
Yacon (Smallanthus Sonchifolius)-Based Product Increases Fecal Short-Chain Fatty Acids Concentration and Up-Regulates T-Bet Expression in the Colon of BALB/c Mice During Colorectal Carcinogenesis
*Corresponding Author(s):
Letícia De Nadai MarconNutritional Biochemistry Laboratory, Department Of Nutrition And Health Of Universidade Federal De Viçosa-UFV, Viçosa, Minas Gerais, Brazil
Tel:+55 31 36125213,
Email:leticiadenadai@gmail.com
Abstract
Colorectal Cancer (CRC) is the third most diagnosed type of cancer worldwide. Prebiotics containing Fructooligosaccharides (FOS) and inulin have been reported to improve CRC in experimental models. We hypothesized whether consumption of the yacon-based product (PBY-Smallanthus sonchifolius concentrate), as a source of FOS and inulin, could mitigate colonic pre-neoplastic lesion development in mice by enhancing fecal Short-Chain Fatty Acid (SCFA) production besides modulating intestinal immune responses. Therefore, we investigate the effects of PBY consumption on anatomical and fecal characteristics, serum biomarkers, fecal SCFA concentration, intestinal lymphocytes population, expression of transcription factors of the adaptive immune response and Aberrant Crypt Foci (ACF) count in the colon of mice chemically induced to pre-neoplastic lesions. Male BALB/c mice were injected intraperitoneally with 1,2-dimethylhydrazine (20mg/kg body weight/week) for 8 weeks. Thereafter, mice were fed either control (AIN-93M) or PBY diet (AIN-93M supplemented with PBY, 6.0% FOS +Inulin) for 8 weeks and then euthanized. PBY was not successful in reducing ACF counts; however, it improved fecal SCFA concentration (formic, acetic, propionic, butyric and valeric), reduced fecal pH and increased humidity and viscosity of feces. Although there were no significant changes in the intestinal lymphocytes population (CD4, CD8, Natural Killer, Treg and Th17), PBY consumption modulates the immune response in the colon reducing the expression of FOXP3 and increasing RORγt and T-bet, which would contribute to activation and proliferation of CD8 T lymphocytes and better CRC prognosis. Therefore, we suggest that PBY might improve intestinal health during the early stages of colorectal carcinogenesis, especially by modulating SCFA production and colonic adaptive immune response.
Keywords
Colorectal cancer; Immunomodulation; Prebiotic; Short-chain fatty acid; Transcription factor; Yacon
INTRODUCTION
Colorectal Cancer (CRC) is the third most diagnosed type of cancer in the world [1]. CRC etiology cross talks with genetic factors, eating habits, immune response and intestinal microbiota composition [2-4]. The tumor-infiltrating lymphocyte profile strongly associates with CRC prognosis [5,6]. CD8 T lymphocytes and Natural Killer (NK) cells, for instance, have been related to disease delay and better prognosis [7-9]. Furthermore, the Th1 immune response may contribute to remission and better CRC prognosis [10,11]. On the other hand, Th17 cells have been associated with worse CRC prognosis and disease progression [12], although it has already been associated with a better prognosis [9]. Regulatory T cells (Treg) also evidence a dual role [13,14], sometimes suppressing the anti-tumor immune response [15], sometimes attenuating the inflammatory immune response [16].
Evidence suggests that prebiotic food consumption induces the growth of probiotic bacteria [17], which improves the anti-inflammatory activity [18] and favors the modulation of cells in the gut-associated lymphoid tissue [19,20]. Yacon is a known source of Fructooligosaccharides (FOS) and inulin and therefore, considered a prebiotic food. This tuberous root is originated from the Andes, but it can easily adapt to different climatic regions, altitudes and soils [21]. For this reason, yacon has been cultivated in several countries such as United States, Japan, Italy, Brazil, Argentina, Bolivia, Czech Republic, Ecuador, Korea, New Zealand and Peru [21]. Most of the yacon content is water, which usually exceeds 70% of fresh weight [22]. In the dry matter, the main component of yacon is FOS, however, highly variable (25.7% [23]; 41.2% [24]; 42% [25]; 52.2% [26]). Lipids and proteins are in low amounts (less than 4% and 0.3 % respectively) [23,26]. Glucose, fructose and sucrose content are around 10 to 20% each [23,26]. Yacon can be incorporated in culinary preparations, juices and salads and also be consumed raw, cooked, dehydrated, or as a syrup [27] or candy [21].
Yacon consumption might modulate the intestinal microbiota by increasing the number of bifidobacteria and lactobacilli [28,29], which directly inhibit the growth of pathogenic bacteria [30,31]. Studies have shown that yacon increase fecal Short-Chain Fatty Acids (SCFA) concentration [20,26,29,32,33], improve intestinal transit [28,34], control satiety [20], contribute to weight loss [27] and reduce glycemia [24], triglycerides and cholesterol [35,36]. Yacon also modulates the intestinal inflammatory response by increasing fecal secretory immunoglobulin A (sIgA) and serum interleukin (IL)-10 and IL-4 [33,37,38], besides preventing pre-neoplastic lesions during colorectal carcinogenesis in animals [26,39]. Recently, our research group verified the role of the yacon-based product (PBY- Smallanthus sonchifolius concentrate) on intestinal immunity. PBY consumption enhanced Treg cells in the colon of BALB/c mice and mitigated the inflammatory profile by down regulating the expression of RORγt [20].
Yacon exhibits antioxidant properties owing to its high polyphenol content, such as chlorogenic acid, caffeic acid, coumaric acid and protocatechuic acid [25,40]. Its content of fructans, mainly FOS and inulin, favor SCFA production by the intestinal microbiota, especially butyrate, which has been reported to be closely linked to neoplastic cells apoptosis [41], Histone Deacetylase (HDAC) inhibition [42,43] and cell cycle blockage, thus impairing colorectal carcinogenesis and tumor progression [44].
The benefits of yacon are directly related to the modulation of intestinal microbiota [29] and intestinal immune response [20,38]. Nevertheless, the immune response profile induced by yacon during colorectal carcinogenesis has still not been elucidated. In this study, we use a mouse model that allows in situ evaluation of pre-neoplastic lesions, known as aberrant crypt foci (ACF) [26,39], as well as we assess biomarkers similar to used in human studies. Therefore, this model supports the evaluating of the effects of prebiotics on colorectal carcinogenesis [45]. We used yacon concentrate (PBY) that provides higher concentrations of FOS and inulin, besides, be lesser perishable than fresh yacon. As recommended by Paula and co-workers [46], PBY was added to the animals' diet to supply 6% FOS + inulin and hence modulate intestinal function. Thus, we hypothesized whether PBY consumption, as a source of FOS and inulin, could mitigate ACF development in mice by enhancing fecal SCFA concentration besides modulating intestinal immune responses. Moreover, the effects of PBY consumption were explored on anatomical and fecal characteristics, serum biomarkers, intestinal lymphocyte populations, and expression of transcription factors of the adaptive immune response in the colon of BALB/c mice chemically induced to pre-neoplastic lesions.
METHODS AND MATERIALS
Animals and experimental design
Thirty-six nine-week-old male BALB/c mice were obtained from the Central Bioterium (Health and Biology Science Center) of the Universidade Federal de Viçosa, Brazil. The animals were housed at the Experimental Nutrition lab in a temperature-controlled room (22±2°C) with a 12-hours light/dark cycle and ad libitum access to water and food. The animal protocol was approved by the Ethics Committee on Animal Experimentation from the Universidade Federal de Viçosa, Brazil, under the process number 30/2016 and performed according to the Guide for the Care and Use of Laboratory Animals, National Academy of Sciences (US). Upon arrival, mice were induced to pre-neoplastic colorectal lesions by intraperitoneal injection of 1,2-Dimethylhydrazine (DMH) (Sigma, Saint Louis, USA) (20mg/kg body weight) once a week during 8 weeks [45]. DMH was dissolved in 0.9% saline solution containing 1mM EDTA and 10mM sodium citrate, at pH 8 [47]. Then, the animals were randomly assigned to two experimental groups: The group receiving control diet (DC; n=17) or the group receiving PBY-supplemented diet (DY; n=19) (Table 1). The sample size was calculated according to the aberrant crypt data reported by de Moura and co-workers [34], to achieve a statistical power of 90%. Diets were offered ad libitum for 8 weeks. Subsequently, mice were anesthetized using 3% isoflurane and blood was collected from the retro-orbital sinus. Mice were euthanized by cervical dislocation and tissue and feces were harvested for analysis.
|
Ingredients (g/100g) |
Control Diet (g/kg) |
PBY Diet (g/kg) |
|
Casein |
155.5 |
145.3 |
|
Dextrinized starch |
155 |
148.2 |
|
Sucrose |
100 |
0 |
|
Soybean oil |
40 |
40 |
|
Fiber (microfine cellulose) |
67 |
0 |
|
Mineral mix |
35 |
35 |
|
Vitamin mix |
10 |
10 |
|
L-Cystine |
1.8 |
1.8 |
|
Choline bitartrate |
2.5 |
2.5 |
|
Cornstarch |
303 |
263.6 |
|
†PBY |
0 |
353.6 |
|
Distilled water |
130.2 |
0 |
|
Total weight (g) |
1000 |
1000 |
|
Total energy (kcal) |
3214 |
3064 |
Table 1: AIN-93M composition in the control and PBY diets.
