
Selected Health Benefits of Spices (Garlic, Ginger, Turmeric)
*Corresponding Author(s):
Martha VergheseDepartment Of Food And Animal Sciences, Alabama Agricultural And Mechanical University, Normal, Alabama, United States
Tel:+1 2563724175,
Email:Martha.verghese@aamu.edu
Abstract
The use of spices has been dated back many centuries ago due to their health-promoting properties and their ability to preserve food. Research suggests that spices have the ability to prevent chronic diseases such as type 2 diabetes and obesity. The objectives of this study were to determine the selected health benefits of spices: garlic, ginger, and turmeric. Total Phenolic Content (TPC), Total Flavonoid Content (TFC), free radical scavenging activity by 1,1-Diphenyl-2Picrylhydrazyll (DPPH), Trolox Equivalent Antioxidant Capacity (TEAC), Ferric Reducing Antioxidant Power (FRAP) and inhibition of α- glucosidase, α-amylase, and lipase were evaluated. The results suggest that spices have great potential in decreasing chronic diseases due to their bioactive compounds. A product named Spiceola Bites was formulated along with the chemical analysis being conducted on the product. Total Phenolic Content (TPC), Total Flavonoid Content (TFC), free radical scavenging activity by 1,1-Diphenyl-2-Picrylhydrazyl (DPPH), Trolox Equivalent Antioxidant Capacity (TEAC), Ferric Reducing Antioxidant Power (FRAP) and inhibition of lipase and α-glucosidase and were evaluated on the Spiceola Bites extracted with water and ethanol. Results suggest incorporation of garlic, turmeric, and ginger into a functional food product (Spiceola Bite) may improve consumers’ antioxidant status. Recently, spices have gained a lot of attention in the food industry due to their health benefits.
Keywords
Enzymes, Obesity; Spices; Type 2 diabetes
Abbreviations
TPC: Total Phenolic Content
TFC: Total Flavonoid Content
DPPH: 1,1-Diphenyl-2Picrylhydrazyl
TEAC: Trolox Equivalent Antioxidant Capacity
FRAP: Ferric Reducing Antioxidant Power
GAE: Gallic Acid Equivalent
DW: Dry Weight
CE: Catechin Equivalent
TE: Trolox Equivalent
SBE: Spiecola Bites Extracted with Ethanol
SBW: Spiceola Bites Extracted with Water
Introduction
It has been suggested from research that spices have multiple bioactive compounds that have a positive effect on the human body along with some cancer-fighting abilities [1]. It has been reported that spices have been used for many centuries due to their known health benefits and antimicrobial activities. Spices are retrieved from the plant’s bulb, stem, and root which are usually found at the bottom of the plant. In addition to phytochemicals, spices also consist of antioxidants, minerals, vitamins, and essential oils that have many medicinal properties. Spices are known as a functional food. The compounds found in spices provide additional health benefits, beyond their nutritional value [2]. The health benefits attributed to spices are stemmed from medicinal properties including hypocholesterolemia, anti-atherogenic, and anti-obesity/thermogenic influence. Garlic, ginger, and turmeric specifically have anti-inflammatory, cardioprotective effects, and anti-diabetic properties through their blood cholesterol-lowering abilities [3].
Phytochemicals are secondary metabolites of plants. They provide them with a defense mechanism against outside influences such as predators and microorganisms. Common phytochemicals include polyphenols, flavonoids, anthocyanidins, and carotenoids [4]. It has been reported that the phytochemicals found within spices including gingerol, allicin, and curcumin are responsible for spices’ color and many health benefits [5]. It has been suggested that the phytochemicals found within spices may regulate the detoxification system of enzymes, and improve the immune system. Beyond spices’ medicinal properties, spices have antibacterial and antiviral capacities. Consumers that incorporate spices within their diet regularly may be able to improve their chemopreventive activities [6].
