
The Influence of Concentrations of Carbon Dioxide and Residual Oxygen on the Growth of Meat Spoilage Moulds
*Corresponding Author(s):
Bjørn CT SchirmerThe Norwegian Veterinary Institute, POB 750 Sentrum, N-0106 Oslo, Norway
Tel:+47 938 94 256,
Email:bjorn-christian.schirmer@vetinst.no
Abstract
This study examined the effect of different concentrations of Carbon Dioxide (CO2) and residual Oxygen (O2) on the growth of specific spoilage moulds (Penicillium solitum, Penicillium nordicum and Aspergillus proliferans) isolated from dried, cured meat products. The objective was to assess whether residual oxygen concentrations commonly found in vacuum or modified atmosphere packed products allow for the growth of typical spoilage moulds and whether the addition of CO2 would inhibit this growth. The three mould species were examined by plate assays. Results showed that even residual O2 concentration of 0.05% allowed for growth of Penicillium solitum and Penicillium nordicum while Aspergillus proliferans grew at 0.25% O2. The incorporation of an O2 absorber in the package completely inhibited the growth of all three species. The addition of CO2 in the packages significantly decreased the growth of all three species, although higher concentrations were needed to inhibit the growth of P solitum (80%) than for P. nordicum (60%) and A. proliferans (40-50%). In conclusion, this study illustrates the importance of controlling residual O2 concentrations to reduce the risk of mould growth as well as the possibility to inhibit mould growth using CO2 as a protective packaging gas.
Keywords
Aspergillus; CO2; Low O2 concentrations; Penicillium
INTRODUCTION
Moulds and yeasts are one of the main causes for microbiological decay of food. Each year, the food industry has to recall or destroy vast amounts of food because of mould contamination. The meat industry in particular regularly experiences problems with the growth of Penicillium spp. and Aspergillus spp. on their products, especially products that require prolonged storage during production, like dried cured meats [1-6]. In order to reduce the losses due to mould contamination, regular cleaning and disinfection routines are implemented at production sites to prevent mould from establishing and subsequently spreading in the production environments. However, as moulds are easily spread through the air, including ventilations systems, the complete eradication of moulds from the production environments has proven to be practically impossible. The second measure is to prevent residual moulds on the products from growing in the packages. Several packaging strategies for meat are employed, from vacuum-packaging to Modified Atmosphere Packaging (MAP) containing Nitrogen (N2), Oxygen (O2) and Carbon Dioxide (CO2) at various concentrations [7]. Utilizing very low levels of residual O2 and high levels of CO2 are suggested as a part of a hurdle technology to prevent fungal spoilage [8].
The main advantage of vacuum-packaging and packaging with pure N2 gas is that the absence of O2 will prevent aerobic organisms from growing [9]. However, it is very difficult to remove all O2 during packaging, and often small O2 residues from leakages or poor film barrier properties remain in the packages or in the product which can be utilized by moulds [10]. It is known that very small amounts of O2 are needed in order for moulds to grow, but during growth the residual O2 is consumed by the organisms [10]. One aim of this study is to investigate the growth of problem moulds from dry cured meat at low residual O2 levels (<0.5%) in an N2 atmosphere.
The second aim of the study is to evaluate the addition of CO2 gas at various concentrations to the same mould species. It is known from several studies that CO2 inhibits microbial growth, however, various mould species tolerate various levels of CO2 [11-13]. One challenge with adding CO2 is the fact that CO2 is readily absorbed by the product, leading to package deformation after packaging. One way to prevent this is to use flexible packaging, like bags, or to partly add a filler gas, like N2. By testing various CO2/N2 ratios and evaluating their effect on mould growth, the goal is to find an optimal packaging gas mixture.
