
Translingual Neurostimulation for Peripheral Motor Control Recovery Post in-Patient Stroke Rehabilitation: A Focus Article
*Corresponding Author(s):
Peter LynchClinical Health And Nutrition Centre Chance, School Of Science, Institute Of Technology (IT) Sligo, Neuroplasticity Research Group, Ireland
Tel:+353 719155222,
Email:peterlynch091@gmail.com
Abstract
Neuroplasticity can be defined as the ability of the Central Nervous System (CNS) to adapt in response to changes in the environment or lesions. Cranial Nerve Non-Invasive Neuromodulation (CN-NINM) is a multi-level rehabilitation therapy that initiates neuroplasticity through a combination of non-invasive brain stimulation combined with physical therapy in the recovery of suppressed brain functions affected by neurological injury. CN-NINM utilises innervations of the tongue (Translingual Neurostimulation) to stimulate the CNS. By supplementing task specific training with additional brain stimulation, the hypothetical value of CN-NINM is enhanced activation of neural pathways directly related to the task, facilitating more efficient ‘learning’ of a task following neurological injury.
Keywords
Crainal nerve non-invasive neuromodulation; Brain stimulation; Translingual neurostimulation; Neuroplasticity; Portable neuromodulation stimulator; PoNS;Stroke
NEUROPLASTICITY
Neuroplasticity can be defined as the ability of the Central Nervous System (CNS) to adapt in response to changes in the environment or lesions [1]. For many decades, it was hypothesized that the brain had a genetically set volume of brain cells which decline respectively with the aging process regardless of intentional efforts to negate the process [2]. It was not until the advent of electron microscopy in the 1960s that it was discovered that neurons could extensively adapt and “unmask” relatively inactive pathways after a neurological event [3]. Using “state-of-the-art” tissue processing methods, it is suggested neuroplastic changes are active in the healthy human adult up to the ninth decade of life [4]. A 2019 review by Kumar and colleagues suggests, human studies objectively identifying/measuring neuroplasticity in neurological patients are seemingly inadequate to date, although animal model studies do provide an insight into the process in the mammalian brain [5]. The “unmasking” of relatively inactive pathways is proposed to be achievable even many years after a neurological injury/lesion with appropriate late intervention [6-7]. The past two decades have seen an increased awareness of neuroplasticity and a new focus on developing means to facilitate this process, focus has shifted centrally from the peripheries to targeted sites to stimulate the CNS.
PERIPHERAL NERVE STIMULATION
Initially, focus for stimulation aimed peripherally via Peripheral Nerve Stimulation (PNS). PNS includes both transcutaneous and subcutaneous devices such as Transcutaneous Electrical Nerve Stimulation (TENS) and Peripheral Subcutaneous Filed Stimulation (PSFS). In 1967, PNS became a well-established and accepted method for treatment of various painful conditions (e.g. migraine, fibromyalgia, low back pain) and later became a neurorehabilitation treatment modality [8]. Recent evidence has found judging the effectiveness of PNS to facilitate neuroplasticity and motor recovery in sites of neurological deficit difficult [9-10]. A systematic review of twenty-one randomised controlled trials and 1481 participants found that PNS, combined with conventional physical therapy, only had a moderate benefit on lower limb motor function in a chronic stroke cohort with no significant difference in gait endurance compared to control groups. These studies overall were evaluated as ‘low risk’ of bias [9].
Danilov and Paltin [8] theorise that as a result of the complexity and diversity of the brain’s damage following neurological injury, various functional systems become inefficient or desynchronized. The spread of neural network malfunctions and the lack of methods for localizing such damages creates a complex complication for efficient neurorehabilitation. The authors also theorise the anatomical specificity and localization of PNS restrains the efficiency of neurostimulation for functional recovery. The small area of affected tissue facilitates changes in the activity of a small proportion within a widely distributed functional network [8]. Recent evidence has progressed toward Cranial Nerve Non-Invasive Neuromodulation (CN-NINM). The synthesised concept of CN-NINM was developed by Yuri Danilov, Mitchell Taylor and Kurt Kaczmarek [11]. Translingual (tongue) Neurostimulation (TLNS) is the route utilised via CN-NINM to induce cranial nerve stimulation and which has been suggested as a potential ‘gateway’ to neural excitability and motor skill learning [12-18]. CN-NINM is multi-level rehabilitation therapy that initiates neuroplasticity through a combination of TLNS with physical therapy in the recovery of suppressed brain functions affected by neurological injury [8].
