silver ion gain electrons from reducing agents and these are converted into metal flakes and aggregated into silver particles. AgNO3 is one of the most widely used salts due to its low cost and other properties [25]. In 2002, Sun and Xia reported the synthesis of monodisperse Silver [26]. The silver particles were created using AgNO3 as a starting point and hydride and trisodium citrate as stabilizers. It has been reported that hydride is an effective reagent for the synthesis of silver particles. Trisodium dicitrate is the best reducing agent for the synthesis of AgNPs [27]. The use of polyvinylpyrrolidon as a size controlling and sealing agent and ethylene glycol as a solvent and reducing agent has been reported to produce silver particles with an average size greater than 10nm [28]. The authors are Patil et al. A synthesis of silver particles using hydrazine hydrate as a reducing agent and polyvinyl alcohol as a stabilizer is shown. The obtained particles have a spherical shape and have important applications in biomedicine and biotechnology [29]. It was found in a study that the silver particles were spherical and had different sizes [30] (Table 5).
|
Reducing Agent |
Precursor Agent |
Capping Agent |
Experimental Conditions |
|
Trisodium citrate |
Silver nitrate |
Trisodium citrate |
Diameter ≈ 10–80 nm; temperature ≈ boiling point |
|
Ascorbic acid |
Silver nitrate |
Daxad 19 |
Diameter ≈ 15–26 nm; temperature ≈ boiling point |
|
Alanine/NaOH |
Silver nitrate |
DBSA (dodecylbenzenesulfonic acid) |
Diameter ≈ 8.9 nm; temperature ≈ 90°C; time ≈ 60 min |
|
Ascorbic acid |
Silver nitrate |
Glycerol/PVP |
Diameter ≈ 20–100 nm; temperature ≈ 90°C |
|
Oleic acid |
Silver nitrate |
Sodium oleate |
Diameter ≈ 5–100 nm; temperature ≈ 100–160°C; time ≈ 15–120 min |
|
Trisodium citrate |
Silver nitrate |
Trisodium citrate |
Diameter ≈ 30–96 nm; temperature ≈ boiling point; pH ≈ 5.7–11.1 |
|
Trisodium citrate |
Silver nitrate |
Trisodiumcitrate/ Tannic acid |
Diameter ≈ 10–100 nm; temperature ≈ 90°C |
Table 5: Chemical methods for the synthesis of monodispersed and quasi-spherical silver nanoparticles.
In the pre-heating method, the AgNO3 solution is heated up to the reaction temperature and the maximum NPs size value is observed, while in the pre-injection method, a silver nitrate solution is injected into the reaction. The temperature is not warm. Particle size should be reduced to achieve monodispersity [31]. The chemical method has an advantage over the physical method. The drugs and chemicals used to make AgNPs such as borohydride and 2-mercaptoethanol are dangerous and toxic because of the high cost of the chemical process. It is difficult to make silver particles of any size [32]. A lot of dangerous and toxic products are formed during mixing. The reducing agent used in this model is toxic [33].
Physical Methods
There are two physical methods of silver particles. The process uses a lot of energy and has a long duration. The synthesis of monodisperse Silver Nanocrystals is caused by thermal degradation of Silver oleate complexes [34]. Small electric motors were used in the study to produce metal particles. Polydispersity of particles can be caused by temperature inconsistencies in heating. The silver particles are spherical and not clustered [35]. Polyol treatment has been shown to produce spherical particles of different sizes [36]. Reducing the laser wavelength was found to reduce the average particle size from 29 to 12nm [37]. To compare the effect and size of silver particles, they were prepared by laser ablation in water. The Femtosecond pulse is designed to be smaller. The colloids that were prepared with a femtosecond pulse were smaller than those that were prepared with a Nanosecond laser pulse [38]. They studied the synthesis of silver particles by converting metal released by the human body into glycerol. This method is an alternative to chemical procedures. The obtained particles are not easy to assemble and have a narrow distribution [39]. The advantage of the physical method is that it is fast, non-toxic and uses electricity as a reducing agent. The disadvantages of physical methods are dispersion, poor performance, non-uniformity and density [40] (Table 6).
|
Type |
Reducing Agent |
Biological Activity |
Characterisation |
|
Polydiallyldimethylammonium chloride and polymethacrylic acid capped silver nanoparticles |
Methacrylic acid polymers |
Antimicrobial |
UV-Vis, reflectance spectrophotometry |
|
Silver nanoparticles |
Ascorbic acid |
Antibacterial |
UV-Vis, EFTEM |
|
Chitosan-loaded silver nanoparticles |
Polysaccharide chitosan |
Antibacterial |
TEM, FTIR, XRD, DSC, TGA |
|
Silver nanoparticles |
Hydrazine, D-glucose |
Antibacterial |
UV-Vis, TEM |
|
PVP-coated silver nanoparticles |
Sodium borohydride |
— |
UV-Vis, TEM, EDS, DLS, FIFFF |
Table 6: Physical and chemical syntheses of silver nanoparticles.
Abbreviations: UV-Vis-ultraviolet-visible spectroscopy; FIFFF- flow field-flow fractionation; DSC-differential scanning calorimetry; TEM-transmission electron microscopy; EDS-energy-dispersive spectroscopy; EFTEM- energy filtered TEM; FTIR- Fourier transform infrared; DLS- dynamic light scattering; XRD- X-ray diffraction; TGA- thermogravimetric analysis.
Biological Methods
Physical and chemical methods are used to make silver particles. It is important to create a chemical-free business and industry to avoid harmful effects on the body [41]. There are many applications of biological processes in medicine. Plant products, as well as the use ofbacteria and yeasts, are included in bioproduction methods. This method is very popular in the treatment of particles. It is reported that plant-based production methods are more economical and less harmful to the environment than chemical synthesis [42]. Plants can collect non-metal ion from the environment [43]. Bacteria and plants are used in the production of silver particles [44] (Figure 2, Table 7).
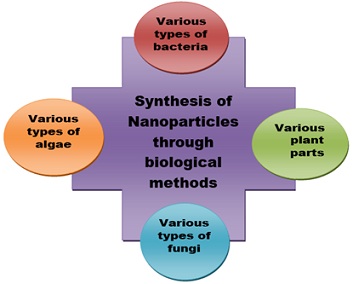 Figure 2: Different biological methods for the synthesis of silver nanoparticles.