Functional food is a term that has gained popularity throughout the past couple of decades. They are used within the food industry to provide consumers with additional health benefits beyond their nutrition such as providing sources of protein, fats, and carbohydrates. Functional food products that have gained a significant amount of popularity are spices, probiotics/prebiotics, and polyunsaturated fatty acids [7]. The terminology of functional foods originates from Japan in the 1980s. This term is commonly misused because not regulated in many countries [8,7]. It has been suggested that functional foods aid in improving one’s health by reducing the risk of dyslipidemia, type 2 diabetes, cancer, and cardiovascular diseases [7,9-11].
Objectives
The objectives of this study were to develop a functional food product utilizing spices. Determination of the antioxidant potential of spices and the product developed from the spices. Determining selected health benefits of spices: Garlic, ginger, and turmeric and the product developed by evaluating the inhibition of selected metabolizing enzymes (α-glucosidase, α-amylase, and lipase).
Materials and Methods
All chemicals were obtained from Sigma Chemical Company, St. Louis, Mo. and Fisher Scientific Company, Waltham, Mass.
Determination of phytochemicals in spices and spiceola bites
Sample preparation and extraction of phenolics in spices: The three spices: Garlic, turmeric, and ginger were extracted with deionized distilled water or 80% ethanol. Triplicates were made for each spice during the aqueous and ethanolic extractions. The spices were stirred for 2 hours at 23oC. The samples were centrifuged using the Thermo Scientific ST8 Benchtop centrifuge (Waltham, MA) at 3000Xg at 4oC for 20 minutes. The samples were evaporated using a Buchi Rotavapor R-100 rotary evaporator (New Castle, DE) and filtered with Whatman no. 4 filter paper. The extracts were stored using a -20oC walk-in until analysis.
Sample preparation and extraction of phenolics in spiceola bites
Spiceola Bites are a granola-like treat that incorporated garlic, turmeric, and ginger at 2% of the total formulation and targets adolescents as a primary market. Spiceola Bites were formulated, freeze-dried, and powdered. Total phenolic and flavonoid content, antioxidant, and metabolizing enzyme inhibition assays were conducted on this product.
Spiceola Bite powder was extracted with water or ethanol. Spiceola Bite was grounded and put into 50mL of 25ºC H20 or 80% ethanol. It was then stirred for 2 hours using an orbital shaker and sonicated for 30 minutes. The sample was centrifuged for 20 minutes at 3000Xg for 20 minutes and was filtered. The samples were evaporated to dryness (BUCHI, Rotavapor); reconstituted. The samples were stored using a -20oC until analysis.
Determination of total phenolic and flavonoid contents in spice extracts and spiceola bite extracts
The total phenolic content was determined using the Folin-Ciocalteu method [12]. Slight changes were made to the method while using gallic acid as the standard. The extracts’ total phenolic content is expressed as mg Gallic Acid Equivalent (GAE) per 100mg dry weight.
The total flavonoid content was determined utilizing the colorimetric assay method [12]. Catechin was used as the standard. The extracts’ total flavonoid content is expressed as mg CE/100g dry weight.
Antioxidant potential of spice extracts and spiceola bite extracts
- 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) radical scavenging activity: The samples’ inhibition to scavenge the free radical was measured by the method published by [12]. The absorbance was read in a microplate reader (Synergy HT, Bio Tek Instruments, Winooski, VT, USA) at 517 nm with 30-minute intervals: 0, 30, 60, and 90 minutes.
- Determination of Ferric Reducing Antioxidative Potential (FRAP): The FRAP assay was determined using the method developed by [12]. The standard for this assay was made using ferrous sulfate. The absorbance was read at 593nm for 4 minutes at 1-minute intervals. The results of the sample extracts’ antioxidant potential are expressed as mg Fe+2 /g dry weight.
- Trolox Equivalent Antioxidant Capacity (TEAC): The sample extracts’ TEAC was measured by using the method by [13], where Trolox vitamin E analog was used as the standard. The absorbance was read at 734nm for 6 minutes at 1-minute intervals.
Determination of lipid and carbohydrate metabolizing enzyme inhibition
- Determination of α-amylase inhibition activity: The α-amylase inhibition activity of the sample extracts was determined using the method by Mutai et al. [14].