MATERIALS AND METHODS
Strains
Three fungal isolates were used in the experiments, strains of Penicillium solitum, Penicillium nordicum and Aspergillus proliferans. All strains were isolated from air samples from production sites for dry-cured meat products and selected as they represented main spoilage organisms at these sites [6] and identified by a polyphasic approach using various growth media [14] and partial sequencing of the ITS and β-tubulin gene as described in Schirmer et al. [6].
For the plate assays, spore solutions (106 CFUml-1) were prepared in 20% glycerol solution. For each solution, three spots of 1µl were inoculated on Malt Extract Agar (MEA, 15 Samson et al.,) or Yeast Extract Agar (YES) [15], for Penicillium and A. proliferans respectively and incubated in the dark at 25ºC for 24h before packaging.
Packaging and gas measurement
The study consisted of two experiments: one with concentrations of residual O2 between 0 and 0.5% and one with concentrations of CO2 between 0 and 80%.
Inoculated agar plates were packaged using pouches of 20x20cm with an ethylene vinyl alcohol laminate of type 3-Seal-Bag M/Pa 72 (Südpack Verpackungen, Ochsenhausen, Germany) with an oxygen permeability of 2.5cm3m-224h-1 at 23°C and 50% relative humidity. The packages of plates were first sealed with air. At one corner, the packages were cut to yield an opening of approximately 5mm and food grade gases of N2 or CO2 or blends of CO2/N2 (AGA, Oslo, Norway) were flushed from bottles at 1.5 bars into the package for up to 60 seconds to exchange the air with CO2/N2 gases with <0.1% residual O2, before the packages were resealed in the corner. Each pouch was filled with approximately 300ml gas. The gas concentrations in the packages were measured after 5 minutes. To obtain packages with elevated levels of O2 in the headspace, variable volumes of air were injected into the packages using syringes with needles through self-sealing septas of type 644.029 (Dansensor, Ringsted, Denmark). After adjustments of gas compositions in the headspace, gas concentrations were measured again after 30 minutes.
Oxygen absorbers of type Fresh Pax (Multisorb Filtration Group, Buffalo, USA) were used in experiments where residual O2 was absent in the packages. All packages in the CO2 experiments initially contained residual oxygen of 0.5 (±0.1)% to potentially allow for mould growth. The packages were all stored in darkness for 7 days at 20°C. Experiments containing samples with CO2 were carried out twice, with two plates for each strain and each CO2 concentration. Samples for determination of gas were taken daily or at termination of experiments.
The concentrations of O2 and CO2 in the headspace of the packages were measured with a Dansensor Check mate 3 instrument (Dansensor) by the use of a small vacuum pump and a needle inserted through self-sealing septas (Dansensor).
RESULTS
Residual oxygen
The results showed that even at residual oxygen concentrations of 0.05% mould growth occurred. Both Penicillium strains produced sporulating colonies at 0.05% O2; however, spore formation was reduced at all O2 concentrations below 0.5% compared to samples stored in air (Figures 1 and 2). Aspergillus proliferans did not show any growth below 0.2% O2.
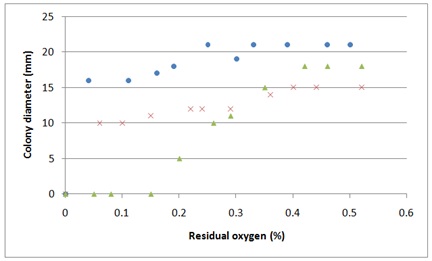 Figure 1: Mould growth after seven days storage depending on initial residual O2 concentration. P. solitum (?), P. nordicum (x), A. proliferans (?)
Figure 1: Mould growth after seven days storage depending on initial residual O2 concentration. P. solitum (?), P. nordicum (x), A. proliferans (?)
 Figure 2: Colony growth of P. nordicum at residual O2 concentrations ranging from 0 (O2 absorber) to 0.5% and air.
Figure 2: Colony growth of P. nordicum at residual O2 concentrations ranging from 0 (O2 absorber) to 0.5% and air.
Oxygen consumption
Results showed that mould colony size increased as long as there was O2 remaining in the package, but growth decreased and stopped when O2 was consumed (Figure 3).