Figure 2: Different biological methods for the synthesis of silver nanoparticles.
|
Silver Salt |
Plant Origin |
Shape |
Silver Size (nm) |
|
|
AgNO3 |
Pinus, Diospyros kaki Ginkgo biloba magnolia and Platanus |
— |
15-500 |
|
|
AgNO3 |
Artocarpus heterophyllus lam |
Irregular |
10.78 |
|
|
AgNO3 |
Prunus yedoensis |
Spherical and oval |
20-70 |
|
|
AgNO3 |
Zingiber officinale |
— |
10-20 |
|
|
AgNO3 |
Morinda citrifolia |
Spherical |
30-55 |
|
|
AgNO3 |
Bunium persicum |
Spherical |
20-50 |
|
|
AgNO3 |
Justicia Adhatoda |
Spherical |
25 |
|
|
AgNO3 |
Adenium obesum |
Spherical |
10-30 |
|
|
AgNO3 |
Coffee arabica |
Spherical and ellipsoidal |
20-30 |
|
|
AgNO3 |
Vigna radiata |
Spherical and oval |
5-30 |
|
|
AgNO3 |
Jatropha curcas |
Spherical |
10-20 |
|
|
AgNO3 |
Emblica officinalis |
— |
10-20 |
|
|
AgNO3 |
Lantana camara |
Spherical |
14-27 |
|
|
AgNO3 |
Sesuvium portulacastrum L. |
Spherical |
5-20 |
|
|
AgNO3 |
Mentha peprita |
Spherical |
90 |
|
|
AgNO3 |
Tribulus terrestris L. |
Spherical |
16-28 |
|
|
AgNO3 |
Nyctanthes arbor-tristis L. |
Spherical |
50-80 |
|
|
AgNO3 |
Azadirachta indica |
Spherical |
50-100 |
|
|
AgNO3 |
Pelargonium sidoides DC. |
Spherical |
16-40 |
|
|
AgNO3 |
Vigna unguiculata |
Spherical |
24.35 |
|
|
AgNO3 |
Cinnamomum camphora |
Spherical |
55-80 |
|
|
AgNO3 |
Aloe barbadensis miller |
Spherical |
15.2 ± 4.2 |
|
|
AgNO3 |
Amaranthus retroflexus |
Spherical |
10-32 |
|
Table 7: Biological method of synthesis of silver NPs using plants extracts as a reducing agent.
Production in Bacteria
Research has been done to form silver particles by reducing Silver ion with water. The method shows that the interaction between Silver ion and cell filtrate can be done in 5 minutes. The reduction of Ag to AgNPs was partially prevented by piperonone [45]. The nitrogen-reducing activity of Enterobacteriaceae was stopped by the natural product piperonone. Klebsiella pneumoniae should have slowed the reduction of Silver ion to Silver particles. Korbekandi et al. The bioreductive synthesis of AgNPs was reported [46]. Liu et al. Stem cells of Bacillus megaterium have been shown to produce NPs [47]. Das et al. The extracellular synthesis of AgNPs by bacterial cells is described. One study found that treating Bacillus strain CS 11 with AgNO3 caused cells to produce AgNPs [48].
Synthesis/Production Based on Fungi
It has been reported that there are many fungi involved in the production of silver particles [49]. The fungus can produce silver particles very quickly. Many researchers have studied the biosynthesis of silver particles by fungi. The interaction of the fungus Fusarium solani with Silver Nitrate was demonstrated in a study [50]. The silver particles were reported by Syed and colleagues. The first drug was Humicola sp. Production of silver particles [51]. Owaid and colleagues reported the production of silver particles from [52]. silver nitrate bioreduction. Xu et al. Attempts have been made to make silver particles with antifungal [53]. properties. The formation of silver particles in the cell wall [54]. is caused by the interaction of nitrate and the fungus Aspergillus flavus. In addition, Bhainsa and D'Souza studied the production of silver particles. Studies show that Silver particles can be produced in a short time [55]. The silver particles are 5 to 50nm in size [56]. In addition, the Silver nitrate solution produced silver particles [57]. The bio reduction of Fusarium oxysporum was reported by Korbekandi and colleagues [58].
Production in Algae
The method can be used instead of physical and chemical methods [59]. Levi can absorb high-speed trains. Some studies show that biological products can have effects. This ability is needed for modern and accurate biosynthesis [60]. Studies have shown that the color change from yellow to brown can be used to show the reduction of Silver ion to silver particles. Also, Rajeshkumar and his associates. The intensity of the dark brown color of the silver particles was directly affected by the time it took for the particles to oxidize [61]. Silver nitrate was reduced with Padina powder and solvent was used to extract the silver particles. The obtained particles have high stability, fast recovery and small size [62]. The Spirogyra variant has been reported to have been involved in the production of silver particles [63]
Production in Yeast
It has been reported that yeast can make silver particles. The yeast-based Silver Nanoparticle production method is cost-effective and eco-friendly. They investigated Saccharomyces cerevisiae. The sample became red-brown with the prolongation of the culture time, after the addition of Silver ion to the yeast culture. The solution's color changed to brownish red [64]. In 2003 there was a report by Kowshik et al. The interaction of Silver with Silver-transfer yeast was reported [65].
Synthesis Based on Plant/Extract
The synthesis of plants is the most suitable for medicine and body because it does not require heat, energy or chemicals, is cost-effective, and is Eco-friendly [66]. There are many components in the leaves of Aloe Vera. Lignin, hemicellulose and pectin are components that play an important role in the reduction of Silver ion [67]. The extract of the Saudi Arabian thyme plant was used to make AgNPs. The reduction of silver ion caused the binding of silver particles. The reaction mixture changes from light brown to dark brown during this process. There was no color change in the absence of plant extract [68]. The results of another study showed that the color of Silver nitrate solution changed slightly from light to yellow-brown after adding different concentrations of neem leaf extract [69]. The rapid synthesis of silver particles using a plant extract [70]. Chinapan et al. A quick and easy method for the synthesis of silver particles was reported by him [71].
In 2016 the results showed that a solution of AgNO3 and Silver nitrate can quickly oxidize the silver. After a few minutes of microwave irradiation, the color was found to change from light yellow to reddish brown due to the presence of silver particles [72]. Lakshmana et al. The plant extract of Cleome viscosa has been shown to reduce Silver nitrate to Silver metal [73]. The book by Prasad et al. A simple and rapid method has been developed for the bio reduction of silver particles. According to their findings, moringa has the ability to reduce the amount of silver in the air [74]. A new discovery shows that using a leaf extract can be used to synthesise silver particles without damaging them [75].