- Determination of α-glucosidase activity: α-glucosidase enzyme inhibition was evaluated from using the method Mutai et al., (14). The absorbance was read at 405nm. The data was reported in percentages using the calculation below.
Inhibition (%) = (Abscontrol–Abssample/Abscontrol) * 100
Determination of lipase inhibition activity
The lipase enzyme inhibition was evaluated using the modified version of the method from Mutai et al. [14], where 2,4 p, nitrophenyl butyrate (2,4 p-NPB) as a substrate.
Functional food product development
A functional food product, named Spiceola Bites, was reformulated using garlic, ginger, and turmeric.
Statistical analysis of spice extracts
All experiments were performed in triplicates and the data was recorded as means ± standard deviation. The factorial design for the chemical analysis portion of this experiment was 3 (garlic, ginger, and turmeric) ´2 (solvent extractions). One-way ANOVA was performed using SAS 9.2 version. Significant differences between means were determined using Tukey's studentized range test, and p ≤ 0.05 was regarded as significant.
Statistical analysis of spiceola bite extracts
All experiments were performed in triplicates and the data was recorded as means ± standard deviation. One-way ANOVA was performed using SAS 9.2 version. Significant differences between means were determined using Tukey’s studentized range test, and p≤0.05 was regarded as significant.
Results And Discussion
Figure 1, displays the total phenolic content of spices to determine the spices’ reducing ability with the presence of the FC reagent. Among the aqueous extracts, a significantly higher amount of phenolic compounds was found in the garlic and ginger extracts compared to the turmeric extract. The opposite trend was found among the ethanolic extracts where both ethanolic ginger and turmeric extracts yielded a significantly higher total phenolic content compared to the ethanolic garlic extract. There was over a three-fold increase in total phenolic content found in the ethanolic turmeric extract compared to the garlic extract. Many studies [15-17], have demonstrated that a lot of the antioxidative potential of plants stems from phenolics along with other vitamins and minerals. The results from figure 1, showed that the ethanolic extraction resulted in higher phenolic compounds with the exception of garlic. Due to spices containing mainly essential oils that are non-polar, utilizing an ethanolic extraction was more sufficient. The findings from figure 1, are in accordance with a study [18], where the ethanoic turmeric extracts had the highest total phenolic followed by turmeric and gingerol. This may be because the structure of alliin is a lot less stable compared to the structures of curcumin and gingerol [18]. 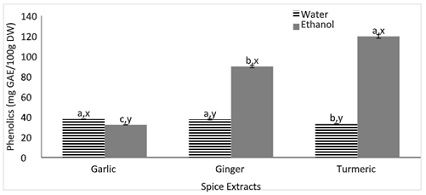
Figure 1: Total phenolic content of spices (garlic, ginger, and turmeric).
Note: Values (n=3) are means ± SEM; Bars with superscripts (ab) without a common letter have a significant difference between spices (p≤0.05). Bars with superscripts (xy) without a common letter have a significant difference between solvents (p≤0.05). GAE: Gallic Acid Equivalent; DW: Dry Weight.
Figure 2, displays the total flavonoid content of spices. Among the aqueous extracts, the aqueous ginger extract yielded a significantly higher TFC compared to both aqueous turmeric and garlic extracts. Although, the aqueous turmeric extract extracted more flavonoid compounds compared to the aqueous garlic extract. Among the ethanoic extracts, the ethanoic ginger extract yielded over a 4-fold increase in TFC compared to both turmeric and garlic extracts. The same trend from figure 1, was found in figure 2, where the ethanolic ginger extract resulted in a significantly higher total flavonoid content while the aqueous garlic extracted yielded in a significantly higher total flavonoid content compared to its aqueous extract. The hydroxyl groups found within spices are responsible for their total flavonoid content and part of their antioxidant content [19]. Due to spices being incorporated into dishes, they are usually consumed with heat treatment. It has been reported that heat treatment of spices along with their combination results in a higher phytochemical content [20]. 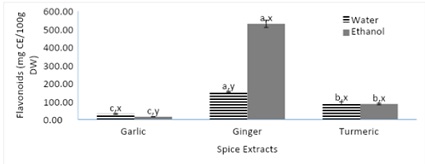
Figure 2: Total flavonoid content of spices (garlic, ginger, and turmeric).