TRANSLINGUAL NEUROSTIMULATION RATIONALE
The tongue, and musculature acting on the organ, occupy a larger surface area than might be first expected. Perception of the tongue is often of the dorsum. Figure 1 highlights the extrinsic muscles of the tongue providing a basic insight into the actual size of the organ.
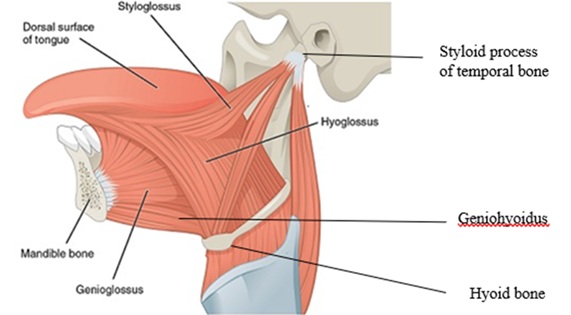 Figure 1:Extrinsic muscles of the tongue [19].
Figure 1:Extrinsic muscles of the tongue [19].
Mu & Sanders described the human tongue as having “extremely dense and complex” nerve innervation, which are not reported in other mammalian tongues [20]. These unique innervations appear to allow for fine motor control of the tongue in humans, with tongue movement suggested to have subsequent effects on other body systems [12,15].
According to a review, certain tongue movements, by and large postero-lateral, are suggested to activate the Anterior Cingulate Cortex (ACC), which plays an important role in sensory, motor, cognitive, emotional and pain processing [15]. The tongue is reported to embody a “gateway” to access other brain pathways and body systems [12]. Findings suggests that a simple movement and conscious control of the tongue can modulate postural control mechanisms. In a cohort of 116 normal healthy male subjects, the mean centre of gravity velocity (i.e. output to maintain balance) decreased significantly when the tongue was positioned against the upper incisors [21].
‘Mapping’ of the tongue via craniotomy and cortical stimulation indicated that innervation of the tongue is reported to have extensive representation at multiple levels in the brain (Cerebral cortex, mesencephalon/midbrain, medulla oblongata, and limbic system), with the highest reported representation reached at the cortical level (responsible for motor, sensory, visual functions and more) [22] (Figure 2). Specific areas of the tongue were found to have ‘dominant’ sensory cortical representation (Figure 3).
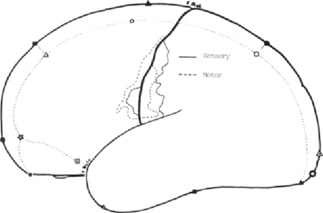 Figure 2:Sensory and Motor tongue responses “mapped” in humans [22].
Figure 2:Sensory and Motor tongue responses “mapped” in humans [22].
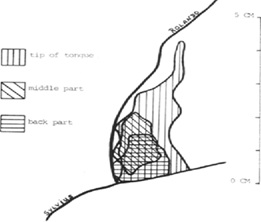 Figure 3:Portions of tongue showing level of cortical representation [22].
Figure 3:Portions of tongue showing level of cortical representation [22].
Such understanding and evidence seemingly would have inspired innovators including Paul Bach-y-Rita, Yuri Danilov, Mitchell Taylor and Kurt Kazcmarek, etc. to further investigate the tongue and integrate and accumulate current understanding into practical intervention, leading to the development of CN-NINM. Years of research and prototypes integrating CN-NINM eventually culminated in the modern-day Portable Neuromodulation Stimulator (PoNSTM) (Helius Medical Technologies).
