
The Impact of Diet on Amphipod, Parhyale Hawaiensis, Production
*Corresponding Author(s):
Susan LaramoreFlorida Atlantic University, Harbor Branch Oceanographic Institute, 5600 US 1 North, Fort Pierce, FL 34946, United States
Email:slaramo1@fau.edu
Abstract
Amphipods have shown to have potential as a live prey item for use in aquaculture operations. The amphipod, Parhyale hawaiensis, was cultured for four weeks using two types of feed, dried Ulva lactuca, and a commercially available shrimp diet. There was a 4.5-fold increase in the final mean population density in P. hawaiensis fed the shrimp diet compared to a 1.5-fold increase in those fed U. lactuca. Juveniles were the most abundant stage in both treatments, followed by hatchlings, then adults in the U. lactuca treatment, and by adults, then hatchlings in the shrimp dietary treatment. There was no significant difference in juvenile or hatchling populations, but the adult population was 14-fold higher in the shrimp diet treatment. The U. lactuca and the shrimp diet were both high in protein, but the lower lipid content of U. lactuca likely accounted for the difference in performance seen. This research demonstrates that although U. lactuca alone can be used to rear P. hawaiensis, production will be increased using a diet with a more complete nutritional profile.
Keywords
Marine fish aquaculture; Parhyale hawaiensis; Ulva lactuca;
Introduction
The marine fish aquaculture industry utilizes a variety of live food organisms, such as artemia, rotifers, copepods, and mysid shrimp, in the production of both fish produced for consumption and ornamental species [1,2]. However, live feeds, such as artemia and rotifers, although whilst easily cultured, are not a natural food source for fish, lacking key nutrients, such as Polyunsaturated Fatty Acids (PUFA) and therefore require enrichment prior to feeding [3].
Live feed is especially important to fish during the larval and juvenile stages, and most fish species reared in aquaculture rely on live feeds during these early stages [4,5]. Larvae possess a rudimentary digestive system and lack certain intestinal enzymes and secretions found in the adult digestive tract [4,6,7]. Live prey is more digestible than formulated diets, increasing nutrient absorption in larvae, leading to enhanced growth and survival. Live feeds are constantly available in the water column and their movement simulates natural feeding behaviors, whereas commercial diets tend to sink to the bottom over time and, if not eaten immediately, leach nutrients and lose palatability, causing fish to lose interest [4].
There is an increased interest in the culture of marine amphipods for use as a natural live food source for fish. A wide variety of fish, including commercially cultured food fish and ornamentals, have been shown to consume caprellid amphipods [2,8]. Fish (Salmo salar, Gadus morhua, Poecilia reticulata, Hippocampus erectus), and cephalopods (Robsonella fontaniana, Octopus maya, Sepia officinalis) have successfully been reared using live [9-12] and frozen amphipods [13]. Dried amphipod meal has also been incorporated into dry diets as a replacement for fish or krill meal without affecting production [14,15]. Amphipods are high in protein, and nutritionally rich in omega-3 Polyunsaturated Fatty Acids (n-3 PUFA), such as Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA), found to be essential for growth and development [4,11,16].
In addition to their potential use as a live or dried feed ingredient for aquaculture, recent research has explored their use as a model organism for studies involving disease resistance and crustacean host defense mechanisms [17]. Amphipods are also widely used in ecotoxicity studies, like sediment toxicity bioassays [18], further strengthening their potential for aquaculture [4,19,20].
Despite the potential uses of amphipods, little emphasis has been placed on establishing optimal abiotic, biotic, and system culture conditions, which will need to be modified for different amphipod species. Some research has been conducted with caprellid [11,21,22] and gammarid species [5,23-26], but it is limited in scope, and may not be suited for larger scale culture.
Parhyale hawaiensis is a hyalidae amphipod and member of the Malacostraca clade found in marine intertidal zones [27]. This species is commonly found among mangroves, macroalgae, and mud flats in coastal Florida waters, including the Indian River Lagoon [27,28].
