
Demonstrating a Novel Experience with Human Periodontium-Derived Stem Cells: A Review
*Corresponding Author(s):
Dr. Med Dent Aous DannanDepartment Of Periodontology, Syrian Private University, Damascus, Syrian Arab Republic
Tel:+963 991117879,
Email:aousdannan@yahoo.com
Abstract
The presence of stem cells in the PDL was supported by several findings where a population of mesenchymal stem cells from the PDL has been isolated and characterized showing the ability to express a variety of stromal cells markers.
In this review, we demonstrated our own experience with human Periodontium-derived Stem Cells (PdSCs) starting by uncovering their characterizations in vitro, and ending up with showing the ability of such cells to regenerate periodontal tissues in vivo. However, a systematic search in the literature for all articles related to PdSCs is not the aim of this paper.
Our experiments showed that human adult PdSCs could differentiate into cementoblasts or osteoblasts to support periodontal regeneration.
After being transplanted into an athymic rat model, PdSCs were able to regenerate periodontal tissue elements at different levels.
More optimized controlled methods should be established when such human PdSCs are to be used.
Keywords
INTRODUCTION
Unlike muscle cells, blood cells, or nerve cells-which do not normally replicate themselves-stem cells may replicate many times by a process called proliferation. A starting population of stem cells that proliferates for many months in the laboratory can yield to millions of cells. If the resulting cells continue to be unspecialized, like the parent stem cells, the cells are meant to be capable of long-term self-renewal.
Properties of stem cells can be illustrated in vitro, using methods such as clonogenic assays, where single cells are characterized by their ability to differentiate and self-renew [2,3]. As well, stem cells can be isolated based on a distinctive set of cell surface markers. However, in vitro culture conditions can alter the behavior of cells, making it unclear whether the cells will behave in a similar manner in vivo.
Considerable debate exists whether some proposed adult cell populations are truly stem cells.
STEM CELLS IN THE PERIODONTIUM
The PDL is derived embryologically from the ecto-mesenchymal tissue of the dental follicle that surrounds the developing tooth in its bony crypt. It contains a unique assortment of cells that are capable of generating and maintaining three distinct tissues, namely the ligament itself as well as the mineralized tissues on either side of it, i.e., the cementum and the alveolar bone. The major cells of the PDL are: the undifferentiated ecto-mesenchymal cells, fibroblasts, macrophages, cementoblasts, cementoclasts, osteoblasts, osteoclasts, cell rests of Malassez, vascular and neural elements.
Moreover, PDL contains heterogeneous cell populations [4,5] that can differentiate into either cementoblasts or osteoblasts [6,7].
Recent findings suggest that PDL cells have many osteoblast-like properties, including the capacity to form mineralized nodules in vitro, expression of the bone-associated markers as alkaline phosphatase and bone sialoprotein, and response to bone-inductive factors such as parathyroid hormone, insulin-like growth factor 1, bone morphogenetic protein 2 and transforming growth factor β1 [8-10]. The presence of multiple cell types within PDL has led to speculation that this tissue might contain progenitor cells that maintain tissue homoeostasis and regeneration of periodontal tissue [11-13].
Using a methodology similar to that utilized to isolate Mesenchymal Stem Cells (MSCs) from deciduous and adult dental pulp, multipotent postnatal stem cells from human PDL or PdSCs have also been isolated and described [14-19]. Cultured cells were expanded from single cell suspensions derived from PDL tissue and the presence of stem cells was determined using antibodies such as STRO-1 and CD146.
In our opinion, PdSCs are not pure mesenchymal, which will be discussed later in this paper. However, the presence of MSCs in the PDL is supported by other findings where a population of MSCs from the PDL has been isolated and characterized showing the ability to express a variety of stromal cells markers (CD90, CD29, CD44, CD166, CD105, CD13) [20,21]. The clinical potential for the use of PdSCs has been further enhanced by the demonstration that these cells can be isolated from cryopreserved periodontal ligaments, thus providing a ready source of MSCs [18].
Moreover, putative stem cells in both healthy and diseased PDL could be identified [14]. They were mainly located in the para-vascular region and small clusters of cells were also found in the extra-vascular region. Wider distributions of these cells were detected in sections of diseased ligament.