The PoNSTM is the latest model in a line of TLNS prototypes. To the principal authors knowledge following extensive reviews of literature, the PoNSTMand initial prototypes are the first of their kind, with no other TLNS devices discussed in the literature reviewed. The theory and technology underpinning the PoNSTM device stemmed from the research of Bach-y-Rita [23].The PoNSTM(Figure 4) is a compact, self-contained device that delivers a fixed sequence of balanced impulses to the anterior-dorsal tongue through a matrix of electrodes. According to Dr. Kim Skinner (Director of Physical Therapy, Helius Medical Technologies), the impulse waveform delivered was optimized via psychophysical experiments to yield maximal stimulus dynamic range, quality of sensation and minimal sensory adaptation. The matrix of electrodes was specifically designed to create a localized and controlled impulse path in the dorsum of the tongue to minimize the amount of energy required to excite the superficial tactile sensory fibres and avoid deep free nerve endings and motor fibres [24].
The device consists of a controller which contains the commands for operating the device and from which impulses are generated. Connected to the controller is a flat surfaced mouthpiece containing the electrodes for administering electrical impulses to the tongue. Original research by Bach-y-Rita considered both tongue and finger stimulation due to dense innervation of both. At that time, the tongue was chosen as it offers an optimal environment, compared to a fingertip. The environment of the tongue offers constant acidity level, temperature and humidity, and low excitability thresholds in comparison to potential surface contaminants and high excitability thresholds of a fingertip [25]. Initial prototypes for TLNS, the Tongue Display Unit (TDU), evolved into the portable BrainPort Vision Device, which was enhanced into the Electrotactile Vestibular Substitution System (EVSS), which itself was commercialised as the portable BrainPort Balance Device, before finally evolving into the modern PoNSTM device(Figure 4).
 Figure 4:Helius Medical Technologies PoNS™ device [26].
Figure 4:Helius Medical Technologies PoNS™ device [26].
TRANSLINGUAL NEUROSTIMULATION AND NEUROPLASTICITY THEORY
The underpinning theory behind CN-NINM is neuroplastic changes induced via non-invasive stimulation of two major cranial nerves innervating the tongue: the trigeminal (Cranial nerve V) and facial (Cranial nerve VII)(Figure 5). This stimulation is proposed to excite a natural flow of neural impulses to the brainstem, Pons/Pons Varolii and Cerebellum [27,28].
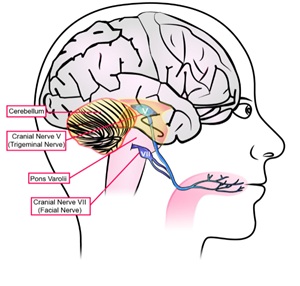 Figure 5:Basic CN-NINM target site illustration [26].
Figure 5:Basic CN-NINM target site illustration [26].
The theory suggests that, during task specific activity, the supplementary concentrated activation of these nerves initiates a subsequent cascade of ‘excitement’ in adjacent and/or connected neurons, by activation of brainstem interneuron circuitries and/or by passive transmission of neurochemical compounds (neurotransmitters) in the synaptic clefts. This stream of impulses leads to activation of corresponding neural networks and further considerable release of neurotransmitters that eventually activate the glial networks of the brainstem (responsible for maintenance of the neuronal environment). This leads to radiating neurochemical and neurophysiological changes affecting impulse focal points responsible for information processing of both afferent and efferent neural signals, including the Cerebellum and neurons of the spinal motor pathways [8,24]. This increased neural activation provides a foundation from which new neural networks are proposed to develop (Figure 6).
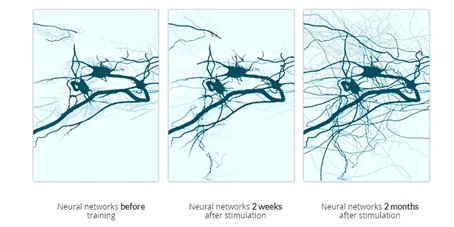 Figure 6:Hypothesised CN-NINM theory [29].
Figure 6:Hypothesised CN-NINM theory [29].