Note: †Centesimal composition and digestible content of carbohydrate, inulin, and FOS on PBY in 100g of product: Fructose: 15.25g; Glucose: 8.59g; Sucrose: 6.35g; FOS: 12.81g; Inulin: 4.16g; Total carbohydrate: 45.49g; Fibers: 1.99g; Humidity: 36.82g; Ashes: 3.39g; Lipids: 0.21g; Protein: 2.89g. AIN: American Institute of Nutrition; FOS: Fructooligosaccharides; PBY: yacon-based product.
Chemical composition of the PBY might vary depending on yacon variety/cultivar, growing conditions, post-harvest treatments, and storage conditions.
Yacon-Based Product (PBY) and experimental diet
Yacon was purchased from a local market in Viçosa-Minas Gerais, Brazil. The PBY was processed according to the methodology proposed by Rodrigues and co-workers [23], which is currently going through patent request PI 1106621-0. The chemical composition of PBY (carbohydrates, proteins, fats, fiber, ash, and humidity) was determined according to the AOAC methodology (AOAC, 1997). FOS and inulin contents were determined by High-Performance Liquid Chromatography (HPLC) with a BIO-RAD brand HPX-87p column (lead stationary phase) using purified water for the mobile phase.
The experimental purified diets were based on the AIN93-M diet, as recommended by the American Institute of Nutrition [49]. The PBY-supplemented diet contained 6.0% FOS + Inulin from PBY (Table 1), as suggested by Paula and co-workers [46]. Casein, sucrose, dextrinized starch, starch, and fiber adjustments were made to control and PBY diets to obtain similar amounts of carbohydrates, lipids, proteins, fibers, and calories. The diets were made in a pellet format and stored at -20°C for a maximum period of thirty days before consumption.
The calculation of the human equivalent amount of dietary PBY consumed by male BALB/c mice was performed according to the body surface area normalization method as previously describe [50]. PBY supplementation was 353.56g PBY/kg of diet. In our study, the average daily consumption was 6g per mouse. This is equivalent to 2,121g of PBY (corresponding to 272mg of FOS and 88mg of inulin) daily for an adult mouse of 45g, which approximately corresponds to 47g PBY/kg body mass/day. Taking into account that the average human adult weight is 70kg, it is thus converted to 229g per day for humans. It is worth noting that the equivalent dose calculation according to Reagan-Shaw and co-workers [50] was only used as a comparative method, with no intention to extrapolate the results for human intake, since the dose-response to nutrient intake may vary according to the experimental model used or to the individual participant in a study.
Body weight and dietary intake
In order to evaluate the weight loss/gain of the animals, individual body weight was weekly recorded using a digital weighing scale. Dietary intake was determined according to the difference between the diet offered and wasted. Quantification was done every 3 to 5 days during the whole dietary intervention on a digital weighing scale. Data represent the diet consumption per cage (7 to 10 animals/cage). Diet consumption (g) was corrected by humidity (fresh diet weight (g)/diet weight (g) in the cage per day).
Fecal characteristics
Fresh feces were harvested and stored at -80ºC for pH determination. For each animal, an aliquot of feces was diluted in distilled water (1:10), homogenized, and the pH was measured ona digital pH meter (Hexis ultra Basic UB-10® duly calibrated) in a temperature-controlled room until pH stabilization [51].
Fecal humidity was determined using approximately 110mg of moist stools. Feces were weighed in Petri dishes, previously dried for 24 hours at 105ºC. Afterward, the material (Petri dish + feces) was placed in a desiccator until it reached room temperature and later weighed for determination of humidity through equation 1 [52].
Equation 1: humidity (%) = (Initial Weight*-Final Weight*) x 100 / (Sample Weight)
*Initial and final weight: weight of the dishes containing the samples, before and after drying, respectively.
In the last week of the experiment, fresh feces were harvested and the fecal score was assessed according to the method used by De Freitas and co-workers [53], with some modifications:
- Firm or normal feces consistency;
- Viscous non-diarrheal feces;
- Watery feces, characteristic of diarrhea.
Anatomical characteristics
After euthanasia, the organs (liver, spleen, small intestine, cecum, colon, and abdominal adipose tissue) were excised, washed in Phosphate-Buffered Saline (PBS) (NaCl: 0.85%, NaH2PO4: 0.023%, NaHPO42H2O: 0.15%, pH 7.2) and weighed using a semi-analytical weighing scale. The hepatosomatic index was obtained by dividing the liver weight by the animal’s body weight. The colon length was obtained by measuring from the end portion of the cecum till the end portion of the rectum on a flat surface using a millimeter ruler.
Serum biomarkers analysis
After euthanasia, blood was collected and centrifuged at 1190 x g/10 minutes/4°C. Total cholesterol (mg/dl), triglycerides (mg/dl), Gamma-Glutamyl Transferase (GGT) (u/L), Aspartate Aminotransferase (AST) (u/L), Alanine Aminotransferase (ALT) (mg/L), albumin (g/dl), alkaline phosphatase (u/L), creatinine (mg/dl), and urea (mg/dl) were assessed in the serum by specific colorimetric assays (Bioclin®, Brazil), using a clinical chemistry analyzer BS-200 (Mindray®).
Fecal SCFA quantification
SCFA quantification was assessed according to the method used by Smiricky-Tjardes and co-workers [54], with some modifications. Fifty mg of frozen feces were weighed and thoroughly vortexed with deionized water (950μL). While incubated on ice for 30 minutes, the samples were homogenized for 2 minutes every 5 minutes. Samples were centrifuged (10,000 x g, 30 minutes, 4°C) three times and the supernatants were collected. The final supernatant from each sample was filtered through a 0.45μm membrane and transferred to vials. SCFA were measured by High-Performance Liquid Chromatography - HPLC (Shimadzu®) on an Aminex HPX 87H column (300 x 7,8mm, Bio-rad®, Rio de Janeiro, Brazil) at 32°C with acidified water (0.005 M H2SO4) as eluent at a flow rate of 0.6mL/minute. The products were detected and quantified by an ultraviolet detector (model SPD-20A VP) at 210nm. Standard curves for formic, acetic, propionic, isobutyric, butyric, isovaleric, valeric, isocaproic, and caproic acids (SUPELCO®) were performed. Results are expressed as μmol SCFA/g feces.
Aberrant Crypt Foci (ACF) counts
After removal, the large intestine was washed in PBS solution, opened along the mesenteric margin, placed in paraffin plates with the mucous facing the top of the plate, and fixed in Carson’s formalin [55], for 24 hours. Following fixation, flat colons were equally divided into three segments (proximal, medium, and distal) and stained with 0.1% methylene blue for 2 minutes to quantify ACF under a BX-60 light microscope (Olympus, Tokyo, Japan) with a magnification of 200X. ACF were counted across the mucosal surface of the large intestine by three independent observers. The ACF categorization was based on the number of aberrant crypts per focus: foci with fewer than or equal to three crypts (ACF≤3) and foci with more than three crypts (ACF>3) [56].