OUR EXPERIMENTS ON PDSCS
Although cells from dental pulp and dental follicle are often defined as ecto-mesenchymal, it is noteworthy that a number of implantation and tissue recombination studies have demonstrated that periodontal tissues, including cementum, are tooth-related but neural crest-derived [24,25]. In addition, Pierret et al., proposed eloquently in a recent review that all adult stem cells might be progeny of the neural crest [26]. Thus, the existence of neural precursors in dental adjacencies might be explained by the ontogeny of human teeth. In this context it is noteworthy that adult teeth are highly innervated, thus the turnover necessitates local neural plasticity. In addition, Obara et al., reported strong expression of Neural Cell Adhesion Molecule (NCAM) in the dental follicle of developing mouse [27,28]-further evidence for the neural character of cells resident within the periodontal tissue. Furthermore, several neuro-trophic factors such as Nerve Growth Factor (NGF), Brain-Derived Neuro-Trophic Factor (BDNF) or glial cell-line derived neuro-trophic factor are reported to be highly expressed in developing human teeth [29].
Thus, due to our early discovery, we considered PdSCs as multi-potential adult progenitor cells [30].
Thereafter, controversial debates regarding PdSCs raised up, and expectations about their real action were under discussions at our side through several papers [15,31].
CHARACTERIZATION OF PDSCS IN VITRO
Imaging of PdSCs’ aggregation on artificial surfaces
Zirconia ceramics (yttrium-stabilized tetragonal poly- crystals) seem to be a suitable material for dental implants because of their tooth-like color, their excellent mechanical properties and their good biocompatibility [32]. They have extensively been used as ball heads in total hip replacements with remarkable clinical outcomes [33].
Animal studies have also shown successful bone healing of dental zirconia implants under both unloaded and loaded conditions [34]. As the conventional fabrication of zirconia rods usually results in relatively smooth surfaces, only few studies have investigated rough surface modifications of zirconia implants. This is a critical aspect, since it has been already demonstrated that surface roughness and topography also influence osseointegration of zirconia implants [35].
In comparison with titanium implants, much less is known about the role played by surface modifications on the osseointegration of zirconia implants as well as the incorporation of stem cell therapy in such methods in dentistry.
We conducted a pilot study aimed at giving primary imaging of the aggregation ability of human PdSCs when coated on Zirconoxid artificial materials [36].
In a serum-free culture medium containing Fibroblast Growth Factor (FGF) and Epidermal Growth Factor (EGF), PdSCs were left to be proliferated for 4 days. Convex-shaped Zirconoxid surfaces were separately incubated in the PdSCs suspension for 3 days. As in normal culture, images taken by optical light microscope showed the ability of PdSCs to form dento-spheres on top of all the material surfaces. Images taken by confocal laser microscope clarified the general adhesion picture of PdSCs. However, an exact detection of the cell-surface region topography was not possible.
According to those primary results, it could be stated that PdSCs showed an initial ability to reside and adhere on Zirconoxid surfaces.
In a similar concept, Kim SY et al., assessed differential gene expression of signaling molecules involved in osteogenic differentiation of PDL stem cells subjected to different Titanium (Ti) surface types [37]. It was concluded that surface roughness and hydrophilicity could affect differential calcium-dependent Wnt signaling molecule pathways and signaling molecules, targeting the osteogenic differentiation of PdSCs.
Morphological characterization of PdSCs
Histological analysis showed differentiation of spheres deriving from the PdSCs with central production of extracellular matrix beginning 3 days after sub-culturing. Isolated PdSCs developed pseudopodia which contained actin. Tubulin was found in the central portion of the cells. Pseudopodia between different cells anastomosed, indicating intercellular transport. Immuno-staining for osteopontin demonstrated a positive reaction in primary spheres and within extracellular matrix vesicles after sub-culturing. It was concluded that PdSCs were capable of differentiating into osteogenic precursor cells, which produced extracellular matrix containing osteopontin. After in vitro expansion and proliferation they might be used for transplantation into the periodontium to enhance periodontal regeneration [38].
UNCOVERING THE ACTION OF PDSCS IN VIVO
Choosing a suitable animal model
In general, animal models have been used to evaluate the pathogenesis of periodontal diseases and various periodontal treatment modalities. Human longitudinal studies of periodontal diseases pose many problems such as determining the level of disease activity, individuals at risk, and susceptibility to disease progression.