Recent evidence has investigated objective measures supporting this theory [16,18]. High-density electroencephalography (EEG) was utilised to measure changes in resting brain activity before, during, and after high and low frequency TLNS using the PoNSTM device [18]. Both frequencies produced significant changes in brain activity (measured via signal output related to the task, i.e. signals related to sedentary breathing and awareness), although it is worth noting that an increase in brain activity following low frequency dosage stimulation only occurred if the subject received high frequency dosage first. The authors concluded that high frequency was required to elicit significant changes in brain activity related to the task, but after initial high frequency, the threshold for activation was lowered and low frequency was then enough to elicit significant changes in brain activity. High frequency dosage was found to also significantly increase attentional microstates, also known as “the atoms of thought” [30], suggesting a possible functional mechanism for neuroplastic improvements. Although this research investigated a ‘healthy’ cohort engaging in breathing and awareness training, not reflecting PoNS intended use for patients with neurological conditions, it supports and builds on previous understanding of neuroplastic changes related to CN-NINM and task specific training.
Research in the treatment of spasticity further supports the theory of neuroplasticity induced by TLNS [16]. Spasticity is defined as a “motor disorder characterised by a velocity-dependent increase in tonic stretch reflexes” [31]. The central lesion causing spasticity is suggested to disrupt the balance of excitation and suggests neuroplastic changes occurring in the spinal cord and the brain [32]. The authors hypothesise the central lesion leads to hypersensitivity following denervation leading to spasticity, which stimulates the creation of new abnormal pathways maintaining spasticity [32,33]. As neuroplastic changes are suggested to occur to maintain spasticity, neuroplastic changes can also be facilitated to treat the condition [16]. The authors examined TLNS in the treatment of six children with Cerebral Palsy in the form of spastic diplegia. The cohort were aged between eight and fourteen years old and were deemed to have intact intellect with no seizures reported in their medical history. The children underwent physical therapy combined with PoNSTMstimulation and were evaluated by various functional outcome measures and measures of spasticity. The cohort underwent functionalMagnetic Resonance Imaging (fMRI) before and after PoNSTMstimulation(Figure 7). The findings indicated positive improvements in a range of dynamics; most of them learned to walk without aids, decreased muscle tonus and improvement in balance and coordination function. For each task related to the physical therapy, fMRI revealed neurostimulation elicited increased brain activation patterns related to the task compared to without the additional stimulation (Figure7). These findings suggest CN-NINM facilitates increased brain activity related to a task allowing for more ‘efficient learning’ of motor function and skills [16].
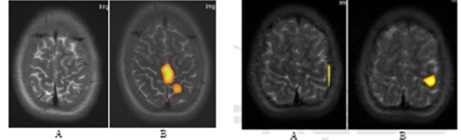 Figure 7:left; Activation pattern in projection of Left-leg motor area (A) before and (B) after neurostimulation. right; Activation pattern in projection of Right-hand motor area (A) before and (B) after neurostimulation [16].
Figure 7:left; Activation pattern in projection of Left-leg motor area (A) before and (B) after neurostimulation. right; Activation pattern in projection of Right-hand motor area (A) before and (B) after neurostimulation [16].
CLINICAL APPLICATION
A review of literature suggests that CN-NINM can significantly improve functional outcomes in neurological patient cohorts with Traumatic Brain Injury (TBI) [17,34], Multiple Sclerosis (MS) [35,36], Spinal Cord Injury (SCI) [13], Cerebellar Degeneration [37],Cerebral Palsy [16] and Stroke [14].By supplementing task specific training with additional brain excitement/activation, the hypothetical value of CN-NINM is stimulation of inactive neural pathways related to the task, facilitating more efficient ‘learning’ of the task.
New research by the authors of this article will be exploring stroke survivors post inpatient rehabilitation only and investigating outcomes following a period of CN-NINM combined with task specific training.
CONSIDERATION FOR FUTURE CLINICAL APPLICATION
According to research, three levels of neuroplastic change transpire during ‘learning’ [38]. There are neurochemical changes during conscious awareness (short-term) memory, structural changes as conscious turns into long-term memory and functional changes for shifting brain areas as memory becomes ‘unconscious’. According to this evidence, the brain is therefore shaped structurally and functionally by behaviour and task repetition, as a neural pathway used more often is increasingly excitable and easy to activate. Therefore, task repetition is crucial for functional ‘learning’ of any new pathways induced during investigation with CN-NINM.