Treatment of silver nitrate and chloroauric acid with neem leaf extracts led to the rapid synthesis of silver and gold particles [76]. Plants were used for the synthesis of NPs [77]. Bonnaruselvam et al. It was found that snail leaf extracts could be used to make silver particles [78]. There are many studies that show that Silver is stable in water [79]. Zarghar and colleagues reported the production of silver particles using the leaf extract of Vitex negundo and the antibacterial activity of silver particles against Gram-positive and Gram-negativebacteria [80].
Synthesis Based on DNA
Reducing agents can be used in the synthesis of silver particles. The high affinity of Silver ion for DNA base pairs makes them a template stabilizer. N-7 and guanine base pairs are found in the DNA helix. A study showed the synthesis of silver particles from bovine DNA [81].
Characterisation Techniques for AgNPs
The main properties of particles are their size, shape, surface area and distribution [82]. Quantitative and qualitative techniques are used for measuring the properties of particles. These include Dynamic Light Scattering (DLS), Scanning Electron Microscopy (SEM), Energy Dissipative Spectroscopy (EDS), UV-Vis Spectroscopy, Transmissive Electron Microscopy (TEM), X-ray Diffraction (XRD), Fu Fourier Transform Infrared Spectroscopy (FT) -IR), Surface Enhanced Raman Spectroscopy (SERS), Atomic Force Microscopy (AFM), High Angle Annular Dark Field (HAADF), Atomic Absorption Spectroscopy [AAS], Inductive Coupled Plasma (ICP), and X-ray photoelectron spectroscopy (XPS) ) [82]. One of these techniques can be used to study the properties of AgNPs, which helps to reveal many parameters such as particle size, shape, crystallinity, fractal size, pore size and surface area [83] (Figure 3). 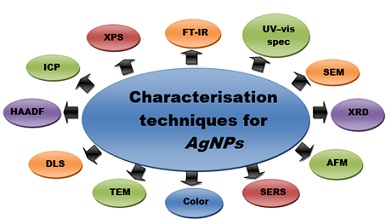
Figure 3: Various techniques used for characterisation of silver nanoparticles.
Qualitative Analysis
FT-IR
The range of chemical activity that can be examined by FT-IR is 4,000 to 400 cm-1 [84]. The purpose of the measurement is to determine the local position of the masking agent and biomolecules that will reduce, seal and stabilizing AgNPs [85].
UV-visible spectrophotometry
The absorption spectrum in the ultraviolet visible spectrum is referred to as the ultraviolet visible spectrum. Depending on the light wavelength, various metal particles can be characterized in the size range of 2 to 100nm [82]. The formation and stability of Silver particles in liquids can be determined using UV-Vis spectroscopy [86]. AgNPs were identified by measuring their absorption at a wavelength [82].
SEM
The output images are produced by using electricity instead of light [87]. The size, shape, and distribution of the AgNPs were characterized with the use of SEM analysis [85]. The purity and polydispersity of the AgNPs were demonstrated by the SEM micrographs [82].
XRD
Information about the atomic structure of materials can be obtained using XRD. XRD can be used to identify minerals in geological samples and mineralogical data [88]. A useful tool that can be used to visualize the formation of AgNPs, determine the crystal structure and calculate the size of crystal particles is a the XRD [85].
AFM
The shape, size and surface area of AgNPs were studied [89]. An improvement of AFM over conventional microscopes is that it uses three-dimensional images that allow height and volume to be determined [83].
SERS
The functional groups of coating agents that are involved in stabilization of particles were determined [90]. A potential technique for in vitro diagnostics is surface-enriched raman spectroscopy. Drug specificity with good sensitivity can be achieved through signal amplification detected when analyte molecules are very close to the metal [91]. SERS uses field plasmons in metal particles to generate a strong field. The SERS spectrum can be seen when the molecule is close to the SERS carriers. SERS technology is widely used in the detection, detection and monitoring of various biochemical processes due to its rapid, label-free, non-invasive and high molecular specificity and sensitivity. SERS gives important information about the adsorption mechanism of biomolecules on metal surfaces by showing functional groups or atoms involved in metal adsorption interactions [92].
Color
A change in the metal saltsolution's color is indicative of the formation of metal particles. The formation of AgNPs is indicated by the distinct color change of the Silver nitrate solution after reduction [93].
Quantitative Analysis
TEM
TEM is an analytical method that can be used to observe the size of materials and evaluate crystal structures at the highest resolution [94]. The particle size and size distribution of the AgNPs were measured [85].
DLS
The DLS technique can be used as a diagnostic tool for the particle size distribution of silver particles in solution or suspension [95]. The average hydrodynamic diameter of a sample can be obtained by varying the difference in light scattering. The size of the particles can be monitored during the measurement of the solution process. This method is used to identify metal ion and cancer biomarkers [96].
HAADF
The interaction between AgNPs bacteria can be studied using various electron microscopy techniques.The size of the particles associated with the pathogen was obtained from the images [97]. A powerful technique for analyzing biological samples is called HAADF. The electron source recovered by Rutherford is the HAADF image. Thus, the ratio of shapes is related to the difference in the number of atoms in density in the ~Z2 range [98].
ICP
The AgNPs can be studied with the help of the SPICPMS in terms of size and number concentration [99]. Ag concentrations in deionized and intact AgNPs solutions can be determined [100]. The amount of Silver is measured using two different methods [101].
XPS
X-ray photoelectron spectroscopy was used to confirm the chemical state of the particles [102]. XPS analyzed AgNPs to find the properties of the adsorbed surfactants on the surface [103]. This provides additional information about the structure of the AgNPs and is used to examine the valence state of the AgNPs. The networks are organic [104].
Applications of AgNPs
The unique properties of silver particles are shown in figure 4. They have been used in many applications.
 Figure 4: Schematic diagram representing various applications of AgNPs.
Figure 4: Schematic diagram representing various applications of AgNPs.