The amphipod P. hawaiensis naturally propagates in the Ulva lactuca tanks that are part of the Land-Based Integrated Multi-trophic Aquaculture (LB-IMTA) system at Florida Atlantic University’s Harbor Branch Oceanographic Institute (FAU-HBOI). Integrated Multi-Trophic Aquaculture (IMTA) systems are used to cultivate fed (e.g., fish), extractive (e.g., bivalves), and assimilative species (e.g., seaweed) [29]. The FAU-HBOI LB-IMTA uses a centralized filtration system to deliver controlled volumes of selected pretreated waste streams to each system component, resolving water quality and flow distribution issues seen with traditional closed IMTA systems [30]. The seaweed cultivated in this system (Ulva lactuca) has been shown to improve water quality through the uptake of nitrogenous compounds, reducing ammonia levels in the system. In addition to improving water quality, the U. lactuca produced has been used as both a replacement and supplement to commercial diets for shrimp [31,32], sea urchins, sea cucumbers and fish (unpublished data) produced in the system.
Commercial seaweed production is increasing globally and serves as an important nutritional source for animal feed, decreasing the dependency of commercial feed pellets as the primary source of protein and nutrition [33]. Seaweed has become a component of both human and animal diets, utilized either in fresh or dried form. Seaweeds, including U. lactuca, are high in fiber and protein, and are relatively low in lipids [31,32,34-38]. Ulva species are also a good source of vitamins, including E, C, A, K, and some B vitamins [34,39-41], carotenoids [31,32,34,38,40,41] macro minerals such as magnesium, sulfur, calcium, potassium, and trace minerals, such as copper, iron, manganese, and zinc [32,34,37,39,40]. Seaweeds are high in polyunsaturated essential fatty acids (PUFA) [31,35-38,41], however, due to their low lipid content they might not provide adequate nutrition compared to commercially available aquaculture diets with a higher lipid content. Studies with shrimp have shown that replacement of a fish meal-based diet with an Ulva-based diet is inadequate when more than 50% of the diet is replaced by Ulva sp. [31,42,43].
Although amphipods are detrivorous in nature [27,28], they readily accept fresh or dried Ulva in a research setting [44], as well as commercial feed [26]. The presumptive diet of the Parhyale hawaiensis found in the U. lactuca tanks in the FAU-HBOI LB-IMTA system consists of fresh Ulva and associated epiphytes. Moreover, it is doubtful that facilities interested in amphipod culture would have a ready source of fresh Ulva to use as a food source. Even though seaweed can be stored for several years if dried properly, some of its nutritional content, such as vitamin C and PUFAS, might be lowered in the process [45]. The use of dried Ulva meal as a potential food source may be an acceptable alternative to fresh seaweed but might not be a nutritionally complete diet due to Ulva’s low lipid content compared to a pelleted diet.
The present study was conducted to evaluate the effect of two diets, dried U. lactuca and commercially available shrimp diet, for use in the production of P. hawaiensis.
Materials And Methods
Experimental design
The 4-week study was conducted at the aquaculture facility located at FAU-HBOI. The systems used consisted of six 60-L circular fiberglass tanks with eight 1-mm mesh fiberglass screens used as an artificial substrate extending the depth of the tank in a radial pattern (Figure 1a). This system had been designed and used previously to culture amphipods. The screens were attached to rods connected to a central disc on top, hung from top to bottom, and kept in place by weighted bottom support pipes (Figure 1b). The tanks were equipped with a central 7.62 cm diameter airlift and two air diffusers, and were fitted with a 2.54 cm drainpipe at the bottom, used for harvest at experimental termination. Finally, the tanks were filled with 55-L of UV filtered salt well water and stocked with 15 g (approximately 192 individuals) adult amphipods each. Amphipods were obtained from the LB-IMTA ex-situ biofloc tank at FAU-HBOI and sieved on a 500-micron screen to ensure that only adults were collected.
 Figure 1: Amphipod culture system a) 60-L tanks and b) fiberglass screens.
Figure 1: Amphipod culture system a) 60-L tanks and b) fiberglass screens.