MOVING FORWARD
To the author’s knowledge, no evidence exists demonstrating the pathophysiological mechanism through which TLNS ‘compensates’ for lost signals/pathways related to the neurological injury. Research has objectively demonstrated that TLNS elicits increased brain activation related to a task and improved motor function as a result [16,18]but the pathophysiological processes and new ‘pathway’ formation occurring via TLNS to compensate for neurological deficit are seemingly hypothetical at present [8]. Future research objectively demonstrating these processes and the positional response and interaction with the tongue as a result would be beneficial to further increase knowledge and understanding of an innovative Neurorehabilitation modality. Potential developments in this area could lead to an innovative device administering translingual stimulation based on biofeedback coming from a frequency measuring device attached to an affected limb to create a ‘direct’ link between limb and CNS which has been disrupted through neurological injury.
REFERENCES
- Sharma N, Classen J, Cohen LG (2013) Neural plasticity and its contribution to functional recovery. Handb Clin Neurol110: 3-12.
- Fuchs E,Flügge G (2014) Adult Neuroplasticity: More Than 40 Years of Research. Hindawi2014: 1-10.
- Raisman, G (1969) Neuronal plasticity in the septal nuclei of the adult rat. Brain Res 14: 25-48.
- Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, et al. (2019) Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med 25: 554-560.
- Kumar A, Pareek V, Faiq M, Ghosh S, Kumari C (2019) Adult neurogenesis in humans: A Review of Basic Concepts, History, Current Research, and Clinical Implications. Innov Clin Neurosci 16: 30-37.
- Bach-y-rita P (2001) Theoretical and practical considerations in the restoration of function after stroke. Top Stroke Rehabil 8: 1-15.
- Bach-y-rita P (2003) Theoretical basis for brain plasticity after a TBI. Brain Inj 17: 643-651.
- Danilov Y, Paltin D (2018) Translingual Neurostimulation (TLNS): Perspective on a Novel Approach to Neurorehabilitation after Brain Injury. Pre-Clinical and Clinical Methods in Brain Trauma Research 139: 307-327.
- Hong Z, Sui M, Zhuang Z, Liu H, Zheng X, et al. (2018) Effectiveness of Neuromuscular Electrical Stimulation on Lower Limbs of Patients with Hemiplegia After Chronic Stroke: A Systematic Review. Arch Phys Med Rehabil 99: 1011-1022.
- Menezes IS, Cohen LG, Mello EA, Machado AG, Peckham PH, et al. (2018) Combined Brain and Peripheral Nerve Stimulation in Chronic Stroke Patients with Moderate to Severe Motor Impairment. Neuromodulation 21: 176-183.
- Danilov Y, Tyler M, Kaczmarek K (2008) Cranial nerve non-invasive neuromodulation (CN-NINM): New approach to neurorehabilitation. International Journal of Psychophysiology 69: 301-302.
- Sbarbati A, Osculati F (2007) Extending the enteric nervous system. Biomedicine & Pharmacotherapy 61: 377-382.
- Chisholm AE, Malik RN, Blouin JS, Borisoff J, Forwell S, et al. (2014) Feasibility of sensory tongue stimulation combined with task-specific therapy in people with spinal cord injury: a case study. Journal of NeuroEngineering and Rehabilitation11: 11-96.
- Galea M, Lizama LEC, Bastani A, Panisset M, Khan F (2017) Cranial nerve non-invasive neuromodulation improves gait and balance in stroke survivors: A pilot randomised controlled trial. Brain Stimulation 10: 1133-1135.
- Bordoni B, Morabito B, Mitrano R, Simonelli M, Toccafondi A (2018) The Anatomical Relationships of the Tongue with the Body System. Cureus10: 3695.
- Ignatova TS, Kolbin VE, Sarana AM, Scherbak SG, Danilov Y, et al. (2018) Translingual Neurostimulation in Treatment of Children with Cerebral Palsy in the Late Residual Stage. Case Study. Proceedings of the 11th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2018) 4: 332-337.
- Tyler M, Skinner K, Prabhakaran V, Kaczmarek K, Danilov Y (2019) Translingual Neurostimulation for the Treatment of Chronic Symptoms Due to Mild-to-Moderate Traumatic Brain Injury. Archives of Rehabilitation Research and Clinical Translation 1: 10026.