Wound Patches
Bank wraps have been used for a long time. For the treatment of burns and other injuries. Although dressings with AgNPs shortened the wound healing time by an average of 3 [105]. Silver particles are used in antimicrobial dressings. The wound dressing is made of two layers of Silver-coated high-density polyethylene mesh with an absorbent polyester core of Silver rayon that retains strong antimicrobial properties [106].
Cardiovascular Implants
The Silicone heart valve is a Silver-coated device. Money can be used to reduce the pressure response of silicone valves. Preliminary clinical trials show that Silver damages patients, fibroblasts, and paravalvular infiltrates. Efforts have been made to incorporate AgNPs into medical devices as a way to provide non-toxic, non-toxic vaccines [106].
Catheters
Infections can be caused by catheters in emergency departments. AgNPs can be used to reduce the formation of biofilm in catheters. Studies have shown that AgNPs-coated catheters can be used for up to 72 hours. A 10-day clinical trial in mice confirmed that catheters coated with AgNPs are non-toxic [105].
Food Industry
Food, nutrition and food products are some of the uses of Nanotechnology, which uses particles as small as one billionth of a meter [107]. AgNPs are slowly released from the coating and can be used to prevent infections [108]. There are packaging materials. The food industry uses AgNPs due to its antibacterial and non-toxic properties. It is not harmful to the human brain to have low concentrations of NanoSilver. It’s widely used to clean food and water and to prevent diseases in medicine. AgNPs are used in a wide range of products, including soap, food, plastic, paste and textiles [109].
Textiles
AgNPs can be used in the manufacture of materials. Blaser et al. The biggest contributors to airborne Silver are fabric and bag working with AgNPs. AgNPs are considered the least expensive due to the high risk of contamination of surgical gowns, but garments such as socks, T-shirts and sportswear have been studied with AgNPs. Different techniques have been used to functionalize fabrics with AgNPs [109].
Water Treatment
Stable AgNPs were developed using fresh sumac leaves that were sprouted at 80°C as a new probe for the detection of chromium ion in tap water. The silver particles were prepared with Prosopis juliflora leaf extract and treated with 100 mL of wastewater for 6 hours [110].
Optical Activity
Eye treatment uses silver particles. It is used in a lot of things [109].
- Applications of AgNPs in Medicine, Pharmacy and Dentistry [111]
- Pharmaceutics and Medicines: It prevents HIV-1 replication. There are treatments for ulcerative colitis and acne. Cell labeling with silver/dendrimer particles. Cancer cells are imaged. The SERS is an enhanced raman scattering. The identification of the cells of the bacterium. The hospital has textile layers. There are stockings for wound dressing.
- Dentistry: There is a patent for dental materials. The SiO2 is loaded with silver. The tube was filled with a sponge with silver particles.
- Other Applications of AgNPs [105]
Silver is used in many products such as water filters and purifiers, as well as in soaps, socks, food and air fresheners. Silver particles attached to Fe3O4 can be used in water purification and can be easily removed using a magnet. Dressings, creams and gels made from silver are effective in reducing inflammation. Silver-impregnated medical equipment, such as surgical masks, has been shown to be effective at sterilizing the skin. Food preservation is one of the uses of silver zeolites. When tested on animal models, silver particles showed better pain relief, better aesthetic and less scarring. Medicine uses AgNPs. Diagnostic and medical applications can be divided. A good relationship between catalysts and surfaces is possible because of the high volume fraction of particles used as catalysts. It’s used in electronic products, antibiotics in the healthcare industry, food storage, textile coating, etc. It is widely used. The environment is used. Many studies examining the application of Inkjet technology have been repeated in recent years.
Toxicity of AgNPs
The unique physical and chemical properties of silver particles make them ideal for many tasks, and their antibacterial and antifungal properties make them ideal for many medical applications. Studies show that the silver can be harmful to humans and the environment. Tons of Silver enters the environment from industrial waste, and the toxicity of Silver is thought to be due to Silver ion in the aqueous phase. The adverse effects of this amount on humans and all living things include toxic effects such as blue-gray skin (argyrosis) or eyes (argyrosis). Changes in cells are related to stomach diseases. Since the 21st century entered, it has become popular and has been used in almost every field. It has been reported that NanoSilver can’t distinguish between different organisms. There are few studies on the toxicity of silver. In one study, exposure to even small amounts of AgNPs caused damage to the cells of rats. The toxicity of silver particles to mouse stem cells has been shown. It is said that the clusters of silver are more harmful than the ones of asbestos. There is evidence that Silver ion can cause changes in the permeability of the cell. The data shows that there may be toxic effects on peripheral blood mononuclear cell proliferation. There can be serious harm to male fertility. Studies show that the blood-testicular barrier can be crossed with the help of the silver in the testicles. Commercial Silver-based dressings have been shown to be cytotoxic in a few experimental models. The study shows that the target of Nano-Silver in mice is the liver. Experiments have shown higher levels of arteriosclerosis with or without fibrosis. Money is released when particles are stored for a long time. It should be said that old AgNPs is more toxic than new. There are many friendly bacteria in the soil. Silver is toxic to denitrifying organisms, so it affects the denitrification process. Eutrophication of water in lakes, ponds and marine ecosystems can be caused by the loss of environmental denitrification. Silver is toxic to aquatic animals because it can interact with fish gills and inhibit fish osmoregulation. A purification experiment on Daphnia magna showed that the World Food Organization's good distribution and good distribution guidelines should apply to the toxicity of AgNPs. The collection of chemicals should be considered carefully. It should be noted that toxicity studies have been conducted on humans under similar conditions, but at different concentrations of AgNPs particles. Before drawing conclusions about the toxicity of AgNPs, more research is needed [112].
Future Outlook
New research continues now that AgNPs have been phased out in some commercial applications. Our recent research has revealed new osteoinductive properties, and AgNPs hold great potential due to their antibacterial, antifungal, antiviral and anti-Inflammatory properties. The administration of AgNPs to healthy people showed promise as a cancer treatment. A new era in cancer treatment and diagnosis will be paved by the biocompatibility and auto fluorescence of green synthetic AgNPswith the normal body. Concentrations of silver particles found in food Additives result in a reduction of nuclear structural complexity over time in isolated oral epithelial cells. Drug reactions and adverse reactions to NPs are an important task for the success of the therapy. Drug delivery using nanomaterials supports both diagnostic and therapeutic functions. This behavior only occurs in the body. Learning in life is important.