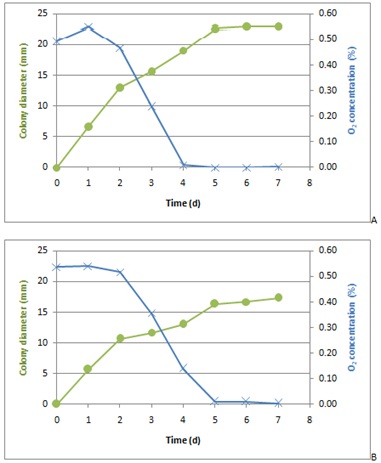 Figure 3: Colony growth (?) of P. nordicum (A) and P. solitum (B) on agar plates depending on oxygen consumption (x) in N2 packaging.
Figure 3: Colony growth (?) of P. nordicum (A) and P. solitum (B) on agar plates depending on oxygen consumption (x) in N2 packaging.
Effect of CO2 on mould growth
Results showed a linear decrease in mould colony size after seven days with increasing initial CO2 concentrations (Figures 4 and 5). All three strains were inhibited by CO2; however, lower concentrations (40-50%) were required to completely inhibit growth of A. proliferans compared to P. nordicum and P. solitum (60 and 80% respectively).
 Figure 4: Mould growth after seven days depending on initial CO2 concentration. P. solitum (?), P. nordicum (x), A. proliferans (?).
Figure 4: Mould growth after seven days depending on initial CO2 concentration. P. solitum (?), P. nordicum (x), A. proliferans (?).
 Figure 5: Colony growth of P. solitum in air and at CO2 concentrations ranging from 0% to 100% (all with 0.5% O2 and N2 as filling gas).
Figure 5: Colony growth of P. solitum in air and at CO2 concentrations ranging from 0% to 100% (all with 0.5% O2 and N2 as filling gas).
DISCUSSION
The results showed that the residual O2 concentrations that are to be expected in products with vacuum-packaging or packaging in N2 (approximately 0.5%) are sufficient to allow growth of undesired mould species on agar rich in nutrients. While the growth of A. proliferans was inhibited at 0.2% O2, P. nordicum and P. solitum grew at O2 concentrations of 0.05%, indicating that vacuum- or N2-packaging alone is not sufficient to prevent mould growth. Hocking [16], previously found that many field and spoilage fungi including Aspergillus and Penicillium species are able to grow in atmospheres containing <1% O2. However, few studies have shown the effect on growth rates at various levels of O2 below 0.5% .One study [17], showed that O2 levels below 0.05% were required to inhibit the growth of Aspergillus fischeranius in fruit puree while another study [18], reported that various mould types could grow at as low as 0.01% O2. Smith et al. [19], showed that 0.4% O2 were required for mould growth in CO2/N2 atmospheres.
In the present study it was observed that mould growth and sporulation at O2 concentrations below 0.5% were delayed compared to growth in air; however, visible Penicillium growth occurred at all O2 concentrations except 0% and sporulation could be observed at 0.2-0.4% O2 after 7 days of storage. Residual O2 was efficiently removed using an O2 absorber and no growth was observed when an absorber was incorporated in the package. O2 absorbers have proven useful for modified atmosphere packaged products. They are, however, less useful in vacuum-packed products. O2 can be located at specific pockets in the package and absorbers may not be able to scavenge all available O2. Also, the addition of an absorber presents an additional cost and is dependent on both consumer acceptance and the practical incorporation of the absorber in the package. Still, mould growth on dry cured meat is unacceptable for the consumer and the results show that in order to inhibit the growth of the spoilage mould the O2 level need to be lower than achievable by most commercial vacuum or N2 packaging alone.