From the point view of comparative biology, non-human primates are similar to humans, having comparable periodontal tissue structures and healthy and diseased periodontal states, as observed in humans [7]. However, most non-human primates used for research purposes are large, expensive, and difficult to handle. Among the species of non-human primates, squirrel monkeys and marmosets are small in size and relatively easy to handle, but unfortunately do not exhibit an inflammatory profile characteristic of human periodontal disease. Periodontal tissue specimens from these animals, unlike humans, exhibit very limited numbers of lymphocytes and plasma cells [39,40].
In 2008 we performed a review of the literature in order to address the most preferred animal models within the field of periodontal research [41]. It was found that dogs were the most animal models used (31.16%), monkeys came at the second level (15.58%), and then rats (10.66%), pigs, ferrets and sheep (1.64%) and finally goats (0.82%).
Though rats came thirdly as animal models in periodontal research according to those results, we preferred to use the athymic nude (Charles River Laboratories®, nomenclature: Crl: NIH-Foxn1 rnu) for the experiments of PdSCs.
The rat is the second most commonly used animal species in biomedical research and testing [42]. Rats possess a number of characteristics which make them ideal animal models: they are readily available from many commercial and private sources, they possess genetic uniformity, they are inexpensive to purchase and maintain, they are easy to handle, they are adaptable to novel situations and environments, they have well-defined physiologic parameters, they have known micro flora, some have spontaneous diseases useful in modeling, and their short life-span affords an opportunity to study long-term effects of experimental treatments on health and wellbeing.
In our study, and because human periodontal stem cells were used, it was important to choose an animal model that has fewer ability to reject foreign transplantations. For this reason, immuno-deficient rats were the method of choice since they have no T-cells in their immune systems [43-45].
Manipulation of PdSCs
Cells were isolated from extracted periodontal tissues and expanded ex-vivo under serum-free conditions as described previously [22]. Shortly, cells were isolated from 10 patients with vertical periodontal defects. Granulation tissues from vertical boney defects were removed and expanded ex-vivo. After they appeared at days 8-10, the primary periodontium-derived spheres were dissociated to derive secondary spheres. The sub-culturing protocol consisted of cell culture passaging every 3-4. The medium was switched to osteogenic differentiation medium. Subsequently, cells (1*105 to 2*105) were seeded onto cover slips and cultured for three weeks in the presence of potassium phosphate, L-ascorbic acid 2-phosphate and dexamethasone 21-phosphate (all chemicals from Sigma-Aldrich, Deisenhofen, Germany) to induce osteogenic differentiation.
in vivo assay of periodontal tissue formation in athymic rat model
For surgical procedures, rats were fully anesthetized, and an artificial bone defect (about 2.5×2.5×2mm) was prepared on the right side of the mandible (as a test side) as well as on the left side (as a control side) at the level of the distal root of the first molars. Four days before transplantation, human adult PdSCs were dissociated and re-suspended in osteogenic differentiation medium (see above). Subsequently, osteogenic pre-differentiated PdSCs (1*105 cells/ml) were plated on OptiMaix® collagen sponges (Matricel, Herzogenrath, Germany), which were previously fit to the shape of the bone defect, and were cultured for additional four days in osteogenic differentiation media. Collagen sponges without PdSCs served as controls in the mouth split- model. The collagen sponge with PdSCs was then transferred to the bone defect created on the test side, whereas the collagen lattice without cells was transferred to the defect created on the control side.
Ten days after culture the isolated stem cells developed three-dimensional spheroids which have been described previously as neurospheres [22]. However, within the center of the spheres extracellular matrix could be found. Therefore it is likely that the spheres were representing mesenchymal cells which may differentiate into fibroblasts or osteoblasts.
Light microscopic sections taken from the control rat clearly demonstrated all histological features related to a normal periodontium; namely the PDL, the supporting bone and the dental hard tissue (here: dentin covered with cementum). Within the PDL, collagen fibers and blood vessels could be shown (Figure 1).
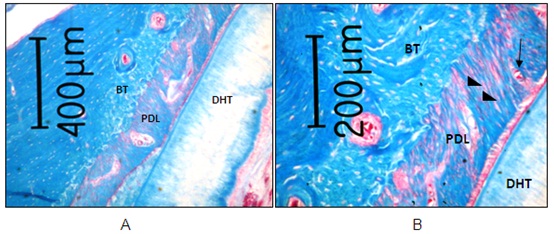 Figure 1: Histological section taken from the untreated control rat demonstrating normal periodontium. Collagen fibers (black triangles) and blood vessels (black arrow) can be shown within PDL (BT: Bone Tissue; DHT: Dental Hard Tissue; PDL: Periodontal Ligament) (orig mag ×2.5) (Azan Blue staining) [48].