Conclusion
Many fields and products use silver particles. Their high reactivity is due to their small size. It is considered the best method as it is cost effective, safe and feasible. The heat and pressure used for mixing are reduced. The use of plant extracts is the fastest way to use medicine. The review discusses various methods for synthesis of silver particles, as well as their biological applications. Many studies have shown that the release of silver into the environment known to cause problems for the environment, as people have expressed concerns about the toxicity of the particles. Care should be taken when using AgNPs to take advantage of its beneficial properties and not harm humans or the environment. AgNPs can be friendly when used correctly, but can be risky and dangerous if used wrong. More research is needed to determine the safe manufacture, use, and disposal of the product. For the benefit of future researchers, we reviewed regular publications on the above topics. More research is needed on the connection and mechanism of action of silver in the human body, as the impact on the environment and human health will be a challenge for its widespread use. More research is needed before using this product outside of the laboratory.
Acknowledgment
This work was supported by the Sri Venkateswara University of Natural Sciences.
Author Statement
Venkataramanaiah Polia: Data curation, Writing - original draft.
Srinivasulu Reddy Motireddy: Conceptualization, Methodology, Software, Writing - review & editing.
Conflicts of interest
The authors declare no conflict of interest. The funders had no role in the study’s design, collection, analyses, and interpretation of data, review manuscript writing, or decision to publish the results.
Funding
The author[s] received no financial support for the research, authorship, and/or publication of this article
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
- Gutte MP, Gaur SR, Hiwale SB (2021) Synthesis of silver nanoparticles by biological and chemical methods. JETIR 8: 271-278.
- Arif R, Jadoun S, Verma A (2020) Synthesis of nanomaterials and their applications in textile industry. Frontiers of Textile Materials 167: 117-133.
- Tiago AJS, Rodrigues RRS, Leonardo PF (2019) Silver nanoparticles An integrated view of green synthesis methods. transformation in the environment. and toxicity. Ecotoxicol Environ Saf 171: 691-700.
- Brobbey KJ, Haapanen J, Gunell M, Mäkelä JM, Eerola E, et al. (2017) One-step flame synthesis of silver nanoparticles for roll-to-roll production of antibacterial paper. Appl Surf Sci 420: 558-565.
- Han HJ, Yu T, Kim WS, Im SH (2017) Highly reproducible polyol synthesis for silver nanocubes. J Cryst Growth 469: 48-53.
- Liu F, Liu J, Cao X (2017) Microwave-assisted synthesis silver nanoparticles and their surface enhancement raman scattering. Rare Met Mater Eng 46: 2395-2398.
- Dutta PP, Bordoloi M, Gogoi K, Roy S, Narzary B, et al. (2017) Antimalarial silver and gold nanoparticles Green synthesis, characterization and in vitro study. Biomed Pharmacother 91: 567-580.
- Hanif M, Juluri RR, Fojan P, Popok VN (2016) Polymer films with size-selected silver nanoparticles as plasmon resonance-based transducers for protein sensing. Biointerface Res Appl Chem 6: 1564-1568.
- Higa AM, Mambrini GP, Hausen M, Strixino FT, Leite FL (2016) Ag-nanoparticle-based nano-immunosensor for anti-glutathione s-transferase detection. Biointerface Res Appl Chem 6: 1053-1058.
- Chien CS, Lin CJ, Ko CJ, Tseng SP, Shih CJ (2018) Antibacterial activity of silver nanoparticles AgNPs): Confined to mesostructured silica against Methicillin-Resistant Staphylococcus Aureus (MRSA). J Alloys Compd 747: 1-7.
- Muhammad Z, Raza A, Ghafoor S, Naeem A, Naz SS, et al. (2016) Peg capped methotrexate silver nanoparticles for efficient anticancer activity and biocompatibility. Eur J Pharm Sci 91: 251-255.
- He H, Tao G, Wang Y, Cai R, Guo P, et al. (2017) In situ green synthesis and characterization of sericin-silver nanoparticle composite with effective antibacterial activity and good biocompatibility. Mater Sci Eng C 80: 509-516.
- Vasantharaj S, Sripriya N, Shanmugavel M, Manikandan E, Gnanamani A, et al. (2018) Surface active gold nanoparticles biosynthesis by new approach for bionanocatalytic activity. Journal of Photochemistry and Photobiology B Biology 179: 119-125.
- Nithya P, Sundrarajan M (2020) Ionic liquid functionalized biogenic synthesis of AgAu bimetal doped CeO2 Nanoparticles from Justicia adhatoda for pharmaceutical applications antibacterial and anti-cancer activities. Journal of Photochemistry and Photobiology B Biology 202: 111706.
- Almatroudi A (2020) Silver nanoparticles synthesis, characterisation and biomedical applications. Open Life Sci 15: 819-839
- Klaus-Joerger T, Joerger R, Olsson E, Granqvist CG (2001) Bacteria as workers in the living factory metalaccumulating bacteria and their potential for materials science. Trends Biotechnol 19: 15-20.
- Sonika D, Saurav K, Aakash G, Uttam L, Ranjita T, et al. (2021) Current research on silver nanoparticles synthesis, characterization, and applications. Journal of Nanomaterials 1-23.
- Mohmmad YW, Mohd AH, Firdosa N, Maqsood AM (2011) Nanotoxicity: Dimensional and morphological concerns. Advances in Physical Chemistry 15: 1-15.
- Saura CS, Wallace HA (2017) Toxicity of nanomaterials found in human environment: A literature review. Toxicology Research and Application 1: 1-13.
- NSET (2010) The National Nanotechnology Initiative. Research and Development Leading to a Revolution in Technology and Industry Supplement to the President's FY 2011 Budget. Washington. DC Subcommittee on Nanoscale Science. Engineering and Technology, Committee on Technology. National Science and Technology Council.
- Sein LS, Fabián FL, Fernando LV (2016) Silver Nanoparticles AgNPs): In the environment a review of potential risks on human and environmental health. Water Air Soil Pollut 227: 1-20.
- Roy A (2017) Synthesis of silver nanoparticles from medicinal plants and its biological application: A review. Res Rev Biosci 12: 138.
- Zewde B, Ambaye A, StubbsIii J, Raghavan D (2016) A review of stabilized silver nanoparticles-synthesis, biological properties, characterization and potential areas of applications. Nanomedicine 4: 1-4.