The two treatments (N=three replicates) consisted of tanks fed Zeigler PL Raceway 40-9 shrimp pellets (400-600 and 600-850 microns) or dried Ulva. The LB-IMTA produced Ulva was collected, rinsed with fresh well water, squeezed dry, then dried in an oven at 65°C for approximately 24 hours prior to use. The amount of daily feed was based on the feed rate for similarly sized juvenile shrimp, which was determined to be 5% of their body weight. The amphipods were fed twice daily, each morning and evening.
Water quality
Temperature (28.4 + 1.0 °C), salinity (32.4 + 1.4 ppt), and dissolved oxygen (6.8 + 0.4 mg L-1) were measured daily using a YSI model 85 (Yellow Springs, OH, USA), and pH (8.0 + 0.05) with a YSI Ecosence PH100 (Yellow Springs, OH, USA). Nitrite (0.65 + 0.74 mg L-1), ammonia (0.15 + 0.17 mg L-1), and alkalinity (171 + 15) were measured twice weekly. Ammonia (TAN) and nitrite (NO2) were measured by colorimetry (HACH DR2500, Method 8155 and 8192). Alkalinity (mg/L as CaCO3) was measured using the digital titration method (HACH Method 8203). A 10% water exchanges were actually performed.
Amphipod production
At the end of four weeks, all tanks were drained, and the amphipods were collected. The total number of amphipods were counted, then subdivided based upon total body length and the presence of secondary sexual characteristics, and lastly recorded as either hatchling (1.5 mm), or adult (>5mm).
Chemical analysis
Proximate analysis of oven dried Ulva (N=3) was conducted by Midwest Laboratories Inc., (Omaha, NE, USA) using the following methods: Crude protein, by combustion, with 6.25 as a conversion factor to calculate protein (AOAC 990.03, 2006), crude fat (AOAC 2003.05, 2006), crude fiber (AOAC ba 6a-05, 2006), and ash (AOAC 942.05, 2006). Proximate analysis of the commercially available shrimp diet fed, PL Raceway 40-9 shrimp feed, is found at www.zeiglerfeed.com (Zeigler Bros., Inc,. Gardners, PA) and was not analyzed for this study.
Statistics
Statistical analysis of total density and subpopulations was performed using a one-way analysis of variance (ANOVA) with a significance level of (P < 0.05).
Results
Ulva meal and feed composition
The proximate compositions of dried Ulva and pelleted feed are presented in (Table 1). The pelleted feed contained significantly higher levels of protein (40.0%) and lipids (9.0%) compared to the Ulva meal (34.1% protein, 2.04% lipid); whereas the Ulva meal contained significantly higher levels of fiber (15.9%) and ash (29.8%) compared to the pelleted feed (3.0% fiber, 13.0% ash).
|
|
Fat |
Protein |
Fiber |
Ash |
|
U. lactuca |
1.4+0.9% |
32+5.5% |
12+7% |
24+1% |
|
Shrimp feed |
9% |
40% |
3% |
13% |
|
|
|
|
|
|
Table 1: Proximate composition of U. lactuca (% dry weight basis) and shrimp feed (Zeigler PL 40-9).
Values for U. lactuca are given as a mean + SD (n = 3); values for shrimp feed used are provided by Zeigler Brothers Inc (Gardners, PA).
Amphipod production
- Mean total density
No significant differences were seen between treatments for mean total density (P = 0.089) (Figure 2). The average amphipod density in tanks fed the pelleted diet was three times the density of amphipods fed the Ulva meal (967 vs 313), but variation among the pelleted tank treatments was also high (393-1320 individuals), compared to that seen in the dried Ulva treatment (219-368 individuals).
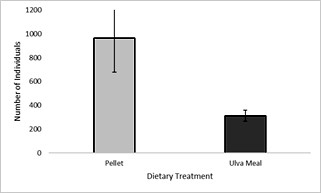 Figure 2: Mean density + SD (n=3) of P. hawaiensis fed two different diets for four weeks.
Figure 2: Mean density + SD (n=3) of P. hawaiensis fed two different diets for four weeks.