Figure 1: Histological section taken from the untreated control rat demonstrating normal periodontium. Collagen fibers (black triangles) and blood vessels (black arrow) can be shown within PDL (BT: Bone Tissue; DHT: Dental Hard Tissue; PDL: Periodontal Ligament) (orig mag ×2.5) (Azan Blue staining) [48].Throughout the experimental time course, healing of the defects in both transplanted and non-transplanted control sides was completed uneventfully. At 2 weeks post-surgery, new connective tissue attachment binding to root surfaces was not observed in either side.Considering test animals, the control sites showed a reformation of periodontal ligament-like (PDL-like) tissue, collagen fibers and elements of bone at different levels in every section after 6 weeks (Figure 2).
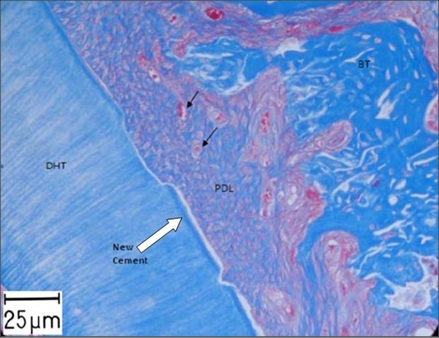 Figure 2: Histological section taken from a control site after 6 weeks showing Bone Tissue elements (BT), Periodontal Ligament-Like Tissue (PDL), Dental Hard Tissue (DHT: Dental Hard Tissues; i.e., Dentin) and integration of collagen fibers within PDL with new blood vessels (black arrows) (orig mag ×40) (Azan Blue staining) [48].
Figure 2: Histological section taken from a control site after 6 weeks showing Bone Tissue elements (BT), Periodontal Ligament-Like Tissue (PDL), Dental Hard Tissue (DHT: Dental Hard Tissues; i.e., Dentin) and integration of collagen fibers within PDL with new blood vessels (black arrows) (orig mag ×40) (Azan Blue staining) [48].At 6 weeks post-surgery, this extensive new bone formation leading to ankylosis with partial root resorption was observed in all control sides. Blood vessels could also be shown in the new PDL tissue and in the bone tissue. The collagen fibers located perpendicularly into the newly formed bone. It seemed to be that the collagen matrix was completely absorbed and replaced with other tissue. Down growth of junctional epithelium was observed to a slight degree over the investigation time period.
At the test sites, where collagen sponges with PdSCs were transplanted, a reformation of PDL-like tissue, elements of bone and osteocyte-lacunae in the bone tissue could be shown after 6 weeks. Some putative transplanted cells were observed to attach onto root dentin surfaces. Blood vessels and collagen fibers could also be shown in the PDL tissue. In the regenerated PDL tissues, immature thin fibers were obliquely arranged parallel to the bone surface and not in a perpendicular direction. This orientation highly resembles native PDL fibers. Such a fibril anchoring was never observed in the control sides (Figure 3). However, a “functional” periodontium seemed not to be existed [48].
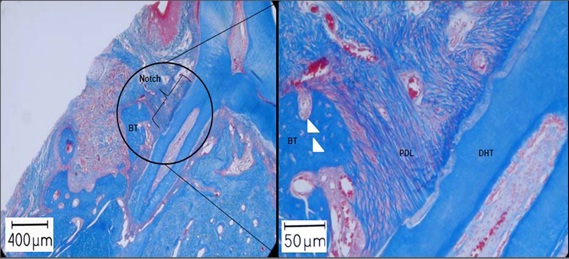
We have successfully demonstrated that human periodontium contains a subpopulation of cells with the phenotypic characterization of ecto-mesenchymal stem cells inducing after transplantation in immuno-compromised rats structures consistent with periodontal tissues. Although the application of human PdSCs to achieve periodontal tissue regeneration had brought limited results in our study, periodontal tissue elements were regenerated at different levels. Although this study had some limitations considering the number of animals, the lack of sufficient statistical analysis, it should be viewed as the first investigation that introduced the in vivo actions of human PdSCs, isolated by means of minimally invasive periodontal surgery, when implanted in artificial periodontal defects in athymic nude rats.