- Shanmuganathan R, Karuppusamy I, Saravanan M, Muthukumar H, Ponnuchamy K, et al. (2019) Synthesis of silver nanoparticles and their biomedical applications: A comprehensive review. Curr Pharm Des 25: 2650-2660.
- Hala MA, Maissa M, Morsi N, Ahmed H, Amal AA, et al. (2021) Comparative analysis of nanosilver particles synthesized by different approaches and their antimicrobial efficacy. Journal of Nanomaterials 1-12.
- Tingting H, Aijuan L, Wenfang L, Chuanpin C (2019) Microdroplet synthesis of silver nanoparticles with controlled sizes. Micromachines 10: 1-10.
- Agnihotri S, Mukherji S (2013) Size-controlled silver nanoparticles synthesized over the range 5-100nm using the same protocol and their antibacterial efficacy. RSC Adv 4: 3974-3983.
- Dang TMD, Le TTT, Fribourg-Blanc E, Dang MC (2012) Influence of surfactant on the preparation of silver nanoparticles by polyol method. Adv Nat Sci Nanosci Nanotechnol 3: 035004.
- Patil RS, Kokate MR, Jambhale CL, Pawar SM, Han SH, et al. (2012) One-pot synthesis of PVA-capped silver nanoparticles their characterization and biomedical application. Adv Nat Sci Nanosci Nanotechnol 3: 015013.
- Amirjani A, Firouzi F, Haghshenas DF (2020) Predicting the size of silver nanoparticles from their optical properties. Plasmonics 15: 1077-1082.
- Reddy SJ (2015) Silver nanoparticles - synthesis, applications and toxic effects on humans a review. International Journal of Bioassays 4: 4563-4573.
- Zhang XF, Liu ZG, Shen W, Gurunathan S (2016) Silver nanoparticles synthesis. characterization. properties. applications. and therapeutic approaches. Int J Mol Sci 17: 1534.
- Ganaie SU, Abbasi T, Abbasi SA (2015) Green synthesis of silver nanoparticles using an otherwise worthless weed mimosa Mimosa pudica): Feasibility and process development toward shape/size control. Part Sci Technol 33: 638-644.
- Mahmuda A, Tajuddin SM, Mostafizur RM, Atique UAKM, Kaniz FBH, et al. (2018) A systematic review on silver nanoparticles-induced cytotoxicity Physicochemical properties and perspectives. Journal of Advanced Research 9: 1-16.
- Dheeksha LR (2021) Recent advances in synthetic methods and applications of silver nanoparticles. Biotechnol Ind J 17: 217.
- Torras M, Roig A (2020) From silver plates to spherical nanoparticles snapshots of microwave-assisted polyol synthesis. ACS Omega 5: 5731-5738.
- Tsuji T, Iryo K, Watanabe N, Tsuji M (2002) Preparation of silver nanoparticles by laser ablation in solution influence of laser wavelength on particle size. Appl Surf Sci 202: 80-85.
- Gutte MP, Gaur SR, Hiwale SB (2021) Synthesis of silver nanoparticles by biological and chemical methods. Res Pharm Sci 8: 271-278.
- Siegel J, Kvítek O, Ulbrich P, Kolska Z, Slepicka P, et al. (2012) Progressive approach for metal nanoparticle synthesis. Mater Lett 89: 47-50.
- Elsupikhe RF, Shameli K, Ahmad MB, Ibrahim NA, Zainudin N (2015) Green sonochemical synthesis of silver nanoparticles at varying concentrations of κ-carrageenan, Nanoscale Res. Lett. 10: 302.
- Iravani S (2014) Bacteria in nanoparticle synthesis current status and future prospects. Int Scholar Res Not 359316.
- Gowramma B, Keerthi U, Rafi M, Rao DM (2015) Biogenic silver nanoparticles production and characterization from native stain of Corynebacterium species and its antimicrobial activity. Biotech 5: 195-201.
- Shah M, Fawcett D, Sharma S, Tripathy SK, Poinern GEJ (2015) Green synthesis of metallic nanoparticles via biological entities. Materials 8: 7278-7308.
- Ahmad S, Munir S, Zeb N, Ullah A, Khan B, et al. (2019) Green nanotechnology a review on green synthesis of silver nanoparticles - an ecofriendly approach, Int J Nanomed 14: 5087-5107.
- Abd El-Raheem RElS, Sobhy EE, Mohamed EE (2011) Extracellular Biosynthesis of Silver Nanoparticles Using Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633, and Streptococcus thermophilus ESh1 and Their Antimicrobial Activities. International Scholarly Research Network ISRN Nanotechnology 1-8.
- Korbekandi H, Iravani S, Abbasi S (2012) Optimization of biological synthesis of silver nanoparticles using Lactobacillus casei subsp. Casei. J Chem Technol Biotechnol 87: 932-937.
- Hitesh C, Shabana B, Inderbir S, Mohammad MH, Muhammad SK, et al. (2022) Green metallic nanoparticles biosynthesis to applications. Front Bioeng Biotechnol 10: 1-29.
- Das VL, Thomas R, Varghese RT, Soniya EV, Mathew J, et al. (2014) Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. Biotech 4: 121-126.
- Guilger-Casagrande M, de Lima R (2019) Synthesis of silver nanoparticles mediated by fungi a review. Front Bioeng Biotechnol 7: 287.
- Atef MM, Sanghoon K (2018) Myco-silver nanoparticles synthesized using Beauveria bassiana and Metarhizium brunneum as a smart pest control. Egypt J Plant Prot Res Inst 1: 1-18.
- Syed A, Saraswati S, Kundu GC, Ahmad A (2013) Biological synthesis of silver nanoparticles using the fungus Humicola sp. and evaluation of their cytoxicity using normal and cancer cell lines. Spectrochim Acta A Mol Biomol Spectrosc 114: 144-147.
- Owaid MN, Raman J, Lakshmanan H, Al-Saeedi SSS, Sabaratnam V, et al. (2015) Mycosynthesis of silver nanoparticles by Pleurotus cornucopiae var. citrinopileatus and its inhibitory effects against Candida sp. Mater Lett 153: 186-190.
- Xue B. He D, Gao S, Wang D, Yokoyama K, et al. (2016) Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal actvity against genera of Candida. Aspergillus and Fusarium. Int J Nanomed 11: 1899-1906.