When viewing the results it must be considered that absolute amount of O2 in the packages is dependent on the total package volume. In the presented work, the total amount of gas in the packages was approximately 300ml; however, the volume was not measured for each package, thereby allowing for minor variations in O2 amount. As results showed that mould growth prevailed until all residual O2 was consumed, the total amount of O2 in large packages (as for instance in 1 or 2kg bags of Pinnekjøtt (dry cured mutton ribs)) with a residual O2 concentration of 0.5% may be substantial. As mould growth may appear unevenly distributed in the packages, the amount of O2 may allow for substantial mould growth on parts of the product.
Previous studies have shown that elevated CO2 concentrations are generally much more efficient in controlling fungal growth than oxygen depletion and that a combination of high CO2 and low O2 concentrations is effective in inhibiting both fungal growth and mycotoxin production [20-23]. In the present study, adding CO2 to the packages significantly decreased mould growth and sporulation. Sixty and 80% CO2 were required to completely inhibit the growth of Penicillium nordicum and P. solitum respectively, while lower concentrations (40-50%) were sufficient to inhibit the growth of A. proliferans. This is in accordance with earlier studies that showed similar inhibiting concentrations and also highlights that different mould species are able to grow at different CO2 concentrations [22,23].
As CO2 dissolves into the product, the use of high CO2 concentrations (>60%) may be unsuitable for use in rigid tray packages with top films, as it may lead to package deformation. It may, however, be suitable for flexible bags.
Our study investigated the growth of three different, yet commonly found mould species from dried cured meat. Results showed that various species react differently to O2 depletion and CO2 exposure. This highlights the importance of customizing packaging methods to specific products and monitoring the mycobiota on the products. If new mould species with higher CO2 tolerances are introduced to the product, mould growth may appear on previously safe products.
In the present study, the three mould types were examined on agar plates with almost ideal growth conditions with abundant access to important nutrients. The results remain to be tested on the meat products and as product properties like water content and nutritional value are of importance for mould growth, analyses have to be carried out for each product in order to determine exact threshold limits for CO2 concentrations. However, results show that even the addition of low levels of CO2 will significantly reduce the growth and sporulation of problem moulds and may hence add additional protection to the product.
In summary, results showed that both reducing the residual O2 concentration to 0% by using an oxygen absorber and adding sufficient levels of CO2 to the packages significantly reduced the growth of undesired mould species, while conventional N2 packaging or vacuum packaging alone may not be enough to prevent mould growth on dry cured meat products.
ACKNOWLEDGEMENT
We would like to thank Lene Øverby at Nofima for excellent assistance in the packaging experiments.
The work was supported by Nortura AS, the Foundation for Research Levy on Agricultural Products (FFL) and the Agricultural Agreement Research Fund of Norway (JA) (all under the Norwegian Research Council grant no. 244627).
REFERENCES
- Lopez-Diaz TM, Santos JA, Garcia-Lopez ML, Otero A (2001) Surface mycoflora of a Spanish fermented meat sausage and toxigenicity of Penicillium Int J Food Microbiol 68: 69-74.
- Comi G, Orlic S, Redzepovic S, Urso R, Iacumin L (2004) Moulds isolated from Istrian dried ham at the pre-ripening and ripening level. Int J Food Microbiol 96: 29-34.
- Sorensen LM, Jacobsen T, Nielsen PV, Frisvad JC, Koch AG (2008) Mycobiota in the processing areas of two different meat products. Int J Food Microbiol 124: 58-64.
- Asefa DT, Gjerde RO, Sidhu MS, Langsrud S, Kure CF, et al. (2009) Moulds contaminants on Norwegian dry-cured meat products. Int J Food Microbiol 128: 435-439.
- Sonjak S, Licen M, Frisvad JC, Gunde-Cimerman N (2011) The mycobiota of three dry-cured meat products from Slovenia. Food Microbiol 28: 373-376.
- Schirmer BC, Wiik-Nielsen J, Skaar I (2018) The mycobiota of the production environments of traditional Norwegian salted and dried mutton (pinnekjøtt). Int J Food Microbiol 276: 39-45.