RECENT FINDINGS REGARDING PDSCS
Regarding the osteogenic differentiation of PDL, Lee JS et al., investigated the osteoinductive effect of Mussel-inspired Polydopamine (PDA) on PdSCs and examined how this phenomenon is encouraged [50]. They found that bioadhesive PDA stimulates osteogenic differentiation of PdSCs via activation of the integrin α5/β1 and protein Phosphatidylinositol-3-kinase (PI3K) signaling pathways.
In 2014, Zhang J et al., investigated human PdSCs for their stem cell characteristics via analysis of cell surface marker expression, colony forming unit efficiency, osteogenic differentiation and adipogenic differentiation, and compared to Bone-Marrow-Derived Human Mesenchymal Stem Cells (BMSCs) [51]. To determine the impact of both inflammation and the NF-κβ signal pathway on osteogenic differentiation, cells were challenged with TNF-α under osteogenic induction conditions and investigated for mineralization, Alkaline Phosphatase (ALP) activity, cell proliferation and relative genes expression. They concluded that BMSCs owned the stronger immunomodulation in local microenvironment via anti-inflammatory functions, compared to PdSCs.
More recently, the expression of specific microRNAs (miRNAs) and their roles in the osteogenic differentiation of human PDL stem cells exposed to mechanical stretch came under focus. In this contest, Wei et al., found in a study that miR-21 is a mechanosensitive gene that plays an important role in the osteogenic differentiation of PdSCs exposed to stretch [52].
CONCLUSION
Our in vitro experiments on PdSCs showed that, after one week, the cultured periodontal stem cells produced extracellular matrix in which osteocalcin was expressed, and this therefore supported the hypothesis that PdSCs could differentiate into cementoblasts or osteoblasts to support periodontal regeneration.
Our in vivo experiments on PdSCs demonstrated that extracting those cells from patients undergoing open flap minimally invasive periodontal surgery seemed to be a simple and reliable method. These cells could be easily propagated ex-vivo, thereby still preserving their stem cell state. Human adult PdSCs being transplanted into an athymic rat model were able to regenerate periodontal tissue elements at different levels. However, prior to the ultimate use of PdSCs in human trials further in vivo animals studies have to be conducted to optimize the cells regenerative capacity. One approach would be to investigate the use of different carrier systems in which human adult PdSCs will be embedded prior to transplantation, in addition to that, more optimized controlled methods should be established when using human PdSCs cells.
REFERENCES
- Fleischmajer R (1967) Epithelial-mesenchymal interactions. Science 157: 1472-1482.
- Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, et al. (1974) Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 2: 83-92.
- Friedenstein AJ, Gorskaja JF, Kulagina NN (1976) Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4: 267-274.
- Lekic P, Rojas J, Birek C, Tenenbaum H, McCulloch CA (2001) Phenotypic comparison of periodontal ligament cells in vivo and in vitro. J Periodontal Res 36: 71-79.
- Murakami Y, Kojima T, Nagasawa T, Kobayashi H, Ishikawa I (2003) Novel isolation of alkaline phosphatase-positive subpopulation from periodontal ligament fibroblasts. J Periodontol 74: 780-786.
- Gould TR, Melcher AH, Brunette DM (1980) Migration and division of progenitor cell populations in periodontal ligament after wounding. J Periodontal Res 15: 20-42.
- Isaka J, Ohazama A, Kobayashi M, Nagashima C, Takiguchi T, et al. (2001) Participation of periodontal ligament cells with regeneration of alveolar bone. J Periodontol 72: 314-323.
- Lekic PC, Rajshankar D, Chen H, Tenenbaum H, McCulloch CA (2001) Transplantation of labeled periodontal ligament cells promotes regeneration of alveolar bone. Anat Rec 262: 193-202.
- Marcopoulou CE, Vavouraki HN, Dereka XE, Vrotsos IA (2003) Proliferative effect of growth factors TGF-beta1, PDGF-BB and rhBMP-2 on human gingival fibroblasts and periodontal ligament cells. J Int Acad Periodontol 5: 63-70.
- Zhao M, Berry JE, Somerman MJ (2003) Bone morphogenetic protein-2 inhibits differentiation and mineralization of cementoblasts in vitro. J Dent Res 82: 23-27.
