
Transforming Food with Acid: Lessons from the Dairy
*Corresponding Author(s):
Patricia O’HaraChemistry Department, Amherst College, Amherst MA, United States
Tel:(+1) 413-542-2732,
Email:pbohara@amherst.edu
Abstract
Whenever a chef adds a bit of starter to warm milk to make yogurt or adds lemon juice to hot milk and goes on to separate the curds and whey to make ricotta cheese, she is utilizing a core concept in Chemistry: the reactive power of protons (acids) in aqueous solution. Few recognize that the transformative chemistry happening in front of them in a kitchen is so similar to the acid/base titrations taught in secondary schools and colleges. In the same way, students with goggles and gloves in an introductory chemistry lab, approach acid/base titrations as if they are purely academic exercises that have no relevance to anything in their own lives. The student of chemistry must learn to recognize and use equipment such as burettes, stopcocks, pipettes, and pH meters. They must learn to safely handle hydrochloric acid, phenolphthalein indicators, and sodium hydroxide base. Most challenging, they need to understand background concepts such as equilibrium, pH, inflection point, end point, and equivalence point. It can seem daunting to the most intrepid student-yet it doesn’t have to be this way. Students learn best when what they are learning is put in a context that is meaningful and familiar to them. When an instructor can convince students that cooking, like chemistry, is a series of transformations that happens not in a lab but in a kitchen, this can do a lot to demystify concepts such as pH and titrations. Students come to understand that the concepts are familiar, though they are cloaked in new language and examined closely using the tools of mathematics. Most importantly, the result of your experiments in the kitchen is delicious and nutritious and not collected up as hazardous waste as they are in the laboratory. What follows is an example of how information from food science and nutrition can be used to motivate basic lessons in chemistry.
Keywords
Acidity; Fermentation; pH
Introduction
Milk is anticarcinogenic, immunomodulatory, antimicrobial, antihypertensive, and can help to prevent tooth decay and high cholesterol [1]. As mammals, it is our original comfort food. While it is mostly water (~87%), whole cow’s milk also includes fat (~4%), proteins (~3.4%), lactose sugars (~4.8%) and minerals (~0.8%). Because the milk fats (which might in another manifestation go on to make butter or butter milk) are not soluble in the water, they form droplets suspended in the milk that are so large they don’t allow light to pass through. Instead, the droplets scatter light thus making the milk appear opaque. In raw milk that is not homogenized or pasteurized, the milk fats and fat soluble proteins will over time separate out from the watery milk solution and rise to the top of “the mixture - the cream rises”. The homogenization process forces raw milk through a small opening at high pressure sheering the large droplets shown in the left images in Figure 1 into smaller droplets that resist aggregation. The milk stays mixed, homogenous. Pasteurization, the heating of milk to 72oC for 15 s, or ultra-pasteurization, heating to 138oC for at least 2 s, are processes for insuring the long-term safety of milk products away from the farm. These processes decrease the droplet size as shown as the right two images in Figure 1.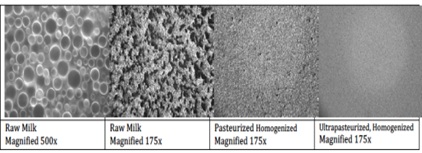 Figure 1: Fat droplet size depends upon milk processing. Microscope images of raw milk shown in the first two images at different magnifications and pasteurized homogenized milk, shown in the third image, and ultra-pasteurized milk, at right. As the milk gets further and further processed, the droplet size gets smaller and smaller.
Figure 1: Fat droplet size depends upon milk processing. Microscope images of raw milk shown in the first two images at different magnifications and pasteurized homogenized milk, shown in the third image, and ultra-pasteurized milk, at right. As the milk gets further and further processed, the droplet size gets smaller and smaller.
Casein is a milk protein that as the name suggests, is the part of milk that can be transformed into cheese. Casein actually represents a family of slightly different proteins (αs1, αs2, β, and κ) all of which have negatively charged phosphate groups attached to them. These phosphate groups on the proteins interact with calcium in the milk to form micellar bundles, like filled soap bubbles, of varying tiny sizes. Figure 2 shows a representation of the casein calcium phosphate micelles adjacent to the much larger fat globule. As we will see, the structure of the casein protein is very dependent on the acidity of the solution [2].