Histopathological score
Paraffin-embedded colonic tissues were sectioned at 5-μm thickness and subjected to Hematoxylin-Eosin (H&E) staining. Each colonic section was scored as previously described by Kangand co-workers [57]. The scoring system was based on three independent parameters: severity of inflammation (0-3), depth of injury (0-3) and crypt damage (0-4). The sum of such scores provides a total histopathological score with 0 being a normal tissue and 10 being the most extensive/severe disease symptoms. The assessment of histopathological score was performed using a BX-60® light microscope (Olympus, Tokyo, Japan) at 200x magnification. Three sections per animal that covered the length of 500μm of the proximal colon were stained and evaluated in a blinded manner.
Determination of leukocytes by immunophenotyping
Leukocytes were quantified and characterized in the colon mucosa as previously described [58], with some modifications. The colon was removed and washed in ice-cold PBS, cut into small fragments and incubated in a cell culture medium, DMEM, pH 7.2 (Sigma-Aldrich ™) for 90 minutes at 37°C. The suspension was centrifuged three times at 42 x g for 5 minutes to collect the supernatant, and lastly at 543 x g for 10 minutes. After the last centrifugation, the remaining pellet was then resuspended with PBS buffer (100μL, pH 7.2). Cell viability was assessed with Trypan blue exclusion and cells were counted in a Neubauer chamber. The obtained leukocytes were incubated with the following antibodies, according to the manufacturer’s instructions: Anti-CD4 (PeCy5), anti-CD25 FITC-conjugated, anti-CD196 (anti-CCR6) PE-conjugated, anti-CD49b (anti-PanNK) APC-conjugated, anti-CD8 PECy7-conjugated (Biolegend, San Diego, CA, USA). Leukocytes (1x104 events) were acquired (FACS Verse™ and BD FAC Suite software; BD Biosciences Phar Mingen San Jose, CA, USA) according to size (forward scatter) and granularity (side scatter). One or two stains were used to identify TCD4 lymphocytes (CD4+), TCD8 lymphocytes (CD8+), regulatory T cells (CD4+CD25+), Th17 lymphocytes (CD4+CD196+) and Natural Killer cells (CD49b+). Results are expressed as means ± Standard Error of the Mean (SEM) of the percentage of each cell subpopulation specifically stained within a selected gated.
Real-time PCR
Total RNA was extracted from the whole colon using Trizol reagent (Invitrogen™, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized using 5μg of RNA through a reverse transcriptase reaction using a specific kit (GoScript™ Reverse Transcription System, Promega, Madison, WI, USA). Random primer and RNAse free water were added to the sample and heated for 5 minutes at 70ºC. Subsequently, a mixture containing the reverse transcriptase dNTPs and ribonuclease inhibitor was added to the sample and heated for 60 minutes at 37ºC. Quantitative mRNA analysis was performed by Real-time PCR on the Applied Biosystems® 7500 Real-Time PCR System. Sybr green (Ludwig, Biotec). Primer sense, primer antisense (400nm/reação), DNase free water and the sample (250ng cDNA/µl) were added at each reaction. Standard PCR conditions were used during the reading (7500 software V2.3, Applied Biosystems®). The sequences of murine primers (Integrated DNA Technologies®) were as follows: FOXP3, sense: 5’-AGG AGC CGC AAG CTA AAA GC- 3’, antisense: 5’-TGC CTT CGT GCC CAC TGT-3’; RORγt, sense: 5’-GGA GCT CTG CCA GAA TGA CC-3’, antisense: 5’-CAA GGT TCG AAA CAG CTC CAC-3’; Tbet, sense: 5’-AGC AAG GAC GGC GAA TGT T-3’, antisense: 5’-GGG TGG ACA TAT AAG CGG TTC-3’; GATA3, sense: 5’-CAA TCT GAC CGG GCA GGT-3’, antisense: 5’-CAG AGA CGG TTG CTC TTC CG-3’; GAPDH, sense: 5’-TCA ACA GCA ACT CCC ACT CTT CCA-3’, antisense: 5’-ACC CTG TTG CTG TAG CCG TAT TCA-3’.mRNA values were calculated according to the constitutive GAPDH gene on the basis of the ΔΔCt algorithm.
Statistical analysis
Results are expressed as means ± SEM. Data were analyzed using Graph Pad Prism version 6.0. Mean values were firstly tested by Kolmogorov-Smirnov test and the groups with normal distribution were tested using unpaired Student’s t-test. Groups that did not show normal distribution were tested using Mann-Whitney test. Statistical differences were considered when the p<0.05 (*) or p<0.001 (**).
RESULTS
PBY-supplemented diet does not alter diet intake or body weight
There was no difference in the weekly weight gain of the animals either during pre-neoplastic lesion induction (1st to 8th week) or dietary intervention (9th to 16th week) (Figure 1A). In addition, there was no change in the average dietary intake between the groups during dietary treatment (Figure 1B).
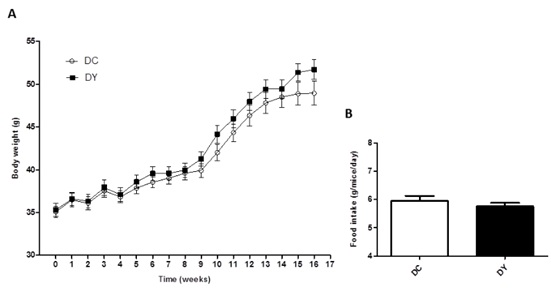
Figure 1: Weight gain and food intake in BALB/c mice induced to colonic pre-neoplastic lesions and submitted to 8 weeks of dietary treatment. (A) Average weight gain of animals over the weeks. The data are expressed as means ± SEM (n=17 (DC); n=19 (DY)). (B) Mean food intake (g/mice/day) of PBY diet or control diet consumed during the period of dietary intervention (8 weeks). The data are expressed as means ± SEM of each experimental group (means referring to the group/cage consumption pool (4 pools/week). Statistical differences between groups were analyzed by the unpaired Student's t-test or Mann Whitney, (*) p<0.05, (**) p<0.001. DC: Dimethylhydrazine-induced mice treated with control diet; DY: Dimethylhydrazine-induced mice treated with PBY diet; PBY: yacon-based product.
PBY-supplemented diet reduces fecal pH and increases fecal humidity
The physical-chemical evaluation of feces contributes to determining the direct and indirect effects of prebiotics on the intestine. In this study, fecal pH was lower in the animals treated with PBY-supplemented diet (p<0.05) (Figure 2A). Fecal humidity was higher in the group receiving the PBY-supplemented diet when compared to control (p<0.001) (Figure 2B). Qualitative analysis through the fecal score shown 70% of PBY-treated animals in score 2 (viscous non-diarrheic stools) and 30% in score 3 (aqueous feces characteristic of diarrhea). Animals fed control diet were 100% in score 2.
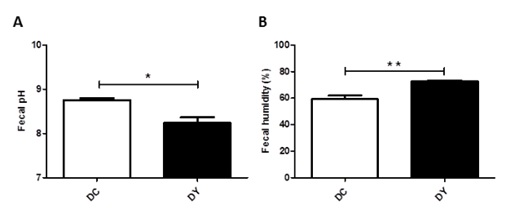
Figure 2: Fecal characteristics in BALB/c mice induced to colonic pre-neoplastic lesions and submitted to 8 weeks of dietary treatment. (A) Fecal pH; (B) Fecal humidity percentage. The data are expressed as means ± SEM (n=10 mice/group). Statistical differences between groups were analyzed by the unpaired Student's t-test or Mann Whitney, (*) p<0.05, (**) p<0.001. DC: Dimethylhydrazine-induced mice treated with control diet; DY: Dimethylhydrazine-induced mice treated with PBY diet; PBY: yacon-based product.
PBY-supplemented diet increases bowel and adipose tissue weight
PBY-supplemented diet increased colon (p<0.05), cecum (p<0.001), small intestine (p<0.05) and abdominal adipose tissue weight (p<0.05) when compared to controls (Figure 3B-E). There was no significant change in colon length, liver and spleen weight or hepatosomatic index between the groups (Figure 3 A-H).