A significant difference was seen between the average mean adult density (P = 0.05). The average number of adults fed the pelleted diet was 350, compared to 25 for amphipods fed the Ulva meal (Figure 3). No significant difference in the number of hatchlings (P = 0.129) or juveniles (P = 0.199) was seen between the pelleted and the Ulva meal dietary treatments, nonetheless the average number of both hatchlings and juveniles were higher in the pelleted diet treatments. For both treatments, the number of juveniles was higher than the number of either hatchings or adults.
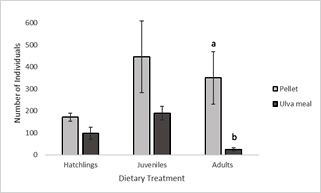 Figure 3: Mean density + SD (n=3) of P. hawaiensis at various life stages fed two different diets for four weeks. Statistical differences (P < 0.05) are denoted by different letters.
Figure 3: Mean density + SD (n=3) of P. hawaiensis at various life stages fed two different diets for four weeks. Statistical differences (P < 0.05) are denoted by different letters.
- Population density
Statistical differences in the population dynamics (P = 0.014) were seen, with adults comprising only 8.5% of the population in the Ulva dietary treatment, compared to 35% in the commercial diet treatment (Figure 4). Although the percentage of adults differed between the two treatments (P = 0.005), the percentage of juveniles (P = 0.077), and hatchlings (P = 0.455) was not. In the pelleted dietary treatment, juveniles made up the highest percentage of amphipods, followed by adults, then hatchlings, while in the Ulva dietary treatment, the highest percentage was juveniles, followed by hatchlings, then adults.
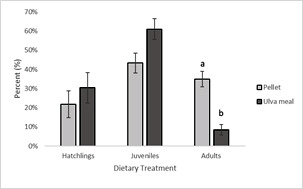 Figure 4: Ratio of three different life stages of P. hawaiensis (n=3) fed two dietary treatments for four weeks. Statistical differences (P < 0.05) are denoted by different letters.
Figure 4: Ratio of three different life stages of P. hawaiensis (n=3) fed two dietary treatments for four weeks. Statistical differences (P < 0.05) are denoted by different letters.
Discussion
The use of marine amphipods as a live food source to culture ornamental species has recently gained interest. For this to be a viable economic endeavor the effects of various abiotic and biotic factors need to be considered. This study sought to determine whether a plant-based diet, consisting of dried Ulva lactuca meal, or a commercially available shrimp diet would be preferrable to produce the amphipod Parhyale hawaiensis. The results of this study show that although dried U. lactuca meal can be used to successfully produce P. hawaiensis, feeding a commercially available pelleted shrimp diet would be preferable.
Despite the average total amphipod density being numerically higher in treatments fed the pelleted shrimp diet, the difference was not significant, due to the low amphipod density that occurred in one of the three replicate treatments fed the pelleted diet. The three-fold lower total density seen with one of the pelleted diet replicates could not be explained by decreased water quality or changes in water chemistry. The temperature was maintained at 28°C in all tanks. Daily recorded salinity, pH, and dissolved oxygen levels were similar in all tanks and were within normal parameters for crustacean culture. Recorded water chemistry measurements were also similar between tanks, and though ammonia and nitrite levels were slightly higher in tanks fed the commercial shrimp diet, there was no significant difference between those tanks and tanks fed the dried Ulva diet. Nitrite levels were highest in week three (over 1 mg/L), reflecting the natural nitrogen cycle progression, but this increase occurred in all tanks, despite daily water exchanges. As water chemistry was monitored twice a week, it is possible that an ammonia or nitrite spike may have been missed. It is more likely that some unknown factor, such as disease (i.e., bacteria), could have been responsible for the low density seen, but no samples of tank water were taken for bacteria analysis during the experiment. It is of interest to note that the tank with the lowest overall density of those fed the pelleted diet varied from the other two replicate tanks in having similar numbers of hatchlings, but fewer juveniles and adults, indicating that juveniles and adults were disproportionately affected.