- Beertsen W, McCulloch CA, Sodek J (1997) The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 13: 20-40.
- Boyko GA, Melcher AH, Brunette DM (1981) Formation of new periodontal ligament by periodontal ligament cells implanted in vivo after culture in vitro. A preliminary study of transplanted roots in the dog. J Periodontal Res 16: 73-88.
- Liu HW, Yacobi R, Savion N, Narayanan AS, Pitaru S (1997) A collagenous cementum-derived attachment protein is a marker for progenitors of the mineralized tissue-forming cell lineage of the periodontal ligament. J Bone Miner Res 12: 1691-1699.
- Chen SC, Marino V, Gronthos S, Bartold PM (2006) Location of putative stem cells in human periodontal ligament. J Periodontal Res 41: 547-553.
- Dannan A, Grimm WD (2008) [Periodontium-derived stem cells; a new Horizon in the Periodontal Tissue Regeneration]. Dental Medium 16: 27-29.
- Ivanovski S, Gronthos S, Shi S, Bartold PM (2006) Stem cells in the periodontal ligament. Oral Dis 12: 358-363.
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, et al. (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364: 149-155.
- Seo BM, Miura M, Sonoyama W, Coppe C, Stanyon R, et al. (2005) Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res 84: 907-912.
- Varga G, Molnar B, Kadar K, Ovari G, Windisch P, et al. (2005) Adult Stem Cells in Primary Culture From Human Dental Tissues. Journal of Dental Research.
- Nagatomo K, Komaki M, Sekiya I, Sakaguchi Y, Noguchi K, et al. (2006) Stem cell properties of human periodontal ligament cells. J Periodontal Res 41: 303-310.
- Trubiani O, Di Primio R, Traini T, Pizzicannella J, Scarano A, et al. (2005) Morphological and cytofluorimetric analysis of adult mesenchymal stem cells expanded ex vivo from periodontal ligament. Int J Immunopathol Pharmacol 18: 213-221.
- Widera D, Grimm WD, Moebius JM, Mikenberg I, Piechaczek C, et al. (2007) Highly efficient neural differentiation of human somatic stem cells, isolated by minimally invasive periodontal surgery. Stem Cells Dev 16: 447-460.
- Widera D, Grimm WD, Möbius JM, Dannan A, Becher S, et al. (2007) Human periodontium-derived neural stem cells: chance or risk? Regenerative Medicine 2: 3-61.
- Hildebrand C, Fried K, Tuisku F, Johansson CS (1995) Teeth and tooth nerves. Prog Neurobiol 45: 165-222.
- Lumsden AG (1988) Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 103: 155-169.
- Pierret C, Spears K, Maruniak JA, Kirk MD (2006) Neural crest as the source of adult stem cells. Stem Cells Dev 15: 286-291.
- Obara N (2002) Expression of the neural cell adhesion molecule during mouse tooth development. Connect Tissue Res 43: 212-215.
- Obara N, Suzuki Y, Nagai Y, Nishiyama H, Mizoguchi I, et al. (2002) Expression of neural cell-adhesion molecule mRNA during mouse molar tooth development. Arch Oral Biol 47: 805-813.
- Nosrat I, Seiger A, Olson L, Nosrat CA (2002) Expression patterns of neurotrophic factor mRNAs in developing human teeth. Cell Tissue Res 310: 177-187.
- Grimm WD, Dannan A, Gassmann G, Kaltschmidt B, Varga G, et al. (2007) Periodontium derived stem cells as multipotential adult progenitor cell. Journal of Dental Research 86: 2463.
- Grimm WD, Arnold WH, Becher S, Dannan A, Gassmann G, et al. (2008) [Stem Cell-based Therapy in Periodontal Regeneration]. Zahnärztliche Mitteilungen 98: 40-48.
- Sennerby L, Dasmah A, Larsson B, Iverhed M (2005) Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin Implant Dent Relat Res 7: 13-20.
- Piconi C, Maccauro G, Muratori F, Brach Del Prever E (2003) Alumina and zirconia ceramics in joint replacements. J Appl Biomater Biomech 1: 19-32.
- Scarano A, Di Carlo F, Quaranta M, Piattelli A (2003) Bone response to zirconia ceramic implants: an experimental study in rabbits. J Oral Implantol 29: 8-12.