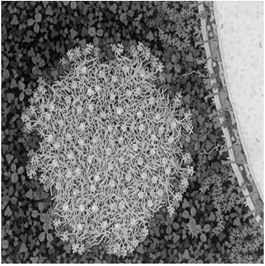 Figure 2: Casein - Calcium Phosphate Micelle approaching a fat globule in Milk.
Figure 2: Casein - Calcium Phosphate Micelle approaching a fat globule in Milk.
To understand the process of transforming milk with acid, we introduce the term, pH, the power of hydrogen. When solutions have a lot of hydrogen ions (H+or protons) they have a low pH and taste sour or tart. Recognizing the taste of sour foods is important for human survival as foods that are unripe or spoiled will often have a very sour taste. Our taste buds contain cells that act as sour chemo-sensors essentially measuring the pH of what we put in our mouth. Proteins in the outer membranes of these cells act as channels for protons. When the protons enter the cell, the movement of charge across the membrane sends an electrical signal to the lingual nerve cells that connect to our brain. If we taste a lemon, which has lots of protons, our brain tells us, WAIT, this food is sour. The normal human response is a grimace and a refusal to consume any further. The most useful pH scale varies from 0 (acidic) to 14 (basic). Counter-intuitively, the lower the number on the scale the higher the acidity. Perhaps you can think of it as like the relationship between altitude and the amount of oxygen available for you to breathe: the higher the elevation, the lower the concentration oxygen- by analogy the higher the pH, the lower the concentration of protons. At sea level where the elevation is the lowest, 0 meters, we have the highest concentration of oxygen. At pH 0, we have an extremely high concentration of protons.
To explore pH more thoroughly, it is useful to remember that a chemist describes the amount of something, or its concentration (amount per unit volume), using the term Molarity. Molarity is defined as moles/liter and given the symbol M also indicated by square brackets around the atom, ion or molecule we are measuring. We can say, for example, that the concentration of protons, or H+ ions is 0.01 M or [H+] = 0.01 M. Remember that since atoms are so very small, and it takes so many of them to even be visible, a standard unit was invented, the mole that is the amount of material that contains 6.02 x 1023 particles. If we have 0.01 M H+, or [H+] = 0.01 M, it means we have 6.02 x 1021 H+ ions per liter of solution. Mathematically, pH = -log [H+], so in the above example, with a 0.01 M solution of H+, the pH = -log (0.01) = 2. The negative logarithmic term, explains why a higher [H+] yields a lower pH. Low pH foods such as Coca Cola or vinegar (both have pH ~ 2.6) are acidic, with a high concentration of H+. High pH foods such as olives and tofu are basic, which means they have a low concentration of H+. Other foods such as fats, starches and sugars are balanced in terms of acid and base and are neutral, pH = 7. At pH 7, the [H+] = 1.0 x 10-7 M, or very low. The foods we eat typically vary in pH from about 3 to 8.
The pH of milk starts out at about 6.8. Making ricotta cheese and fermenting milk to make yogurt, both use acids to sour the milk and cause a transformation in the structure of the milk protein casein.
Ri-cotta or twice cooked cheese has its origins in the kitchens of the Mediterranean where frugal households would have processed milk to extract its curds (milk solids) not once but twice. In a modern kitchen, my favorite recipe for ricotta is made by heating up a mixture of 95% milk; 5% cream to just about 93oC, turning off the heat and adding lemon juice with stirring until. I see the beginnings of the separation of the yellowish watery whey from the fluffy white curd. The molecular structure of citric acid is shown in Figure 3. This weak acid from lemons and other citrus fruit causes the pH of the milk to drop from its normal value of about 6.8 to about 5.4. This drop in pH causes the casein proteins to unfold, as the added H+ ions bind to phosphate and other groups on the protein disrupting their interactions with each other and with the calcium in the protein micelle. This unfolding causes them to precipitate out of solution and form curds. As we add more and more lemon juice, we are essentially titrating in H+ and we proceed until the endpoint at which all of the curd forming protein has come out of the suspension.
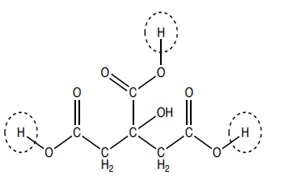 Figure 3: Citric acid is a weak acid found in lemons and other citrus fruits. The bonds to the circled H atoms are easily broken, producing hydrogen ions, H+, and lowering the pH. The remaining H atoms in the structure do not dissociate because they have different environments.