Along with a higher total numerical density, the density of all three life stages (hatchlings, juveniles, and adults) was higher in treatments fed the pelleted diet, though this was only significant for adults. Considering that the density and proportion of adults were significantly higher in the tanks fed the shrimp diet, suggests that initial reproduction rates were higher in this treatment group, and that a brood-to-brood life cycle was achieved within four weeks. Juveniles comprised the highest percentage of assigned life stages and were found in higher numbers in both treatments, indicating that the hatchlings that were produced within this study grew in both treatment groups. The average number of hatchlings was similar between the two groups, but the higher proportion of hatchlings and juveniles in the Ulva treatment group, and the lower numbers of juveniles and adults indicates that this diet led to reduced growth and survival compared to the shrimp diet. As the average number of adults was 7% of those fed the pelleted diet, it is unclear whether the complete life cycle was achieved in the Ulva treatment; the few adults seen (~25) may have been the remnants of the initial starting population of 200.
Density and population differences may be explained by the fact that the pelleted shrimp diet had a more complete nutritional profile than the Ulva diet. Although protein levels were similar between diets, lipids were much lower in the Ulva meal, which likely impacted reproduction as well as growth. Lipids, particularly the PUFA, are necessary for enhanced egg production [46]. Highly Unsaturated Fatty Acids (HUFA), specifically Arachidonic Acid (ARA), Eicosapentaenoic Acid (EPA), and Docosahexaenoic Acid (DHA) have been confirmed to be necessary for vitellogenesis, embryogenesis, and pre-feeding larval development in shrimp [46,47].
Few dietary studies have been conducted with amphipods and, to our knowledge, none have compared an Ulva sp. diet to a commercially available diet. Alberts-Hubatsch [25] conducted an amphipod plant-based dietary study while Vargas-Abúndez [26] compared three commercial diets. The amphipods, Gammarus locusta, fed a plant-based diet of either dried Ulva spp., carrot leaf, or blue lupin for 10 weeks had significantly higher growth when fed the blue lupin diet compared to an Ulva spp. diet [25]. Survival, however, was significantly higher in G. locusta fed carrot leaf compared to both blue lupin and Ulva, (49% vs 14% and 5%, respectively), which was thought to have been due to the carotenoid levels found in carrot leaf [25]. Of the three diets, carrot leaf also had the highest amounts of PUFA, but neither carrot leaf nor blue lupin had detectable levels of EPA, DPA or ARA; no proximate analysis was conducted. Vargas-Abúndez [26] saw no significant growth differences of juvenile P. hawaiensis fed three commercially available diets (two shrimp, one fish), for 30 days, but did not report survival and no dietary analysis was conducted [48].
Studies with shrimp fed partial or full Ulva sp. replacement diets have shown decreased production. Shrimp fed only wet Ulva clathrata for six weeks showed no significant difference in survival compared to those fed a pelleted diet but saw a significant decrease in growth (final weight, weight gain) [36]. Laramore [31] saw no difference in survival with either a 25% or 50% replacement of a shrimp pelleted diet with wet Ulva lactuca, but a significant decrease in final weight compared to shrimp fed a pelleted diet exclusively. Pallaoro [43] showed that shrimp fed only U. lactuca had significantly less survival (16.7% vs 86.7%) than partial replacement diets, and both a 75% and 100% replacement diet resulted in lower growth (final weight, weight gain) [49]. A similar trend was seen in the present study conducted with P. hawaiensis, although dried Ulva, rather than wet, was compared to a pelleted shrimp diet. While not significant, higher numbers of P. hawaiensis were produced in the shrimp diet treatment, and a significantly higher number of adults were produced, indicating that reproduction was enhanced, and survival and growth of progeny was increased by feeding a more nutritionally complete diet [50,51].
Conclusion
The amphipod, P. hawaiensis can be reared on a diet of dried Ulva, but a more nutritionally complete diet with a higher lipid content, such as that of the pelleted shrimp diet utilized in this study, results in increased production. It would be of interest to compare a dried versus wet Ulva diet, and the addition of Ulva as a supplement to a pelleted diet. Even though this study was concerned with the production of P. hawaiensis, it would be of additional interest to know whether these two treatments impacted the nutritional profile of P. hawaiensis, and how that would ultimately affect the production of fish fed this amphipod.