Note: Values (n=3) are means ± SEM; Bars with superscripts (abc) without a common letter have a significant difference between spices (p≤0.05). Bars with superscripts (xy) without a common letter have a significant difference between solvents (p≤0.05). CE: Catechin Equivalent; DW: Dry weight.
Figure 3, displays the 2,2 Diphenyl-1-dicrahydrazyl radical scavenging (DPPH) of the spices (garlic, ginger, and turmeric). DPPH is a stable free radical whereupon reduction the dark color purple is changed to a pale-yellow color [21]. Overall all of the spice extracts exhibited DPPH percent inhibition. The same trend from figure 2, was found in figure 3 where both ginger extracts resulted in a significantly higher DPPH percent inhibition. Among the aqueous extracts, a 30% increase of DPPH percent inhibition was found between the aqueous ginger extract and turmeric extract. Among the ethanol extracts, the ethanol ginger extract had a significantly higher DPPH inhibition of 87% compared to both ethanolic turmeric and garlic extracts. All ethanoic extracts yielded a higher DPPH percent inhibition compared to their respective aqueous extracts. The finding of this study from the figure is in accordance with another study [22]. In that study the same trend was found among the ethanolic extracts of the spices where the DPPH percent inhibition are in the following order from least to greatest: Ginger > turmeric > garlic.
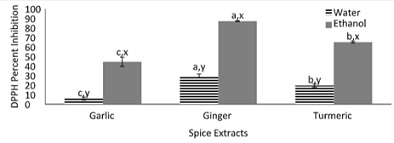 Figure 3: DPPH inhibition by spices (garlic, ginger, and turmeric).
Figure 3: DPPH inhibition by spices (garlic, ginger, and turmeric).
Note: Values (n=3) are means ± SEM; Bars with superscripts (abc) without a common letter have a significant difference between spices (p≤0.05). Bars with superscripts (xy) without a common letter have a significant difference between solvents (p≤0.05). DPPH: 2,2 Diphenyl-1-dicrahydrazyl radical scavenging.
Figure 4, displays the ferric reducing antioxidant power of spices (garlic, ginger, and turmeric). The FRAP assays determine the spices’ ability to reduce the oxidized form of iron (ferric) into the reduced form (ferrous) through the transfer of electrons. The aqueous garlic extract had a significantly higher ferric reducing ability compared to both aqueous ginger and turmeric extracts. The aqueous ginger extract had over a 3-fold higher FRAP value compared the aqueous turmeric extract. Both ethanolic turmeric and ginger extract yielded over a 70% significantly higher ferric reducing ability compared to the garlic extract. Out of the ginger and turmeric extracts, their ethanolic extracts were more successful in reducing the oxidized form of iron into its reduced form of ferrous while no significant difference was found between the garlic extracts. The findings from figure 4, are not in accordance with a study [23], which may have been attributed to the origin of the spices, storage, processing, and method of extraction. These factors have a significant impact on spices’ reducing ability and antioxidant content.
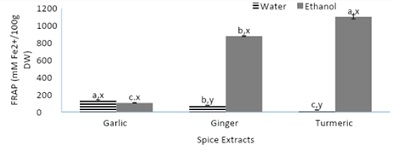 Figure 4: Ferric reducing antioxidant power of spices (garlic, ginger, and turmeric).
Figure 4: Ferric reducing antioxidant power of spices (garlic, ginger, and turmeric).
Note: Values (n=3) are means ± SEM; Bars with superscripts (abc) without a common letter have a significant difference between spices (p≤0.05). Bars with superscripts (xy) without a common letter have a significant difference between solvents (p≤0.05). FRAP- Ferric reducing antioxidant power. Fe (II): Ferrous Iron; DW: Dry Weight.