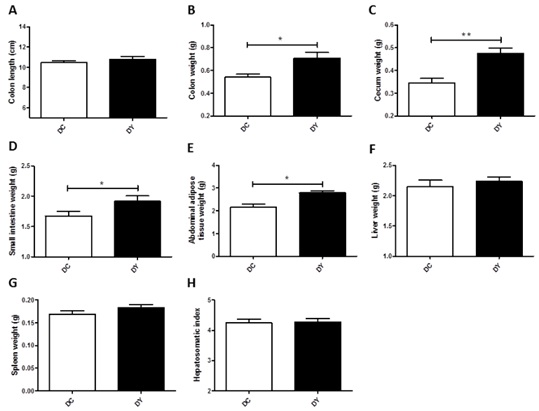
Figure 3: Anatomical characteristics of visceral organs of BALB/c mice induced to colonic pre-neoplastic lesions and submitted to 8 weeks of dietary treatment. (A) Colon length (cm); (B) Colon weight (g); (C) Cecum weight (g); (D) Small intestine weight (g); (E) Abdominal adipose tissue weight (g); (F) Liver weight (g); (G) Spleen weight (g); (H) Hepatosomatic index. The data are expressed as means ± SEM (n=17 (DC); n=19 (DY)). Statistical differences between groups were analyzed by the unpaired Student's t-test or Mann Whitney, (*) p<0.05, (**) p<0.001. DC: Dimethylhydrazine-induced mice treated with control diet; DY: Dimethylhydrazine-induced mice treated with PBY diet; PBY: yacon-based product.
PBY-supplemented diet increases serum albumin and alkaline phosphatase
Serum biomarkers analysis was performed to verify possible changes in lipid profile and hepatic and renal function. Total cholesterol, triglycerides, GGT, AST, ALT, creatinine, and urea remained unchanged between the groups (Figure 4 A-I). However, PBY-treated mice exhibited higher albumin (p<0.05) and alkaline phosphatase (p<0.05) when compared to controls (Figure 4).
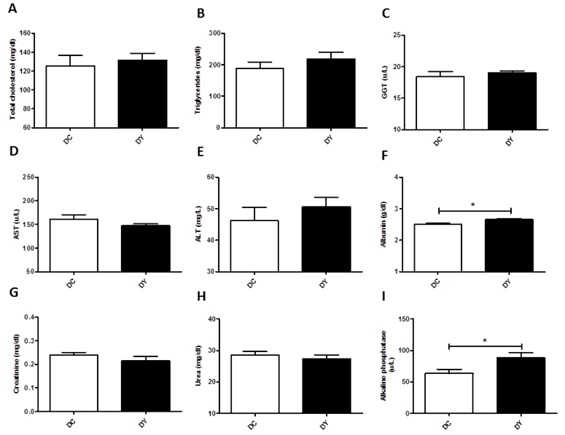
Figure 4: Serum biomarkers level in BALB/c mice induced to colonic pre-neoplastic lesions and submitted to 8 weeks of dietary treatment. (A) Total cholesterol (mg/dl); (B) Triglycerides (mg/dl); (C) GGT (gamma-glutamyltransferase) (u/L); (D) AST (aspartate aminotransferase) (u/L); (E) ALT (alanine aminotransferase) (mg/dl); (F) Albumin; (G) Creatinine; (H) Urea; (I) Alkaline phosphatase. The data are expressed as means ± SEM (n=10 mice/group). Statistical differences between groups were analyzed by the unpaired Student's t-test or Mann Whitney, (*) p<0.05, (**) p<0.001. DC: Dimethylhydrazine-induced mice treated with control diet; DY: Dimethylhydrazine-induced mice treated with PBY diet; PBY: yacon-based product.
PBY-supplemented diet increases fecal SCFA concentrations
The intestinal bacterial activity was assessed through fecal SCFA concentrations. PBY-treated animals showed increased formic (p<0.001), acetic (p<0.05), propionic (p<0.05), butyric (p<0.001), and valeric (p<0.05) acid concentrations when compared to controls (Figure 5A-C, E and G). There was no difference in isobutyric, isovaleric, isocaproic, and caproic acid concentrations between the groups (Figure 5).
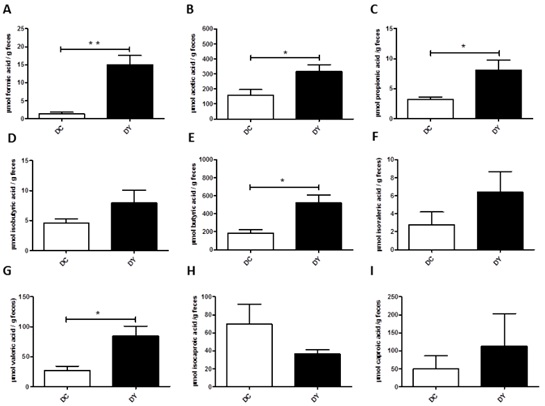
Figure 5: Fecal SCFA concentration in BALB/c mice induced to colonic pre-neoplastic lesions and submitted to 8 weeks of dietary treatment. (A) Formic acid (µmol); (B) Acetic acid (µmol); (C) Propionic acid (µmol); (D) Isobutyric acid (µmol); (E) Butyric acid (µmol); (F) Isovaleric acid (µmol); (G) Valeric acid (µmol); (H) Isocaproic acid (µmol); (I) Caproic acid (µmol). The data are expressed as means ± SEM (n=10 mice/group). Statistical differences between groups were analyzed by the unpaired Student's t-test or Mann Whitney, (*) p<0.05, (**) p<0.001. DC: Dimethylhydrazine-induced mice treated with control diet; DY: Dimethylhydrazine-induced mice treated with PBY diet; PBY: yacon-based product.
PBY-supplemented diet does not change ACF counts
ACF was assessed to confirm pre-neoplastic lesions formation in DMH-induced mice and to detect the effectiveness of PBY diet in reducing its formation. In both groups, a greater number of ACF categorized as ≤3 were observed concerning ACF >3 (Figure 6). In ACF ≤3 categorization, ACF were more abundant in the proximal colon, followed by medial and distal colon (Figure 6). There was no significant difference in total ACF count or categorized (ACF ≤3 and ACF >3) per colon segment between the groups (Figure 6).
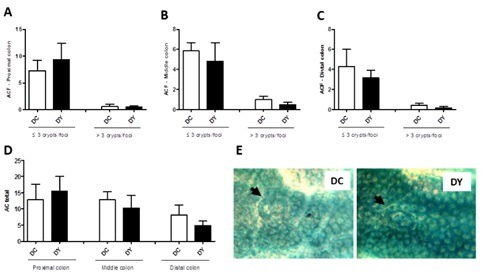
Figure 6: Colonic Aberrant Crypt Foci (ACF) in BALB/c mice fed with PBY or control diet for 8 weeks after DMH-induced pre-neoplastic lesions. (A) Proximal colon; (B) Middle colon; (C) Distal colon; (D) Total Aberrant Crypt (AC). The data are expressed as means ± SEM (n=7 (DC); n=6 (DY)). Statistical differences between groups were analyzed by the unpaired Student's t-test or Mann Whitney, (*) p<0.05, (**) p<0.001. (E) Photomicrographs representative of colon luminal surface: DC - Dimethylhydrazine-induced mice treated with control diet; DY - Dimethylhydrazine-induced mice treated with PBY diet; The black arrows indicate ACF containing three aberrant crypts (DC) and two aberrant crypts (DY). 100x magnification. PBY: yacon-based product.
PBY-supplemented diet does not alter the colonic histopathological score
In both groups, DMH was able to promote colonic histopathological changes at crypt level (Figure 7B), inflammation (Figure 7C), depth of damage (Figure 7D), and histopathological score (Figure 7A). Both groups showed crypt damages. Damage was considered when the crypt light does not reach the intestinal lumen, besides the oval aspect. The control group mostly presented crypts with 2/3 of damage (Figure 7E). PBY-treated animals showed crypts with 1/3 of damage. Inflammatory infiltrates were found in both groups and were minimally expressive (Figure 7E). Damage was mostly restricted to the mucosa.