Acknowledgements
The authors would like to thank Erica Rose for her assistance during the feeding trial. This is FAU contribution #2321.
Funding
This study was funded by the Florida Aquaculture Specialty License Plates funds, which are granted through the Harbor Branch Oceanographic Institute Foundation and by the Link Foundation.
References
- Holt GJ, Cato JC, Brown CL (2023) In: Marine ornamental species: Collection, culture and conservation 251-254.
- Woods CMC (2009) Caprellid amphipods: An overlooked marine finfish aquaculture resource? Aquaculture 298: 199-211.
- Sargent JR, McEvoy LA, Bell JG (1997) Requirements, presentation and sources of polyunsaturated fatty acids in marine fish larval feeds. Aquaculture 155: 117-127.
- Conceicão LEC, Yúfera M, Makridis P, Morais S, Dinis MT (2010) Live feeds for early stages of fish rearing. Aquac Res 41: 613-640.
- Guerra-García JM, Hachero-Cruzado I, González-Romero P, Jiménez-Prada P, Cassell C, et al. (2016) Towards Integrated Multi-Trophic aquaculture: Lessons from Caprellids (Crustacea: Amphipodia). PLoS ONE 11: e0154776.
- Govoni JJ, Bochlert GW, Watanabe Y (1986) The physiology of digestion in fish larvae. Environ Biol Fishes 16: 59-77.
- Riberiro L, Hubert FN, Rodrigues V, Rojas-Garcia C, Dinis MT (2022) Understanding fish larvae’s feeding biology to improve aquaculture feeding protocols. Oceans 3: 94-113.
- Russell BC (1983) The food and feeding habits of rocky of rocky reef fish of north-eastern New Zealand. N Z J Mar Freshwater Res 17: 121-145.
- González ML, Perez-Schultheiss J, Lopez DA (2001) Exotic amphipods in aquaculture systems: Presence and potential use. Crustaceana 84: 769-755.
- Baeza-Rojano E, Domingues P, Guerra-García JM, Capella S, Noreña-Barroso E, et al. (2013a) Marine gammarids (Crustacea: Amphipoda): A new live prey to culture Octopus maya Aquac Res 44: 1602-1612.
- Baeza-Rojano E, Hachero-Cruzado I, Guerra-García JM (2014) Nutritional analysis of freshwater and marine amphipods from the Strait of Gibraltar and potential aquaculture applications. J Sea Res 85: 29-36.
- Vargas-Abúndez JA, López-Vázquez HI, Mascaro M, Martinez-Moreno GL, Simões N (2021a) Marine amphipods as a new live for ornamental aquaculture: exploring the potential of Parhyale hawaiensis and Erasmus pectenicrus. PeerJ 9: e10840.
- Vargas-Abúndez JA, Simoes N, Mascaro M (2018) Feeding the lined seahorse Hippocampus erectus with frozen amphipods. Aquaculture 491: 82-85.
- Moren M, Suotama J, Hemre GI, Karlsen Ø, Olsen RE, et al. (2006) Element concentrations in meals from krill and amphipods, - Possible alternative protein sources in complete diets for farmed fish. Aquaculture 261: 174-181.
- Appadoo C, Saudagur R (2007) A Low-Cost Amphipod-Based Feed for Rearing of Ornamental Aquarium Fish, Poecilia reticulata (Peters). Univ Maurit Res J 13: 123-135.
- Kolanowski W, Stolyhwo A, Grabowski M (2007) Fatty acid composition of selected fresh water Gammarids (Amphipoda, Crustacea): A potentially innovative source of omega-3 LC PUFA. J Am Oil Chem Soc 84: 827-833.
- Rallis J, Kapai G, Pavlopoulos (2021) Handbook of Marine Model Organisms in Experimental Biology. 1st
- Podlesinska W, Dabrowska H (2019) Amphipods in estuarine and marine quality assessment- a review. Oceanologia 61: 179-196.