- Bächle M, Butz F, Hübner U, Bakalinis E, Kohal RJ (2007) Behavior of CAL72 osteoblast-like cells cultured on zirconia ceramics with different surface topographies. Clin Oral Implants Res 18: 53-59.
- Dannan A, Dittmar T, Grimm WD (2009) Imaging of Periodontium-derived Stem Cells’ Aggregation on Zirconoxid Surfaces. Proceeding of the 11th Essen Symposium on Biomaterials and Biomechanics: Fundamental and Clinical Applications, Essen, Germany.
- Kim SY, Yoo JY, Ohe JY, Lee JW, Moon JH, et al. (2014) Differential expression of osteo-modulatory molecules in periodontal ligament stem cells in response to modified titanium surfaces. Biomed Res Int 2014: 452175.
- Arnold WH, Becher S, Dannan A, Widera D, Dittmar T, et al. (2010) Morphological characterization of periodontium-derived human stem cells. Ann Anat 192: 215-219.
- Hosokawa R, Kikuzaki K, Kimoto T, Matsuura T, Chiba D, et al. (2000) Controlled local application of basic Fibroblast Growth Factor (FGF-2) accelerates the healing of GBR. An experimental study in beagle dogs. Clin Oral Implants Res 11: 345-353.
- Ruskin JD, Hardwick R, Buser D, Dahlin C, Schenk RK (2000) Alveolar ridge repair in a canine model using rhTGF-beta 1 with barrier membranes. Clin Oral Implants Res 11: 107-115.
- Dannan A, Alkattan F (2008) Animal models in periodontal research: A mini-review of the literature. The Internet Journal of Veterinary Medicine.
- Jordan HV (1971) Rodent model systems in periodontal disease research. J Dent Res 50: 236-242.
- Becher S, Dannan A, Grimm WD (2007) Athymic Nude Rat Model for the Transplantation of Human Periodontal Stem Cells. Parodontologie 18: 281.
- Grimm WD, Arnold WH, Becher S, Dannan A, Gassmann G, et al. (2009) Periodontal Stem Cell Plasticity in Athymic Nude Rat Model. Journal of Dental Research.
- Grimm WD, Becher S, Dannan A, Varga G, Dittmar T, et al. (2009) Evaluation of an in vivo Animal Model for the Transplantation of Human Periodonium-derived Stem Cells (PdSCs) 36: 30.
- Gassmann G, Grimm WD (2006) Minimal-invasive regenerative und plastisch-rekonstruktive Parodontalchirurgie. Dentale Implantologie & Parodontologie 10: 90-97.
- Dannan A (2011) Minimally invasive periodontal therapy. J Indian Soc Periodontol 15: 338-343.
- Grimm WD, Dannan A, Becher S, Gassmann G, Arnold W, et al. (2011) The ability of human periodontium-derived stem cells to regenerate periodontal tissues: a preliminary in vivo investigation. Int J Periodontics Restorative Dent 31: 94-101.
- Liu J, Wang L, Liu W, Li Q, Jin Z, et al. (2014) Dental follicle cells rescue the regenerative capacity of periodontal ligament stem cells in an inflammatory microenvironment. PLoS One 9: 108752.
- Lee JS, Yi JK, An SY, Heo JS (2014) Increased osteogenic differentiation of periodontal ligament stem cells on polydopamine film occurs via activation of integrin and PI3K signaling pathways. Cell Physiol Biochem 34: 1824-1834.
- Zhang J, Li ZG, Si YM, Chen B, Meng J (2014) The difference on the osteogenic differentiation between periodontal ligament stem cells and bone marrow mesenchymal stem cells under inflammatory microenviroments. Differentiation 88: 97-105.
- Wei F, Liu D, Feng C, Zhang F, Yang S, et al. (2015) microRNA-21 mediates stretch-induced osteogenic differentiation in human periodontal ligament stem cells. Stem Cells Dev 24: 312-319.
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Post natal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97: 13625-13630.
- Liu Y, Zheng Y, Ding G, Fang D, Zhang C, et al. (2008) Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 26: 1065-1073.
Citation: med dent Aous Dannan (2015) Demonstrating a Novel Experience with Human Periodontium-Derived Stem Cells: A Review. J Stem Cell Res Dev Ther 2: 006.
Copyright: © 2015 Dr. med dent Aous Dannan, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