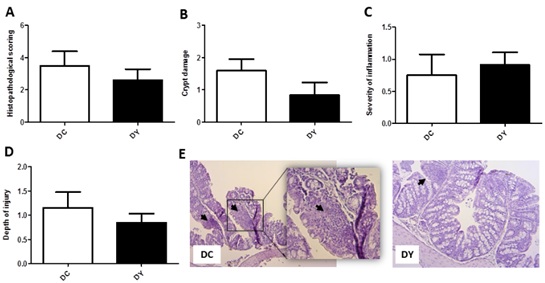
Figure 7: Histopathological score of the proximal colon of BALB/c mice induced to pre-neoplastic colorectal lesions and submitted to 8 weeks of dietary treatment. (A) Histopathological score; (B) Crypt damage; (C) Severity of inflammation; (D) Depth of injury. The data are expressed as means ± SEM (n=7 (DC); n=6 (DY)). Statistical differences between groups were analyzed by the unpaired Student's t-test or Mann Whitney, (*) p<0.05, (**) p<0.001. DC, control diet; DY, PBY diet. (E) Photomicrographs representative of proximal colon mucosa: DC - Dimethylhydrazine-induced mice treated with control diet; DY -Dimethylhydrazine-induced mice treated with PBY diet; The black arrows indicate inflammatory infiltrates. 100x magnification; Highlight: 200x magnification. PBY: yacon-based product.
PBY-supplemented diet does not alter the percentage of colonic lymphocytes
Immunophenotyping of colonic immune cells was performed to evaluate the intestinal lymphocyte population. There were no significant changes in the percentage of CD4+ and CD8+ T cells, Treg cells (CD4+CD25+), Th17 cells (CD4+CCR6+), NK cells, and CD4/CD8 ratio between the groups (Figure 8). Although not significant, there was a reduction on the percentage of CD4+ T lymphocytes (30.4%), and increments in CD8+ T cells (37.7%), CD8+/CD4+ ratio (156.9%) and Treg cells (84.8%) in the colon of PBY-treated animals when compared to controls.
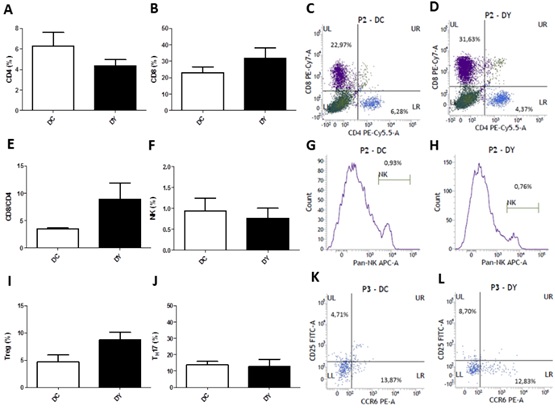
Figure 8: Phenotypic profile of colon lymphocytes of BALB/c mice induced to colonic pre-neoplastic lesions and submitted to 8 weeks of dietary treatment. (A) % CD4+ cells; (B) % CD8+ cells; Flow cytometry plots from forward scatter / side scatter-gated lymphocytes cells marked with anti-CD8 (PE-Cy7) on the upper left side quadrants (% CD8 cells) and anti-CD4 (PE-Cy5) on the lower right side quadrants (% CD4 cells) on DC (C) and DY groups (D); (E) % CD8+/CD4+ ratio; (F) % NK cells (Pan NK+ cells); Histogram plots of gated lymphocytes cells marked with anti-Pan-NK (APC) on DC (G) and DY groups (H); (I) % Treg cells (CD4+CD25+ cells); (J) % Th17 cells (CD4+CCR6+). Flow cytometry plots from gated CD4+ cells marked with anti-CD25 (FITC) on the upper left side quadrants (% Treg cells), and anti-CCR6 (PE) on the lower right side quadrants (% Th17 cells) on DC (K) and DY groups (L). The results are represented as means ± SEM of each experimental group (n=5 (DC); n=6 (DY)). Statistical differences between groups were analyzed by the unpaired Student's t-test or Mann Whitney, (*) p<0.05, (**) p<0.001. DC: Dimethylhydrazine-induced mice treated with control diet; DY: Dimethylhydrazine-induced mice treated with PBY diet; PBY: yacon-based product; APC: allophycocyanin; FITC: Fluorescein isothiocyanate; PBY: yacon-based product; PE: Phycoerythrin.
PBY-supplemented diet increases the expression of RORγt and T-bet in the colon
Transcription factors of the adaptive immune response were evaluated to verify the pattern of the intestinal immune response in the colon. PBY-treated animals showed down-regulation of FOXP3 (p<0.05) and up-regulation of RORγt (p<0.05) and T-bet (p<0.05) when compared to controls (Figure 9). GATA-3 expression, although up-regulated in the PBY-treated groups (p <0.05), should not be considered biologically relevant due to the very low expression in both groups (DC: 0.023; DY: 0.046).
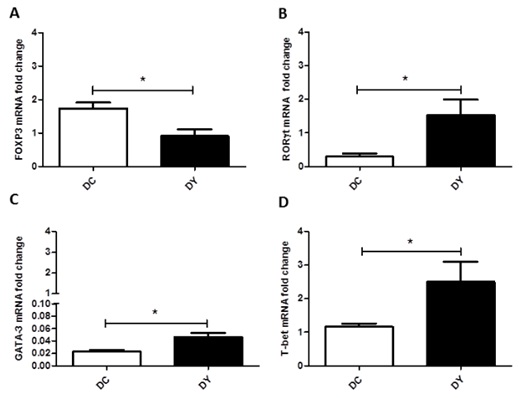
Figure 9: Transcription factors expression of the adaptive immune response in the colon of BALB/c mice induced to colonic pre-neoplastic lesions and submitted to 8 weeks of dietary treatment. Real-time PCR analysis of the colonic gene expression of FOXP3 (A), RORγt (B), GATA-3 (C) and T-bet (D). Gene expression was calculated in relation to the constitutive GAPDH gene and presented as a relative variation of the control group. The data are expressed as means ± SEM (n=6 (DC); n=4 (DY)). Statistical differences between groups were analyzed by the unpaired Student's t-test or Mann Whitney, (*) p<0.05, (**) p< 0.001. DC: Dimethylhydrazine-induced mice treated with control diet; DY: Dimethylhydrazine-induced mice treated with PBY diet; PBY: yacon-based product.
DISCUSSION
Yacon has been associated with the reduction of pre-neoplastic lesions in experimental CRC models [26,39], especially by its high content of FOS and inulin. The benefits of yacon are directly related to the growth of probiotic bacteria and their metabolite production, such as SCFA [59]. Despite the promising role of yacon on the immune system modulation, as indicated by increased IgA production [33,37,38], reduction of pathogenic bacteria [28], increased fecal SCFA, enhanced Treg cells, and down-regulation of RORγt in healthy BALB/c mice [20], it is still unknown the adaptive immune response induced by yacon consumption during colorectal carcinogenesis. Thus, this study aimed at investigating the effects of PBY consumption on ACF improvement in DMH-induced mice as well as to assessing the immunological mechanisms behind this process.
We observed reduced fecal pH and increased humidity and viscosity of feces in PBY-treated mice. Yacon-supplemented diet has already been shown to reduce fecal pH in rodents [26] and improve fecal consistency and intestinal transit in humans [28]. In obese individuals, yacon consumption also improves satiety and decreases body weight [27]. However, in our study, dietary intake and body weight remained unchanged between the groups during the dietary intervention, probably because such effects should be more pronounced in overweight conditions. The increased weight of colon, cecum, and small intestine verified in PBY-treated mice are supposedly due to the trophic effect of yacon on the intestinal mucosa. Yacon is demonstrated to increase the number and depth of intestinal crypts [31,60] and enhance SCFA production, like butyric acid, by intestinal microbiota metabolism. This SCFA can be used as an energetic substrate for colonocytes [61], thus favoring the trophic effect on the intestine.
Metabolic function biomarkers are essential to assess health, especially during carcinogenesis. In this study, the PBY diet did not alter biomarker concentrations of lipid metabolism and hepatic and renal function. However, it was verified increased albumin and alkaline phosphatase in PBY-treated animals. Higher albumin levels may be interesting for carcinogenesis-induced animals, as albumin levels decrease during inflammation, which is considered a negative reagent of the acute-phase inflammatory response [62]. Also, albumin levels might have increased owing to higher fecal SCFA concentration in PBY-treated mice, once albumin is responsible to carry fatty acids in the bloodstream [63]. The increase in alkaline phosphatase can be attributed to its role in osteogenesis [64], as yacon promotes an increase in intestinal calcium absorption and bone deposition [60].