- Steniford GD, Neil DM, Peeler EJ, Sheilds JD, Small HJ, et al. (2012) Disease will limit future food supply from the global crustacean fishery and aquaculture sectors. J Invert Path 110: 141-157.
- Kao D, Lai AG, Stamataki E, Rosic S, Konstantindes N, et al. (2016) The genome of the crustacean Parhyale hawaiensis, a model for animal development, regeneration, immunity, and lignocellulose digestion. elife 5 e20062.
- Nakajima K, Takeuchi I (2008) Rearing method for Caprella mutica (Malacostraca: Amphipoda) in an exhibition tank in the port of Nagoya public aquarium, with notes on reproductive biology. J Crustacean Biol 28: 171-174.
- Baeza-Rojano E, Calero-Cano S, Hachero-Cruzado I, Guerra-Garcia JM (2013b) A preliminary study of the Caprella scaura amphipod culture for potential use in aquaculture. J Sea Res 83: 146-151.
- Xue S, Fang J, Zhang J, Jiang Z, Mao Y, et al. (2013) Effects of temperature and salinity on the development of the amphipod crustacean Eogammarus sinenis. Chin J Oceanol Limn 31: 1010-1017.
- Xue S, Mao Y, Li J, Zhu L, Fang J, et al. (2018) Life history responses to variations in temperature by the marine amphipod Eogammarus possjeticus (Gammaridae) and their implications for productivity in aquaculture. Hydrobiologia 813: 133-145.
- Alberts-Hubatsch H, Slater MJ, Beermann J (2019) Effect of a diet on growth, survival and fatty acid profile of marine amphipods: implications for utilization as a feed ingredient for sustainable aquaculture. Aquac Environ Interact 11: 481-490.
- Vargas-Abúndez JA, Martínez-Moreno GL, Simões N, Noreña-Barroso E, Mascaró M (2021b) Marine amphipods (Parhyale hawaiensis) as an alternative feed for the lined seahorse (Hippocampus erectus, Perri 1810): Nutritional value and feeding trial. PeerJ 9: e12288.
- Serejo CS (1999) Taxonomy and distribution of the family of Hyalidae (Amphipoda, Talitroidea) on the Brazilian coast. Proceedings of the Fourth International Crustacean Congress, Amsterdam, The Netherlands, Koninklijke Brill NV, Leiden pp 591-616.
- Nelson WG (1995) Amphipod crustaceans of the Indian River Lagoon: Current status and threats to biodiversity. Bull Mar Sci 57: 143-152.
- Chopin T, Cooper JA, Ried G, Cross S, Moore C (2012) Open-water integrated multi-tropic aquaculture: environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture. Rev Aquac 4: 209-220.
- Wills PS, Beiser G, Scarpa J, Hanisak D, Laramore S, et al. (2012) A new design concept for land-based integrated multi-trophic aquaculture system. In: Rakestraw TT, and Lawson LS, editors. Proceed ninth Int Conference Recirculating aquaculture, Department Food Sci Technol, Virgina Polytechnic Institute and State College, Roanoke. Virginia 178-180.
- Laramore S, Baptiste R, Wills PS, Hanisak MD (2018) Utilization of IMTA-produced Ulva lactuca to supplement or partially replace pelleted diets in shrimp (Litopenaeus vannamei) reared in a clear water production system. J Appl Phycol 30: 3603-3610.
- Laramore S, Wills PS, Hanisak MD (2022) Seasonal variation in the nutritional profile of Ulva lactuca produced in a land-based IMTA system. Aquac Int 30: 3067-3079.
- FAO (2020) The state of world fisheries and aquaculture 2020. Sustainability in Action. Rome; Food and Agriculture Organization of the United Nations.
- McDermid KJ, Stuercke B (2003) Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol 15: 513-524.
- Ortiz J, Romero N, Robert P, Araya J, Lopez-Hernández J, et al. (2006) Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Druvillaea antarctica. Food Chem 99: 98-104.