Figure 5, displays the Trolox equivalent antioxidant capacity of spices to evaluate the spice’s ability to scavenge the ABTS radical. Starting with the first comparison, among the aqueous extracts, the ginger extract had a significantly higher Trolox equivalence antioxidant capacity as compared to both garlic and turmeric aqueous extracts. The aqueous garlic extract had over a 30% higher Trolox equivalence compared to the turmeric extract. Among the ethanolic extracts, both ethanoic turmeric and ginger extracts had over a 24-fold higher reducing ability compared to the garlic ethanolic extract. With the exception of garlic, all ethanolic extracts (ginger and turmeric) had a significantly higher TEAC compared to their respective aqueous extracts. The results from figure 5, show that the ethanolic extracts of turmeric and ginger have a high reducing ability through scavenging the ABTS radical. This may be attributed to the complex structures of the phytochemicals, curcumin, and gingerol, found within turmeric and ginger.
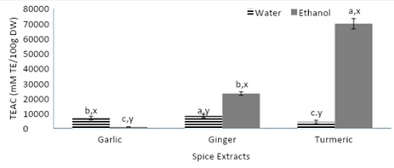 Figure 5: Trolox equivalent antioxidant capacity of spices (garlic, ginger, and turmeric).
Figure 5: Trolox equivalent antioxidant capacity of spices (garlic, ginger, and turmeric).
Note: Values (n=3) are means ± SEM; Bars with superscripts (abc) without a common letter have a significant difference between spices (p≤0.05). Bars with superscripts (xy) without a common letter have a significant difference between solvents (p≤0.05). Trolox Equivalent Antioxidant Capacity (TEAC).TE: Trolox Equivalence; DW: Dry Weight.
The graph for α-amylase inhibition by spices (garlic, ginger, and turmeric) is not shown. α-amylase is an enzyme that is induced upon consumption of carbohydrates. Due to the western diet being high in simple carbohydrates, possible inhibition of this enzyme may be beneficial in preventing chronic diseases such as type 2 diabetes and obesity [24]. No inhibition was reported among the aqueous and ethanolic extracts. The presence of the spices may have not interfered with the binding of the substrate to the enzyme in this assay. The results from figure 6 show that non-competitive enzyme inhibition may have taken place, while competitive inhibition was desired [25].
Figure 6, displays the α-glucosidase inhibition by spices (garlic, ginger, and turmeric). Among the ethanolic extracts α-glucosidase inhibition was reported, while no α-glucosidase inhibition was reported among the aqueous extracts. The highest α-glucosidase percent inhibition was found within the ginger extract. The ethanolic ginger extract had over an 18% higher enzyme percent inhibition compared to the ethanolic turmeric extract. The ethanolic turmeric extract had a significantly higher enzyme percent inhibition of 61% compared to the garlic extract. The results from figure 6, show possible implications of spices being able to reduce blood glucose levels, which may be beneficial in preventing chronic diseases.
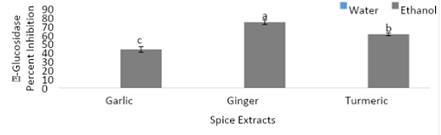 Figure 6: α-glucosidase inhibition by spices (garlic, ginger, and turmeric).
Figure 6: α-glucosidase inhibition by spices (garlic, ginger, and turmeric).
Note: Values (n=3) are means ± SEM; Bars with superscripts (abc) without a common letter have a significant difference between spices (p≤0.05).
Figure 7, displays the lipase inhibition by spices (garlic, ginger, and turmeric). The lipase enzyme is responsible for the hydrolysis of triglycerides into free fatty acids. Inhibition of the lipase enzyme may be beneficial towards preventing obesity within individuals. Lipase percent inhibition was reported among both aqueous and ethanolic extracts. In figure 7, the opposite trend was found compared to the previous figures where the aqueous extracts of the spices resulted in a higher lipase percent inhibition. Overall the aqueous garlic and ginger extracts resulted in a significantly higher lipase percent inhibition followed by the aqueous turmeric extracts.