Fecal SCFA concentration reflects bacterial activity in the colon, an important indicating of intestinal health. PBY-supplemented diet promoted increased formic, acetic, propionic, butyric, and valeric acid concentrations on feces. Such an increase may be attributed to the prebiotic role of yacon, which contains high concentrations of FOS and inulin [65], stimulating probiotic bacteria growth such as lactobacilli and bifidobacteria [28,29]. The use of yacon flour has already been shown to increase propionic and butyric acid concentrations in rodents feces [26]. Besides the trophic effect on the intestinal mucosa, butyrate has also apoptotic action on cancer cells [66]. SCFA, mainly butyric acid, and to a lesser extent propionic acid, are supposed to act as histone deacetylase (HDAC) inhibitors [42,43]. HDAC inhibitors can trigger histone hyperacetylation, which allows the opening of chromatin and, therefore, the gene transcription activation. This process promotes the expression of silenced genes related to apoptosis and cell cycle arrest, contributing to the suppression of colon cancer cell growth [41,44]. The SCFA-induced cell growth blockade is associated with increased expression of the p21 cell cycle inhibitor and down regulation of cyclin B1 in colon cancer cells [42]. In addition, the role of the SFCA- SFCA receptor axis in the suppression of bacterial invasion, chronic inflammation and inflammation-associated intestinal carcinogenesis has recently been demonstrated [67].
ACF is considered to be pre-neoplastic lesions or a previous event to CRC development [56]. In the present study, DMH-induced colorectal tumorigenesis in male BALB/c mice resulted in a greater amount of ACF in the proximal colon, followed by the medial colon, and in lesser number in the distal colon. Most of crypts foci was containing 3 aberrant crypts utmost. ACF induction also compromised the structure of intestinal mucosa, thus affecting the architecture of intestinal crypts, which usually leads to the recruitment of inflammatory infiltrates. PBY-supplemented diet did not alter intestinal ACF counts. Although an improvement in the crypt structure could be noticed, the histopathological score remained unchanged. Some authors have shown that DMH-induced animals fed with yacon-containing diets present a reduction of ACF count [26,39]. Yacon flour, containing 20.4% FOS, added to the diet at 1%, offered for 13 weeks after DMH-induction, significantly reduced the lesions in rats [39]. Similarly, a yacon flour-supplemented diet (7.5% FOS) offered for 8 weeks to DMH-induced rats (25mg/kg body weight) showed more than 40% reduction in ACF count [26]. The lack of changes in ACF counts in our study might be justified by the lesser percentage of FOS and inulin (6%) in PBY-supplemented diet, yacon processing type, or the short period of dietary intervention (8 weeks), which only started after ACF induction.
The profile of immune cells in the colon has been studied as a prognostic factor in CRC. Regarding CD4+ and CD8+ T lymphocytes, Treg cells (CD4+CD25+), Th17 cells (CD4+CCR6+), NK cells and CD4/CD8 ratio, no significant differences between the groups were noticed in our study. Nevertheless, PBY-treated animals had an increase in CD8+/CD4+ T cells ratio (156.9%), which denotes a higher prevalence of CD8+ T over CD4+ T cells. CD8+ T lymphocytes are strongly related to the anti-tumor role in CRC [9]. There was also an increase in Treg cells (84.8%) that might be attributed to the modulatory role of PBY, recently described by our research group [20]. However, the role of Treg cells is still controversial in CRC, as they are related to either tumor progression [68-70] or better CRC prognosis [16,71,72]. Treg cells play a regulatory role in the immune response, secreting cytokines and immune-modulating factors [73], able to suppress the effector function of both cytotoxic T lymphocytes and NK cells, which act eliminating tumor cells [74]. Furthermore, Treg cells may not only mediate the immunosuppression of immune responses to the tumor [15], but also attenuate the inflammatory immune response, especially when triggered by intestinal bacteria [16]. This fact denotes the strong immunosuppressive role of Treg cells during intestinal dysbiosis, usually seen in the CRC. Similarly to Treg cells, Th17 lymphocytes might have a dual role in CRC [9]. Although related to tumor progression owing to its main cytokine profile (IL-17A, IL-17F, IL-21, IL-22 and, IL-6) [12], Th17 lymphocytes may also favor neutrophils recruitment through IL-8 secretion, and drive highly cytotoxic CCR5+CCR6+CD8+ T cells into the tumor tissue, through CCL5 and CCL20 release [9].
Concomitantly, we investigate what could be the main immune pathways induced by PBY diet during colon carcinogenesis. Thus, the expression of transcription factors key to the adaptive immune response were assessed. The PBY-supplemented diet was able to down-regulate FOXP3 expression and up-regulated both RORγt and T-bet expression in the colon. FOXP3 is the key transcription factor for Treg cell differentiation. The differentiation of Th17 lymphocytes, in turn, requires the RORγt transcription factor [75], which is expressed in CD4+T cells in an opposite manner of FOXP3 expression. High amounts of transforming growth factor-β (TGF-β) and pro-inflammatory cytokines, such as IL-6, IL-21, and IL-23 promote RORγt expression, whereas a low amount of pro-inflammatory cytokines and high amount of TGF-β induce FOXP3 expression [75]. Intestinal FOXP3 down-regulation in PBY-treated animals could be contributing to reduce Treg cells, which may be beneficial since such cells are be involved in anti-tumor immune response suppression [15]. Interestingly, cancer cells may also express FOXP3 [76], thus generating a confounding factor in the overall tissue analysis. Somehow, FOXP3 down-regulation may indicate a positive effect of PBY supplementation; however, it is necessary to differentiate FOXP3+ cancer cells from FOXP3+ lymphocyte populations in intestine tissue for better comprehension of this data. Regarding Th17 cells, we did not observe changes between the groups; nevertheless, RORγt up-regulation might suggest a possible stimulation of theTh17 profile in the immune response.
T-bet transcription factor initiates the development of the Th1 cell line, which secretes interferon-gamma (IFN-γ), the main cytokine of this lineage. It also produces IL-2 and TNF-α [77]. In contrast, GATA-3 is the specific transcription factor for Th2 cell line development, which secretes the key cytokine IL-4 in addition to IL-5, IL-6, IL-9, and IL-13 [77-79]. It is widely recognized that IFN-γ-producing Th1 cells, as well as CD8 T lymphocytes, play an important role in inhibition and death of tumor cells [80]. Moreover, Th1 cells produce IL-2, which is essential for cytotoxic T lymphocyte proliferation [81]. Th1 cells have already been shown to be associated with better CRC prognosis [10,11]. Therefore, increased T-bet expression in PBY-treated animals could be contributing to impairing tumor growth and, thus, to better disease prognosis.
In this study, we confirmed our hypothesis that PBY may increase fecal SCFA concentration and modulate the intestinal immune response against colorectal carcinogenesis, verified by FOXP3 down-regulation, and RORγt and T-bet up-regulation. Despite our findings, and be knowing the benefits of SCFA (butyric and propionic acids) in neoplastic cell apoptosis, as well as the role of Th1 cells (T-bet expression) in CRC control, it was not possible to verify changes in ACF counts with PBY consumption.
Therefore, certain limitations in this study should be considered, for instance, the amount of FOS and inulin in PBY-supplemented diet, and the short period of dietary intervention. Besides, the restricted number of samples per analysis that required the entire colon, each one (ACF counts, immunophenotyping, and Real-time PCR) may have contributed to the absence of statistical significance, especially concerning immunophenotyping analysis. We have also considered that the human equivalent amount of dietary PBY (229g PBY per day for a 70kg adult) might be difficult to be achieved at once. Therefore, we suggest that PBY dosage could be spread throughout the day, thus favoring the inclusion of small portions in the usual diet, for example, in juices, shakes or bakery products. Interestingly, Santana and co-workers (de Souza Lima Sant’Anna et al., 2015) reported that in humans a lower amount of PBY (52g PBY twice daily, containing 10g FOS + inulin), mixed with orange juice for 30 days was effective in improving intestinal constipation and microbiota modulation. This fact suggests that prebiotic consumption long term may be more effective for a healthy gut.
CONCLUSION
In summary, PBY consumption did not reduce colonic ACF count; however, it promoted the reduction of fecal pH, and increased humidity and viscosity of feces, improving fecal characteristics. PBY also increased fecal SCFA concentration (formic, acetic, propionic, butyric, and valeric acids), thus indicating probiotic activity stimuli. Besides, the increase in SCFA, mainly butyric acid, can contribute to the activation of tumor suppressor genes. PBY consumption also down-regulated FOXP3 expression and up-regulated RORγt and T-bet expression in the colon, which would contribute to activation and proliferation of CD8 T lymphocytes and better CRC prognosis. Thus, we might infer that during the early stages of colorectal carcinogenesis in mice, PBY consumption contributes to driving the anti-tumor immune response. Therefore, we suggest that PBY, as a functional food, might improve intestinal health during the early stages of colorectal carcinogenesis, especially by modulating both SCFA production and colonic adaptive immune response. Studies concerning cell cycle arrest genes and apoptosis could contribute to the comprehension of this research.