- Cruz-Suárez LE, León A, Peña-Rodríguez A, Moll B, Ricque-Marie D (2010) Shrimp/Ulva co-culture: a sustainable alternative to diminish the need for artificial and improve shrimp quality. Aquaculture 301: 64-68.
- Yaich H, Garna H, Besbes S, Paquot M, Blecker C, et al. (2011) Chemical composition and function properties of Ulva lactuca seaweed collected in Tunisia. Food Chem 128: 895-901.
- Peña-Rodríguez A, Mawhinney TP, Ricque-Marie D, Cruz-Suárez LE (2011) Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chem 129: 491-498.
- Taboada C, Millán R, Míguez I (2009) Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. J Sci Food Agri 90: 445-449.
- Yildiz G, Celikler S, Vatan O, Dere S (2012) Determination of the anti-oxidative capacity and bioactive compounds in green seaweed Ulva Rigida Agardh. Int J Food Prop 15: 1182-1189.
- Debbarma J, Madhusudana Rao B, Narasimha Murthy L, Mathew S, Venkateshwarlu G, et al. (2016) Nutritional profiling of the edible seaweeds Gracilaria edulis, Ulva lactuca and Sargassum sp. Indian J Fish 63: 81-87.
- Gamboa-Delgado J, Peña-Rodríguez A, Ricque-Marie D, Cruz-Suárez LE (2011) Assessment of nutrient allocation and metabolic turnover rate in Pacific white shrimp Litopenaeus vannamei co-fed live macroalgae Ulva clathrata and inert feed: dual stable isotope analysis. J Shellfish Res 30: 969-978.
- Pallaoro MF, Vieira FN, Hayashi L (2016) Ulva lactuca (Chlorophyta Ulvales) as co-feed for Pacific white shrimp. J Appl Phycol 28: 3659-3665.
- Macko SA, Lee WY, Parker PL (1982) Nitrogen and carbon isotope fractionation by two species of Marine amphipods: laboratory and field studies. J Exp Mar Biol Ecol 63: 145-149.
- Chan JCC, Cheung PCK, Ang Jr. PO (1997) Comparative studies on the effect of three drying methods on the nutritional composition of seaweed Sargassum hemiphyllum (Turn.) C Ag. J Agric Food Chem 45: 3056-3059.
- Cardona E, Lorgeoux B, Chim L, Goguenheim J, Le Delliou H, et al. (2016) Biofloc contribution to antioxidant defense status, lipid nutrition and reproductive performance of broodstock of the shrimp Litopenaeus stylirostris: Consequences for the quality of eggs and larvae. Aquaculture 452: 252-262.
- Palacios E, Racotta IS, Heras H, Marty Y, Moal J, et al. (2001) Relationship between lipid and fatty acid composition of eggs and larval survival in white pacific shrimp (Penaeus vannamei, Boone, 1931). Aquac Int 9: 531-543.
- Baeza-Rojano E, García S, Garrido D, Guerra-García JM, Domingues P (2010) Use of Amphipods as alternative prey to culture cuttlefish (Sepia officinalis) hatchlings. Aquaculture 300: 243-246.
- Elizondo-González R, Quiroz-Guzmán E, Escobedo-Fregoso C, Magallón-Servín P, Peña-Rodríguez A (2018) Use of seaweed Ulva lactuca for water bioremediation and as feed additive for white shrimp Litopenaeus vannamei. PubMed 6: e4459.
- Fleurence J, Morançais M, Dumay J, Decottignies P, Turpin V, et al. (2012) What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture?. Trends Food Sci Technol 27: 57-61.
- Khoi L V, Fotedar R (2011) Integration of western prawn (Penaeus latislcatus Kishinouye, 1896) and green seaweed (Ulva lactuca Linnaeus, 1753) in a closed recirculating aquaculture system. Aquaculture 322: 201-209.
Citation: Laramore S, Albright E, Sinacore CM (2023) The Impact of Diet on Amphipod, Parhyale Hawaiensis, Production. J Aquac Fisheries 7: 079.
Copyright: © 2023 Susan Laramore, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