- McMillin KW (2017) Advancements in meat packaging. Meat Sci 44: 153-162.
- Rico-Munoz E, Samson RA, Houbraken J (2019) Mould spoilage of foods and beverages: Using the right methodology. Food Microbiol 81: 51-62.
- Walker GM, White NA (2005) Introduction to fungal physiology. In: Kavanagh K (ed.). Fungi: Biology and Applications. John Wiley & Sons, Chichester, UK.
- Pitt JJ, Hocking AD (1999) Fungi and Food Spoilage. Aspen Publisher, Maryland, USA.
- El Halouat A, Debevere JM (1997) Effect of water activity, modified atmosphere packaging and storage temperature on spore germination of moulds isolated from prunes. Int J Food Microbiol 35: 41-48.
- Taniwaki MH, Hocking AD, Pitt JJ, Fleet GH (2009) Growth and mycotoxin production by food spoilage fungi under high carbon dioxide and low oxygen atmospheres. Int J Food Microbiol 132: 100-108.
- Nguyen Van Long N, Vasseur V, Couvert O, Coroller L, Burlot M, et al. (2017) Modeling the effect of modified atmospheres on conidial Germination of fungi from dairy foods. Front Microbiol 8: 2109.
- Frisvad JC, Samson RA (2004) Polyphasic taxonomy of Penicillium subgenus Penicillium-a guide to identification of food and air-borne terverticillate penicillia and their mycotoxins. Stud Mycol 49: 1-174.
- de Boer E (2004) Introduction to Food- and Airborne Fungi. ASM Press, Washington, DC, USA.
- Hocking AD (1990) Responses of Fungi to Modified Atmospheres. In: Champ BR, Highley E, Banks HJ (eds.). Fumigation and Controlled Atmosphere Storage of Grain, Proceedings of an International conference held at Singapore, 14-18 February 1989. ACIAR Proceedings, Canberra, Australia.
- Dos Santos JLP, Samapundo S, Djunaidi S, Vermeulen A, Sant'Ana AS, et al. (2020) Effect of storage temperature, water activity, oxygen headspace concentration and pasteurization intensity on the time to growth of Aspergillus fischerianus (teleomorph Neosartorya fischeri). Food Microbiol 88: 103406.
- Dos Santos JLP, Samapundo S, Pimentel GC, Van Impe J, Sant'Ana AS, et al. (2019) Assessment of minimum oxygen concentrations for the growth of heat-resistant moulds. Food Microbiol 84: 103243.
- Smith JP, Ooraikul B, KoersenWJ, Jachson ED, Lawrence RA (1986) Novel approach to oxygen control in modified atmosphere packaging of bakery products. Food Microbiol 3: 315-320.
- van den Tempel T, Nielsen MS (2000) Effects of atmospheric conditions, NaCl and pH on growth and interactions between moulds and yeasts related to blue cheese production. Int J Food Microbiol 57: 193-199.
- Hoogerwerf SW, Kets EPW, Dijksterhuis J (2002) High-oxygen and high-carbon dioxide containing atmospheres inhibit growth of food associated moulds. Lett Appl Microbiol 35: 419-422.
- Taniwaki MH, Hocking AD, Pitt JI, Fleet GH (2001) Growth of fungi and mycotoxin production on cheese under modified atmospheres. Int J Food Microbiol 68: 125-133.
- Taniweaki MH, Hocking AD, Pit JI, Fleet GH (2009) Growth and mycotoxin production by spoilage fungi under high carbon dioxide and low oxygen atmospheres. Int J Food Microbiol 132: 100-108.
Citation: Schirmer BCT, Sørheim O, Skaar I, Kure CF (2020) The Influence of Concentrations of Carbon Dioxide and Residual Oxygen on the Growth of Meat Spoilage Moulds. J Food Sci Nutr 6: 064.
Copyright: © 2020 Bjørn CT Schirmer, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