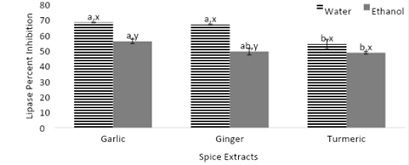 Figure 7: Lipase inhibition by spices (garlic, ginger, and turmeric).
Figure 7: Lipase inhibition by spices (garlic, ginger, and turmeric).
Note: Values (n=3) are means ± SEM; Bars with superscripts (abc) without a common letter have a significant difference between spices (p≤0.05). Bars with superscripts (xy) without a common letter have a significant difference between solvents (p≤0.05).
Figures 8-13, display the chemical analysis that was conducted on the Spiceola Bites, a granola-like product formulated with garlic, ginger, and turmeric. In each of the assays conducted, the spciecola bite extracted with ethanol results in higher phytochemical content, antioxidant capacity, reducing ability, and enzyme inhibition. The Spiceola Bites were formulated to be marketed toward adolescents. The results show that the Spiceola Bites have a great potential in decreasing the risk and prevalence of obesity and type 2 diabetes in individuals, especially adolescents.
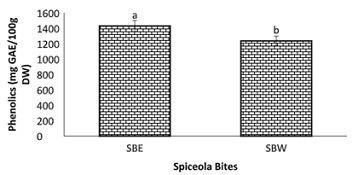 Figure 8: Total Phenolic Content of Spiceola Bites.
Figure 8: Total Phenolic Content of Spiceola Bites.
Note: Values (n=3) are means ± SEM; Bars with superscripts (abc) without a common letter have a significant difference between spiceola bite extracts (p≤0.05). Abbreviations: GAE: Gallic Acid Equivalents; SBE: Spiecola Bites extracted with Ethanol; SBW: Spiceola Bites Extracted with water.
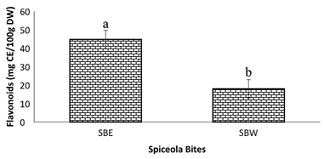 Figure 9: Total flavonoid content of spiceola bites.
Figure 9: Total flavonoid content of spiceola bites.
Note: Values (n=3) are means ± SEM; Bars with superscripts (abc) without a common letter have a significant difference between spiceola bite extracts (p≤0.05). Abbreviations: CE: Catechin Equivalents; SBE: Spiecola Bites extracted with ethanol; SBW: Spiceola Bites Extracted with water; DW: Dry weight
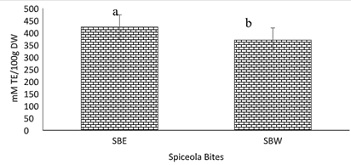 Figure 10: Trolox equivalent antioxidant capacity of spiceola bites.
Figure 10: Trolox equivalent antioxidant capacity of spiceola bites.
Note: Values (n=3) are means ± SEM; Bars with superscripts (abc) without a common letter have a significant difference between spiceola bite extracts (p≤0.05). Abbreviations: TE: Trolox Equivalents; SBE: Spiecola Bites extracted with ethanol; SBW: Spiceola Bites Extracted with water; DW: Dry Weight.
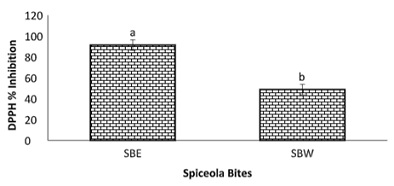 Figure 11: DPPH percent inhibition by spiceola bites.
Figure 11: DPPH percent inhibition by spiceola bites.
Note: Values (n=3) are means ± SEM; Bars with superscripts (abc) without a common letter have a significant difference between spiceola bite extracts (p≤0.05). Abbreviations: DPPH: 2,2-Diphenyl-1-Picrylhydrazyl; SBE: Spiecola Bites extracted with ethanol; SBW: Spiceola Bites Extracted with water.