- Priyamvada G, Nilesh R, Ashish V, Dimple S, Surya PS, et al. (2022) Green-based approach to synthesize silver nanoparticles using the fungal endophyte penicillium oxalicum and their antimicrobial, antioxidant, and in vitro anticancer potential. ACS Omega 7: 46653-46673.
- Kareem MA, Bello IT, Shittu HA, Awodele MK, Adedokun O, et al. (2020) Green synthesis of silver nanoparticles (AgNPs) for optical and photocatalytic applications a review. IOP Conf. Series Materials Science and Engineering 805: 1-27.
- Aleksandra Z, Magdalena KO (2017) Fungal synthesis of size-defined nanoparticles, Adv Nat Sci Nanosci Nanotechnol 8: 1-10.
- Karthikeyan P, Mohan D, Abishek G, Priya R (2015) Synthesis of silver nanoparticles using Phytoplankton and its characteristics. International Journal of Fisheries and Aquatic Studies 2: 398-401.
- Korbekandi H, Ashari Z, Iravani S, Abbasi S (2013) Optimization of biological synthesis of silver nanoparticles using Fusarium oxysporum. Iran J Pharm Res 12: 289-298.
- Hamouda RA, Hussein MH, Abo-elmagd R.A, Bawazir SS (2019) Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci Rep 9: 13071.
- Kapoor RT, Salvadori MR, Rafatullah M, Siddiqui MR, Khan MA, et al. (2021) Exploration of Microbial Factories for Synthesis of Nanoparticles-A Sustainable Approach for Bioremediation of Environmental Contaminants. Front Microbiol 12: 1-16.
- Rajeshkumar s, Malarkodi C, Venkat kumar S (2017) Synthesis and characterization of silver nanoparticles from marine brown seaweed and its antifungal efficiency against clinical fungal pathogens. Asian J Pharm Clin Res 10: 190-193.
- AbdelRahim K, Mahmoud SY, Ali AM, Almaary KS, Mustafa AEZMA, et al. (2017) Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J Biol Sci 24: 208-216.
- Salari Z, Danafar F, Dabaghi S, Ataei SA (2016) Sustainable synthesis of silver nanoparticles using macroalgae Spirogyra varians and analysis of their antibacterial activity. J Saudi Chem Soc 20: 459-464.
- Niknejad F, Nabili M, Daie Ghazvini R, Moazeni M (2015) Green synthesis of silver nanoparticles advantages of the yeast Saccharomyces cerevisiae model. Curr Med Mycol 1: 17-24.
- Hoda S, Ashraf E, Amira D (2018) Antimicrobial activity of silver nanoparticles biosynthesised by Rhodotorula sp. strain ATL72, Egyptian Journal of Basic and Applied Sciences. 5: 228-233.
- Jagessar RC (2021) Nanotechnology and Nanoparticles in Contemporary Sciences. Journal of Nanosciences Research & Reports 3: 1-7.
- Patcharaporn T, Nutthakritta P, Parichart B, Apiwat C (2016) Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. Peer J 1-15.
- Shaik MR, Khan M, Kuniyil M, Al-Warthan A, Alkhathlan HZ, et al. (2018) Plant-extract-assisted green synthesis of silver nanoparticles using Origanum vulgare L. extract and their microbicidal activities, Sustainability 10: 913.
- Ahmed SS, Ahmad M, Swami BL, Ikram S (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci 9: 1-7.
- Lopez-Miranda JL, Vázquez M, Fletes N, Esparza R, Rosas G (2016) Biosynthesis of silver nanoparticles using a Tamarix gallica leaf extract and their antibacterial activity. Mater Lett 176: 285-289.
- Chinnappan S, Kandasamy S, Arumugam S, Seralathan KK, Thangaswamy S, et al. (2018) Biomimetic synthesis of silver nanoparticles using flower extract of Bauhinia purpurea and its antibacterial activity against clinical pathogens. Environ Sci Pollut Res 25: 963-969.
- Ibraheim MH, Ibrahiem AA, Dalloul TR (2016) Biosynthesis of silver nanoparticles using Pomegranate juice extract and its antibacterial activity. Int J Appl Sci Biotechnol 4: 254.
- Lakshmanan G, Sathiyaseelan A, Kalaichelvan PT, Murugesan K (2018) Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L. assessment of their antibacterial and anticancer activity. Karbala. Int J Mod Sci 4: 61-68.
- Abiola GFA, Adewumi OD, Kabir OO, Adeyinka OA, Ojo PF (2019) Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia Pectinata (Willd.) C. Presl.) characterization and antimicrobial studies. Heliyon 5: 1-18.
- Alan KOL, Arthur AV, José JVSJ, Silvia KSE, Gerson N, et al. (2019) Green synthesis of silver nanoparticles using amazon fruits. Int J Nanosci Nanotechnol 15: 179-188.
- Lata R, Sourja G (2018) Metallic nanoparticle synthesised by biological route safer candidate for diverse applications. IET Nanobiotechnol 12: 392-404.
- Francis JO, Victor MK, Victor MW, Sanjay K, Bruno N, et al. (2019) Photochemical synthesis and catalytic applications of gold nanoplates fabricated using quercetin diphosphate macromolecules. ACS Omega 4: 6511-6520.
- Ponarulselvam S, Panneerselvam C, Murugan K, Aarthi N, Kalimuthu K, et al. (2012) Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac J Trop Biomed 2: 574-580.
- Kanchan K (2019) Applications of silver nanoparticles: A review. J Biol Chem Chron 5: 140-146.
- Shriniwas PP, Subhash TK (2020) Vitex negundo assisted green synthesis of metallic nanoparticles with different applications: A mini review. Patil and Kumbhar Future Journal of Pharmaceutical Sciences 6: 1-11.
- Kasyanenko N, Varshavskii M, Ikonnikov E, Tolstyko E, Belykh R, et al. (2016) DNA modified with metal nanoparticles preparation and characterization of ordered metal-DNA nanostructures in a solution and on a substrate. J Nanomater 1-12.
- Mohammadlou M, Maghsoudi H, Jafarizadeh-Malmiri H (2016) A review on green silver nanoparticles based on plants Synthesis, potential applications and eco-friendly approach. International Food Research Journal 23: 446-463.
- Xu L, Wang YY, Huang J, Chen CY, Wang ZX, et al. (2020) Silver nanoparticles Synthesis, medical applications and biosafety. Theranostics 10: 8996-9031.