ACKNOWLEDGEMENT
The study was supported by the National Council of Scientific and Technological Development-CNPq, Coordination for the Improvement of Higher Education Personnel-CAPES, Research Support Foundation of the State of Minas Gerais-FAPEMIG, and Microscopy and Microanalysis Division (NMM) of the Universidade Federal de Viçosa-UFV.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVAL
All the trials and experimental protocols were approved by the Ethics Committee on Animal Experimentation of Universidade Federalde Viçosa, Brazil, under process number 30/2016, and performed according to the Guide for the Care and Use of Laboratory Animals, National Academy of Sciences (US).
REFERENCES
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, et al. (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66: 683-691.
- Schwabe RF, Jobin C (2013) The microbiome and cancer. Nat Rev Cancer 13: 800-812.
- van Engeland M, Derks S, Smits KM, Meijer GA, Herman JG (2011) Colorectal cancer epigenetics: Complex simplicity. J Clin Oncol 29: 1382-1391.
- Watson AJM, Collins PD (2011) Colon cancer: A civilization disorder. Dig Dis 29: 222-228.
- Lee W-S, Park S, Lee WY, Yun SH, Chun H-K (2010) Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer 116: 5188-5199.
- Lee WS, Kang M, Baek JH, Lee JI, Ha SY (2013) Clinical impact of tumor-infiltrating lymphocytes for survival in curatively resected stage IV colon cancer with isolated liver or lung metastasis. Ann Surg Oncol 20: 697-702.
- Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L (2012) Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 12: 239-252.
- Fountzilas E, Kotoula V, Tikas I, Manousou K, Papadopoulou K, et al. (2018) Prognostic significance of tumor genotypes and CD8+ infiltrates in stage I-III colorectal cancer. Oncotarget 9: 35623-35638.
- Amicarella F, Muraro MG, Hirt C, Cremonesi E, Padovan E, et al. (2017) Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut 66: 692-704.
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, et al. (2011) Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, Th2, Treg, Th17) in patients with colorectal cancer. Cancer Res 71: 1263-1271.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, et al. (2018) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313: 1960-1964.
- De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, et al. (2015) Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 34: 3493-3503.
- Zhuo C, Xu Y, Ying M, Li Q, Huang L, et al. (2015) FOXP3+ Tregs: Heterogeneous phenotypes and conflicting impacts on survival outcomes in patients with colorectal cancer. Immunol Res 61: 338-347.
- Wolf D, Sopper S, Pircher A, Gastl G, Wolf AM (2015) Treg(s) in cancer: Friends or foe? J Cell Physiol 230: 2598-2605.
- Chen X, Du Y, Lin X, Qian Y, Zhou T, et al. (2018) CD4+ CD25+ regulatory T cells in tumor immunity. Int Immunopharmacol 34: 244-249.
- Ladoire S, Martin F, Ghiringhelli F (2011) Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: The paradox of colorectal cancer. Cancer Immunol Immunother 60: 909-918.
- Vandeputte D, Falony G, Vieira-Silva S, Wang J, Sailer M, et al. (2017) Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 66: 1968-1974.
- Walia S, Kamal R, Dhawan DK, Kanwar SS (2018) Chemoprevention by Probiotics During 1,2-Dimethylhydrazine-Induced Colon Carcinogenesis in Rats. Dig Dis Sci 63: 900-909.
- Mariman R, Tielen F, Koning F, Nagelkerken L (2015) The probiotic mixture VSL#3 has differential effects on intestinal immune parameters in healthy female BALB/c and C57BL/6 mice. J Nutr 145: 1354-1361.
- Marcon LDN, de Sousa Moraes LF, Cruz BC dos S, Teixeira MD de O, Vidon Bruno TC, et al. (2019) Yacon (Smallanthus sonchifolius)-based product increases fecal short-chain fatty acids and enhances regulatory T cells by downregulating RORγt in the colon of BALB/c mice. J Funct Foods 55: 333-342.
- Ojansivu I, Ferreira CL, Salminen S (2011) Yacon, a new source of prebiotic oligosaccharides with a history of safe use. Trends Food Sci Technol 22: 40-46.
- Lachman J, Fernández EC, Orsák M (2003) Yacon [Smallanthus sonchifolia (Poepp. et Endl.) H. Robinson] chemical composition and use-a review. Plant Soil Environ 49: 283-290.
- Rodrigues FC, Castro ASB, Rodrigues VC, Fernandes SA, Fontes EAF, , et al. (2012) Yacon flour and bifidobacterium longum modulate bone health in rats. J Med Food 15: 664-670.
- Scheid MMA, Genaro PS, Moreno YMF, Pastore GM (2014) Freeze-dried powdered yacon: effects of FOS on serum glucose, lipids and intestinal transit in the elderly. Eur J Nutr 53: 1457-1464.
- Campos D, Betalleluz-Pallardel I, Chirinos R, Aguilar-Galvez A, Noratto G, et al. (2012) Prebiotic effects of yacon (Smallanthus sonchifolius & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem 135: 1592-1599.
- Grancieri M, Costa NMB, Vaz Tostes MDG, de Oliveira DS, Nunes LDC, et al. (2017) Yacon flour (Smallanthus sonchifolius) attenuates intestinal morbidity in rats with colon cancer. J Funct Foods 37: 666-675.
- Genta S, Cabrera W, Habib N, Pons J, Carillo IM, et al. (2009) Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin Nutr 28: 182-187.
- de Souza Lima Sant’Anna M, Rodrigues VC, Araújo TF, de Oliveira TT, do Carmo Gouveia Peluzio M, et al. (2015) Yacon-based product in the modulation of intestinal constipation. J Med Food 18: 980-986.
- Utami NWA, Sone T, Tanaka M, Nakatsu CH, Saito A, et al. (2013) Comparison of Yacon (Smallanthus sonchifolius) Tuber with Commercialized Fructo-oligosaccharides (FOS) in Terms of Physiology, Fermentation Products and Intestinal Microbial Communities in Rats. Biosci Microbiota, Food Heal 32: 167-178.
- Veiga P, Pons N, Agrawal A, Oozeer R, Guyonnet D, et al. (2014) Changes of the human gut microbiome induced by a fermented milk product. Sci Rep 4: 6328.
- Sant’Anna M de SL, Rodrigues VC, Araújo TF, Oliveira TT de, Pelúzio M do CG, et al. (2018) Yacon Product (PBY) modulates intestinal constipation and protects the integrity of crypts in wistar rats. Food Nutr Sci 9: 1391-1407.
- Lobo AR, Cocato ML, Borelli P, Gaievski EHS, Crisma AR, et al. (2011) Iron bioavailability from ferric pyrophosphate in rats fed with fructan-containing yacon (Smallanthus sonchifolius) flour. Food Chem 126: 885-891.
- Vaz-Tostes M das G, Viana ML, Grancieri M, Luz TC dos S, Paula H de, et al. (2014) Yacon effects in immune response and nutritional status of iron and zinc in preschool children. Nutrition 30: 666-672.
- Geyer M, Manrique I, Degen L, Beglinger C (2008) Effect of yacon (Smallanthus sonchifolius) on colonic transit time in healthy volunteers. Digestion 78: 30-33.
- Roselino MN, Pauly-Silveira ND, Cavallini DCU, Celiberto LS, Pinto RA, et al. (2012) A potential synbiotic product improves the lipid profile of diabetic rats. Lipids Health Dis 11: 114.
- Habib NC, Honoré SM, Genta SB, Sánchez SS (2011) Hypolipidemic effect of Smallanthus sonchifolius (yacon) roots on diabetic rats: Biochemical approach. Chem Biol Interact 194: 31-39.
- Grancieri M, Machado PA, Oliveira DF, Marcon LN, Vaz Tostes MG, et al. (2016) Efeito da farinha de yacon (Smallanthus sonchifolius) na resposta imunológica intestinal no câncer colorretal. Braspen J 31: 335-339.