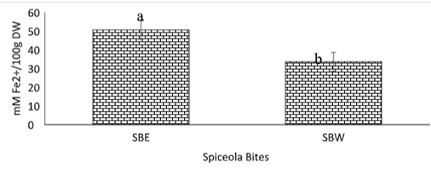 Figure 12: Ferric reducing antioxidant power of spiceola bites.
Figure 12: Ferric reducing antioxidant power of spiceola bites.
Note: Values (n=3) are means ± SEM; Bars with superscripts (abc) without a common letter have a significant difference between spiceola bite extracts (p≤0.05). Abbreviations: FE2+- Ferrous Sulfate; SBE: Spiecola Bites extracted with Ethanol. SBW: Spiceola Bites Extracted with water; DW: Dry Weight.
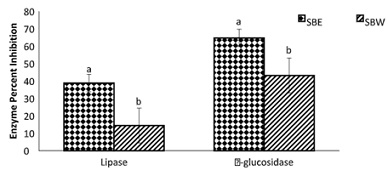 Figure 13: Inhibition of lipase and α-glucosidase activities by spiceola bites.
Figure 13: Inhibition of lipase and α-glucosidase activities by spiceola bites.
Note: Values (n=3) are means ± SEM Bars with superscripts (abc) without a common letter have a significant difference between spiceola bite extracts (p≤0.05). SBE: Spiecola Bites extracted with ethanol; SBW: Spiceola Bites Extracted with water.
Discussion
The Total Phenolic Content (TPC), Total Flavonoid Content (TFC), 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) free radical scavenging, Trolox Equivalent Antioxidant Capacity (TEAC), Ferric Reducing Antioxidant Power (FRAP), α-amylase, α-glucosidase, and lipase enzyme inhibition was measured from the spice extracts and Spiceola Bite extracts. The extraction method of both extracts had a great impact on the results, which demonstrated that the use of 80% ethanol was more sufficient in extracting the phytochemical compounds in the extracts. The findings of this study are in accordance with a study [26], where the ethanolic extractions of ginger and turmeric resulted in higher bioactivity compared to the aqueous extracts. The phytochemicals found in ginger and turmeric are more stable than the phytochemicals found in garlic due to their presence of benzoic rings, double bonds, and hydroxyl groups.
Conclusion
To conclude, the results from this study, demonstrate that spices and the Spiceola Bites functional food product has great potential in preventing chronic diseases within individuals. Overall, the ginger extracts exhibited a higher antioxidant content while the opposite trend was found among the garlic extracts. The results from the assays performed on the Spiceola Bites also demonstrate that spices combined in a food matrix have a great synergistic effect in regards to phytochemical content and antioxidant capacity. Due to blood glucose levels and free fatty acids being significantly elevated within diabetic and obese individuals, this study investigated the spices’ and Spiceola Bites’ ability to inhibit the metabolizing enzymes: α-amylase, α-glucosidase, and lipase. The results showed promising results towards possibly decreasing blood glucose and free fatty acid levels within individuals. Spices should be incorporated into more functional foods within the food industry to improve the prevalence of chronic diseases.
Future Work or Recommendations
Future work will involve determining the anti-diabetic potential of spices using a cell culture model.
References
- Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Alvarez JA (2011) Spices as functional foods. Crit Rev Food Sci Nutr51: 13-28.
- Dini I (2018) Food Quality: Balancing Health and Disease. Academic Press, Massachusetts, USA.
- Srinivasan K (2019) Cardio protective influence of dietary spices mediated through their hypolipidemic and antioxidant potential. Oxidative Stress in Heart Diseases 46: 173-189.
- Jimenez-Garcia SN, Vazquez-Cruz MA, Garcia-Mier L, Contreras-Medina LM, Guevara-González RG, et al. (2018) Phytochemical and pharmacological properties of secondary metabolites in berries. Therapeutic Foods 397-427.
- Guldiken B, Ozkan G, Catalkaya G, Ceylan FD, Ekin Yalcinkaya I, et al. (2018) Phytochemicals of herbs and spices: Health versus toxicological effects. Food Chem Toxicol 119: 37-49.