- Frehlick Z, Lakhani B, Fickling SD, Livingstone AC, Danilov Y,et al. (2019) Human translingual neurostimulation alters resting brain activity in high-density EEG. Journal of NeuroEngineering and Rehabilitation 16: 2-7.
- https://commons.wikimedia.org/wiki/File:1109_Muscles_that_Move_the_Tongue_Extrinsic.png
- Mu L, Sanders I (2010) Human tongue neuroanatomy: Nerve supply and motor endplates. Clinical Anatomy23: 777-791.
- Alghadir AH, Zafar H, Iqbal ZA (2015) Effect of tongue position on postural stability during quiet standing in healthy young males. Somatosens Mot Res32: 183-186.
- Picard C, Olivier A (1983) Sensory cortical tongue representation in man. J Neurosurg59: 781-789.
- https://www.meddeviceonline.com/doc/good-money-no-longer-goes-to-die-in-neuroscience-0001
- Kaczmarek K (2017) The Portable Neuromodulation Stimulator (PoNS) for neurorehabilitation. Scientia Iranica 24: 3171-3180.
- Bach-y-Rita P, Kaczmarek KA, Tyler ME, Garcia-Lara J (1998) Form perception with a 49-point electrotactile stimulus array on the tongue: A technical note. J Rehabil Res Dev35: 427-430.
- https://heliusmedical.com/
- Ptito M, Moesgaard SM, Gjedde A, Kupers R (2005) Cross-modal plasticity revealed by electrotactile stimulation of the tongue in the congenitally blind. Brain128: 606-614.
- Wildenberg JC, Tyler ME, Danilov YP, Kaczmarek KA, Meyerand ME (2010) Sustained cortical and subcortical neuromodulation induced by electrical tongue stimulation. Brain Imaging Behav 4: 199-211.
- https://www.cognifit.com/brain-plasticity-and-cognition
- Koenig T, Lehmann D, Merlo MC, Kochi K, Hell D, et al. (1999) A deviant EEG brain microstate in acute, neuroleptic-naive schizophrenics at rest. Eur Arch Psychiatry Clin Neurosci249: 205-211.
- https://www.worldcat.org/title/spasticity-disordered-motor-control/oclc/6734642
- Trompetto C, Marinelli L, Mori L, Pelosin E, Currà A, et al. (2014) Pathophysiology of Spasticity: Implications for Neurorehabilitation. Biomed Res Int2014: 354906.
- Roper S (1976) The acetylcholine sensitivity of the surface membrane of multiply innervated parasympathetic ganglion cells in the mudpuppy before and after partial denervation. J Physiol 254: 455-473.
- Bramlett HM, Dietrich WD (2015) Long-Term Consequences of Traumatic Brain Injury: Current Status of Potential Mechanisms of Injury and Neurological Outcomes. J Neurotrauma32: 1834-1848.
- Tyler ME, Kaczmarek KA, Rust KL, Subbotin AM, Skinner KL, et al. (2014) Non-invasive neuromodulation to improve gait in chronic multiple sclerosis: A randomized double blind controlled pilot trial. J Neuroeng Rehabil 11: 79.
- Leonard G, Lapierre Y, Chen JK, Wardini R, Crane J, et al. (2017)Noninvasive tongue stimulation combined with intensive cognitive and physical rehabilitation induces neuroplastic changes in patients with multiple sclerosis: A multimodal neuroimaging study. Mult Scler J Exp Transl Clin 3: 2055217317690561.
- Bastani A, Cofré Lizama LE, Zoghi M, Blashki G, Davis S, et al. (2018) The combined effect of cranial-nerve non-invasive neuromodulation with high-intensity physiotherapy on gait and balance in a patient with cerebellar degeneration: A case report. Cerebellum Ataxias 5: 6.
- https://www.youtube.com/watch?v=LNHBMFCzznE
Citation: Lynch P, Roberts D, Monaghan K (2020) Translingual Neurostimulation for Peripheral Motor Control Recovery Post in-Patient Stroke Rehabilitation: A Focus Article. J Alzheimers Neurodegener Dis 6: 038.
Copyright: © 2020 Peter Lynch, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