- Bonet MEB, Meson O, Leblanc ADM De, Dogi CA, Kortsarz A, et al. (2010) Prebiotic effect of yacon (Smallanthus sonchifoliusi) on intestinal mucosa using a mouse model. Food Agric Immunol 21: 175-189.
- de Moura NA, Caetano BFR, Sivieri K, Urbano LH, Cabello C, et al. (2012) Protective effects of yacon (Smallanthus sonchifolius) intake on experimental colon carcinogenesis. Food Chem Toxicol 50: 2902-2910.
- Sousa S, Pinto J, Rodrigues C, Gião M, Pereira C, et al. (2015) Antioxidant properties of sterilized yacon (Smallanthus sonchifolius) tuber flour. Food Chem 188: 504-509.
- Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, et al. (2012) Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am J Physiol Liver Physiol 302: 1405-1415.
- Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA (2002) The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr 132: 1012-1017.
- McBain JA, Eastman A, Nobel CS, Mueller GC (1997) Apoptotic death in adenocarcinoma cell lines induced by butyrate and other histone deacetylase inhibitors. Biochem Pharmacol 53: 1357-1368.
- Wilson AJ, Byun D-S, Popova N, Murray LB, L’Italien K, et al. (2006) Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem 281: 13548-13558.
- Gomides AFF, de Paula SO, Gonçalves RV, de Oliveira LL, Ferreira CL de LF, et al. (2014) Prebiotics prevent the appearance of aberrant crypt foci (ACF) in the colon of BALB/c mice for increasing the gene expression of p16 protein. Nutr Hosp 30: 883-890.
- Paula HAA, Martins JFL, Sartori SSR, Castro ASB, Abranches MV, et al. (2012) The yacon product PBY: which is the best dose to evaluate the functionality of this new source of prebiotic fructans? In: Functional Foods Forum Probiotics. Turku. Finland.
- Newell LE, Heddle JA (2004) The potent colon carcinogen, 1,2-dimethylhydrazine induces mutations primarily in the colon. Mutat Res Toxicol Environ Mutagen 564: 1-7.
- Association of Official Analytical Chemists (1999) Official Methods of Analysis. 16th edition. Gaitherburg, USA.
- Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition Ad Hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939-1951.
- Reagan-Shaw S, Nihal M, Ahmad N (2007) Dose translation from animal to human studies revisited. FASEB J 22: 659-661.
- Bedani R, Pauly-Silveira ND, Cano VSP, Valentini SR, de Valdez GF, et al. (2011) Effect of ingestion of soy yogurt on intestinal parameters of rats fed on a beef-based animal diet. Brazilian J Microbiol 42: 1238-1247.
- Cecchi HM (2007) Fundamentos Teóricos e Práticos em Análise de Alimentos. 2a. editora Unicamp, editor. São Paulo.
- De Freitas LS, Lopes DC, De Freitas AF, Carneiro JDC, Corassa A, et al. (2006) Avaliação de ácidos orgânicos em dietas para leitões de 21 a 49 dias de idade. Rev Bras Zootec 35: 1711-1719.
- Smiricky-Tjardes MR, Grieshop CM, Flickinger EA, Bauer LL, Fahey GC (2003) Dietary galactooligosaccharides affect ileal and total-tract nutrient digestibility, ileal and fecal bacterial concentrations, and ileal fermentative characteristics of growing pigs. J Anim Sci 81: 2535-2545.
- Carson FL, Martin JH, Lynn JA (1973) Formalin fixation for electron microscopy: A re-evaluation. Am J Clin Pathol 59: 365-373.
- Bird RP (1987) Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett 37: 147-151.
- Kang Y, Xue Y, Du M, Zhu MJ (2017) Preventive effects of Goji berry on dextran-sulfate-sodium-induced colitis in mice. J Nutr Biochem 40: 70-76.
- Belkaid Y, Jouin H, Milon G (1996) A method to recover, enumerate and identify lymphomyeloid cells present in an inflammatory dermal site: A study in laboratory mice. J Immunol Methods 199: 5-25.
- Caetano BFR, de Moura NA, Almeida APS, Dias MC, Sivieri K, et al. (2016) Yacon (Smallanthus sonchifolius) as a food supplement: Health-promoting benefits of fructooligosaccharides. Nutrients 8: 436.
- Lobo AR, Colli C, Alvares EP, Filisetti TMCC (2007) Effects of fructans-containing yacon (Smallanthus sonchifolius Poepp & Endl.) flour on caecum mucosal morphometry, calcium and magnesium balance, and bone calcium retention in growing rats. Br J Nutr 97: 776-785.
- Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N (2013) Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol 13: 869-874.
- Fox P, Hudson M, Brown C, Lord S, Gebski V, et al. (2013) Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer 109: 147-153.
- Jafari N, Ahmed R, Gloyd M, Bloomfield J, Britz-McKibbin P, et al. (2016) Allosteric Sensing of Fatty Acid Binding by NMR: Application to Human Serum Albumin. J Med Chem 59: 7457-7465.
- Fauran-Clavel MJ, Oustrin J (1986) Alkaline phosphatase and bone calcium parameters. Bone 7: 95-99.
- Choque Delgado GT, da Silva Cunha Tamashiro WM, Maróstica Junior MR, Pastore GM (2013) Yacon (Smallanthus sonchifolius): A functional food. Plant Foods Hum Nutr 68: 222-228.
- Williams EA, Coxhead JM, Mathers JC (2003) Anti-cancer effects of butyrate: use of micro-array technology to investigate mechanisms. Proc Nutr Soc 62: 107-115.
- Kim M, Friesen L, Park J, Kim HM, Kim CH (2018) Microbial metabolites, short-chain fatty acids, restrain tissue bacterial load, chronic inflammation, and associated cancer in the colon of mice. Eur J Immunol 48: 1235-1247.
- Zeng JC, Zhang Z, Li TY, Liang YF, Wang HM, et al. (2013) Assessing the role of IL-35 in colorectal cancer progression and prognosis. Int J Clin Exp Pathol 6: 1806-1816.
- Liu Z, Huang Q, Liu G, Dang L, Chu D, et al. (2014) Presence of FOXP3(+)Treg cells is correlated with colorectal cancer progression. Int J Clin Exp Med 7: 1781-1785.
- Katz SC, Bamboat ZM, Maker AV, Shia J, Pillarisetty VG, et al. (2013) Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann Surg Oncol 20: 946-955.
- Salama P, Phillips M, Grieu F, Morris M, Zeps N, et al. (2009) Tumor-Infiltrating FOXP3+ T Regulatory Cells Show Strong Prognostic Significance in Colorectal Cancer. J Clin Oncol 27: 186-192.
- Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, et al. (2010) High frequency of tumor-infiltrating FOXP3+ regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer 126: 2635-2643.
- Vignali DAA, Collison LW, Workman CJ (2008) How regulatory T cells work. Nat Rev Immunol 8: 523-532.
- Cao X, Cai SF, Fehniger TA, Song J, Collins LI, et al. (2007) Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 27: 635-646.
- Zhu J, Paul WE (2008) CD4 T cells: Fates, functions, and faults. Blood 112: 1557-1569.
- Kim M, Grimmig T, Grimm M, Lazariotou M, Meier E, et al. (2013) Expression of Foxp3 in Colorectal Cancer but Not in Treg Cells Correlates with Disease Progression in Patients with Colorectal Cancer. PLoS One 8: 53630.
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, et al. (2000) A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100: 655-669.
- Zheng W, Flavell RA (1997) The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89: 587-596.
- Zhang DH, Cohn L, Ray P, Bottomly K, Ray A (1997) Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem 272: 21597-21603.
- Zamarron BF, Chen W (2011) Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 7: 651-658.
- Loose D, Van de Wiele C (2009) The Immune System and Cancer. Cancer Biother Radiopharm 24: 369-376.
Citation: Marcon LDN, Moraes LFS, Cruz BCS, Teixeira MDO, Gomides AFF, et al. (2020) Yacon (Smallanthus Sonchifolius)-Based Product Increases Fecal Short-Chain Fatty Acids Concentration and Up-Regulates T-Bet Expression in the Colon of BALB/c Mice During Colorectal Carcinogenesis. J Food Sci Nutr 6: 069.
Copyright: © 2020 Letícia De Nadai Marcon, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