- Dharmasoth RD, Ganga RB (2019) Qualitative Phytochemical Screening and FTIR Spectroscopic Analysis of Grewia Tilifolia (VAHL) Leaf Extracts. Int J Curr Pharm Re 11: 100-107.
- Ekaterina OM (2020) Silver nanoparticles mechanism of action and probable bio-application. J Funct Biomater 11: 1-26.
- Tiara A, Windri H (2021) The UV-VIS Spectrum Analysis From Silver Nanoparticles Synthesized Using Diospyros maritima Blume. Leaves Extract. Advances in Biological Sciences Research 14: 411-419.
- Akintelu SA, Bo Y, Folorunso AS (2020) A review on synthesis, optimization, mechanism, characterization, and antibacterial application of silver nanoparticles synthesized from plants. Journal of Chemistry 1-12.
- Amargeetha A, Velavan S (2018) X-ray Diffraction (XRD) and Energy Dispersive Spectroscopy (EDS) Analysis of Silver Page 2 of 5 Nanoparticles Synthesized from Erythrina Indica Flowers. Nanosci Technol 5: 1-5.
- Kumara V, Lakhawat SS, Kumar S, Chaudhary AA, Rudyni HA, et al. (2022) Rapid biogenic fabrication of silver nanoparticles using Ziziphus nummularia under optimised conditions and evaluation of their antimicrobial synergy. Digest Journal of Nanomaterials and Biostructures 17: 421-430.
- Guerrini L, Alvarez-Puebla RA, Pazos-Perez N (2018) Surface modifications of nanoparticles for stability in biological fluids. Materials 11: 1-28.
- Wang F, Cao S, Yan R, Wang Z, Wang D, et al. (2017) Selectivity/specificity improvement strategies in surface-enhanced raman spectroscopy analysis. Sensors 17: 1-26.
- Xia J, Li W, Sun M, Wang H (2022) Application of SERS in the Detection of Fungi. Bacteria and Viruses, Nanomaterials 12: 1-24.
- Mohammad A, Bosung K, Kevin DB, David N, Mary B, et al. (2016) Green synthesis and characterization of silver nanoparticles using Artemisia absinthium aqueous extract-A comprehensive study. Materials Science and Engineering C 58: 359-365.
- Satoshi H, Hiromitsu F, Takashi G, Daisuke H, Noritaka H, et al. (2020) Electron tomography imaging methods with diffraction contrast for materials research. Microscopy 69: 141-155.
- Li Z, Wang Y, Shen J, Liu W, Sun X (2014) The measurement system of nanoparticle size distribution from dynamic light scattering data. Optics and Lasers in Engineering 56: 94-98.
- Ma LN, Liu DJ, Wang ZX (2014) Gold nanoparticle-based dynamic light scattering assay for mercury ion detection. Chinese Journal of Analytical Chemistry 42: 332-336.
- Anike PV, Ferreyra M, Sónia G, Nuno CS, Beatriz A, et al. (2019) Studies on interaction of green silver nanoparticles with whole bacteria by surface characterization techniques. BBA- Biomembranes 1861: 1086-1092.
- Hu Z, Bao Q, Haocheng G, Kai J, Zhaoping L, et al. (2017) Characterization of Li-rich layered oxides by using transmission electron microscope. Green Energy & Environment 2: 174-185.
- Bin L, Sew Lay C, Dingyi Y, Sheot Harn C, Angela L (2022) Detection, identification and size distribution of silver nanoparticles (AgNPs) in milk and migration study for breast milk storage bags. Molecules 27: 1-21.
- Monica MR, Christina MS, John S, Shane RS, George LD, et al. (2021) The mechanism of cell death induced by silver nanoparticles is distinct from silver cations. Part Fibre Toxicol 18: 1-24.
- Yongjun H, Shao-Wei F, Yi-Ling C, Chin CL (2016) A reaction study of sulfur vapor with silver and silver–indium solid solution as a tarnishing test method. J Mater Sci Mater Electron 27: 10382-10392.
- Krishna DNG, John P (2022) Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS) Recent developments and challenges. Applied Surface Science Advances 12: 100332.
- Wilson D, Langell M (2014) XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Applied Surface Science 303: 6-13.
- Xiong J, Wu X, Xue Q (2013) One-step route for the synthesis of monodisperse aliphatic amine-stabilized silver nanoparticles. Colloids and Surfaces A Physicochemical and Engineering Aspects 423: 89- 97.
- Maxwell M, Kang T, Xinli Z, Chia S, Zhong Z (2015) Current Development of Silver Nanoparticle Preparation. Investigation. and Application in the Field of Medicine. Hindawi Publishing Corporation 12.
- Amit KM, Uttam CB (2016) Current status and future prospects of nano biomaterials in drug delivery. Nanobiomaterials in Drug Delivery 147-170.
- Prasanta KB, Subhadip D (2015) Effects and applications of silver nanoparticles in different fields. International Journal of Recent Scientific Research 6: 5880-5883.
- Adnan H, Inn-Kyu K (2015) Preparation of silver nanoparticles and their industrial and biomedical applications: A Comprehensive Review. Advances in Materials Science and Engineering 1-17.
- Rajasekharreddy P, Rani PU (2014) Biofabrication of Ag nanoparticles using Sterculia foetida L. seed extract and their toxic potential against mosquito vectors and HeLa cancer cells. Mater Sci Eng C 39: 203-212.
- Yeasmin S, Datta HK, Chaudhuri S, Malik D, Bandyopadhyay A (2017) In-vitro anti-cancer activity of shapecontrolled silver nanoparticles AgNPs): in various organ specific cell lines. J Mol Liq 242: 757-766.
- Krishnanand SI, Sekhar JC (2018) Biological synthesis of silver nanoparticles and their antimicrobial properties: A Review. Int J Curr Microbiol App Sci 7: 2896-2911.
- Aditya V, Swati D, Ninian PPP, Divakar K, Rama RB (2020) A review on synthesis, applications, toxicity, risk assessment, and limitations of plant extracts synthesized silver nanoparticles. NanoWorld Journal 6: 35-60
Citation: Poli V, Motireddy SR (2023) Review on Synthesis and Applications of Silver Nanoparticles, their Characterization and Toxicity Effects: Future Outlook. J Food Sci Nutr 9: 173.
Copyright: © 2023 Venkataramanaiah Poli, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