Figure 3: Citric acid is a weak acid found in lemons and other citrus fruits. The bonds to the circled H atoms are easily broken, producing hydrogen ions, H+, and lowering the pH. The remaining H atoms in the structure do not dissociate because they have different environments.
Letting the warm milk/lemon juice mixture sit for 30 minutes or so, allows the curds to further aggregate and they can now be easily separated from the mixture with cheesecloth or a strainer. The watery substance left behind, the whey is actually full of nutritionally valuable material and can be used instead of water in breads, soups, or smoothies (think whey powder that is an add-in at your favorite smoothie store.)
Fermentation is a process by which larger biological molecules are broken down into smaller molecules by the action of yeasts or bacteria. Yogurt is a fermented food made from milk that traces its history back to about 5000 BC [3]. Regional fermented dairy products in addition to yogurt include sour cream, buttermilk, cheese, kefir, lassi, labneh, and ayran - a small sampling of the rich array of liquid and solid products from milk each of which resists spoilage when compared with fresh milk. Fermentation starts with the breakdown of complex milk sugars known as lactose, a disaccharide of galactose and glucose to lactic acid, the structures of which are both shown in Figure 4. The microbial fermentation process that creates yogurt relies on the slow digestion of lactose by the microbes in the live starter culture (typically Lactobacillus bulgaricus and Streptococcus thermophilus.) [4] these bacteria work together to continue the fermentation process for 4-12 hours. Given its preference for growing in media at neutral pH and high oxygen content, the S. thermophilus kicks off the show. As the sugars and proteins are broken down, the pH and the oxygen content both drop, which leads to the perfect conditions for growth of the second bacteria, L. bulgaricus. Fermentation continues until the lactose is used up. The lactic acid product is a weak acid is responsible for the drop in pH which in turn produces physical changes in the milk. What we call yogurt is a soft gel like matrix formed as the milk proteins gradually unfold. Tart flavor compounds characteristic of yogurt are also produced during fermentation.
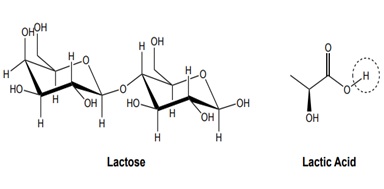 Figure 4: The disaccharide lactose is made up of galactose and glucose. Also shown is the weak acid, lactic acid, which is produced upon fermentation of lactose. The bond to the circled H atom is easily broken, producing hydrogen ion, H+, and lowering the pH. The remaining H atoms in the structure do not dissociate because they have a different environment.
Figure 4: The disaccharide lactose is made up of galactose and glucose. Also shown is the weak acid, lactic acid, which is produced upon fermentation of lactose. The bond to the circled H atom is easily broken, producing hydrogen ion, H+, and lowering the pH. The remaining H atoms in the structure do not dissociate because they have a different environment.
To make yogurt, milk is heated with stirring to just about 85oC, enough to kill off any existing competing bacteria in the milk but not high enough to denature the protein and form curds. Next, when the milk cools to about 45oC, it is “inoculated” with active cultures from a yogurt starter. The two bacteria in the culture consume the sugar and proteins of the milk, producing the breakdown product lactic acid and after 4-12 hours at around 30-40oC, the yogurt is done.
Yogurt will typically have a much lower pH (about 4.6) than the ricotta (about 5.4). Remember if the pH differs by 1, the H+ differs by 10. While each product has been created by the action of a weak acid, in one, the acid was added directly and in the other the acid is generated by microbial action from added cultures. Both are good for you, both are delicious.
Acknowledgement
Author declares no conflict of interest.
References
- Davoodi SH, Shahbazi R, Esmaeili S, Sohrabvandi S, Mortazavian A, et al. (2016) Health-Related Aspects of Milk Proteins. Iran J Pharm Res Summer 15: 573- 591.
- https://www.clemson.edu/extension/food/food2market/documents/ph_of_common_foods.pdf
- Tribby D (2009) "Yogurt" In Clark C, et al. (eds.). The Sensory Evaluation of Dairy Products. Springer Science & Business Media 191.
- http://milkfacts.info/Milk%20Processing/Yogurt%20Production.htm
Citation: O’Hara P (2022) Transforming Food with Acid: Lessons from the Dairy. J Food Sci Nutr 8: 139.
Copyright: © 2022 Patricia O’Hara, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