- Lampe JW (2003) Spicing up a vegetarian diet: Chemopreventive effects of phytochemicals. Am J Clin Nutr78: 579-583.
- Granato D, Barba FJ, Kovacevik DB, Lorenzo JM, Cruz AG, et al. (2020) Functional foods: Product development, technological trends, efficacy testing, and safety. Annu Rev Food Sci Technol 11: 93-118.
- Ye Q, Georges N, Selomulya C (2018) Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends in Food Science & Technology 78: 167-179.
- Brown L, Caligiuri SP, Brown D, Pierce GN (2018) Clinical trials using functional foods provide unique challenges. Journal of Functional Foods 45: 233-238.
- Cassidy YM, McSorley EM, Allsopp PJ (2018) Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. Journal of Functional Foods 46: 423-439.
- Mak KK, Tan JJ, Marappan P, Balijepalli MK, Choudhury H, et al. (2018) Galangin’s potential as a functional food ingredient. Journal of Functional Foods 46: 490-503.
- Shackelford L, Mentreddy SR, Cedric S (2009) Determination of total phenolics, flavonoids and antioxidant and chemopreventive potential of basil (Ocimum basilicum L. and Ocimum tenuiflorum L.). International Journal of Cancer Research 5: 130-143.
- Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA (1996) Antioxidant activities of carotenes and xanthophylls. FEBS Letters 384: 240-242.
- Mutai E, Vizcarra J, Walker LT, Verghese M (2015) Antioxidant, enzyme inhibitory and anti-obesity potential of sorrel calyx extracts in 3T3-L1 adipocytes. Food and Nutrition Sciences 6: 452.
- Wang H, Cao G, Prior RL (1996) Total antioxidant capacity of fruits. J Agric Food Chem 44: 701-705.
- Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, et al. (1999) Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem47: 3954-3962.
- Kaur C, Kapoor HC (2001) Anti-oxidant activity and total phenolic content of some Asian vegetables. International Journal of Food Science and Technology37: 153-161.
- Temitope AO, Olufemi AG, Alaba FT (2010) Effect of heat treatment on antioxidant activity of some spices. Continental J Food Science and Technology4: 53-59.
- Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13: 572-584.
- Otunola GA, Afolayan AJ (2013) Evaluation of the polyphenolic contents and some antioxidant properties of aqueous extracts of garlic, ginger, cayenne pepper and their mixture. Journal of Applied Botany and Food Quality 86: 66-70.
- El-Refai AA, Ghoniem GA, El-Khateeb AY, Hassaan MM (2018) Eco-friendly synthesis of metal nanoparticles using ginger and garlic extracts as biocompatible novel antioxidant and antimicrobial agents. Journal of Nanostructure in Chemistry8: 71-81.
- Panpatil VV, Tattari S, Kota N, Nimgulkar C, Polasa K (2013) In vitro evaluation on antioxidant and antimicrobial activity of spice extracts of ginger, turmeric and garlic. Journal of Pharmacognosy and Phytochemistry 2: 143-148.
- Azzini E, Durazzo A, Foddai MS, Temperini O, Venneria E, et al. (2014) Phytochemicals content in Italian Garlic bulb (Allium sativum L.) Varieties. Journal of Food Research3:
- Kopp W (2019) How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes12: 2221-2236.
- Lewis T, Stone WL (2021) Biochemistry, Proteins Enzymes. In Stat Pearls. Stat Pearls Publishing.
- Mushtaq Z, Nadeem MT, Arshad MU, Saeed F, Ahmed MH, et al. (2019) Exploring the biochemical and antioxidant potential of ginger (Adric) and turmeric (Haldi). International Journal of Food Properties 22: 1642-1651.
Citation: Nash D, Kaur R, Ward V, Hester F, Willis S, et al. (2022) Selected Health Benefits of Spices (Garlic, Ginger, Turmeric). J Food Sci Nutr 8: 135.
Copyright: © 2022 Dawn Nash, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

