
Overview of Cellular Stromal Vascular Fraction (cSVF) & Biocellular Uses of Stem/Stromal Cells & Matrix (tSVF + HD-PRP) in Regenerative Medicine, Aesthetic Medicine and Plastic Surgery
*Corresponding Author(s):
Robert W AlexanderDepartment Of Surgery, Institute Of Regenerative Medicine, University Of Washington, 715 Main Street, Suite B, Stevensville, MT 59870, United States
Tel:+1 4067775312,
Fax:+1 8667665458
Email:rwamd@cybernet1.com
Abstract
Paper presents an overview of cellular and biocellular uses of both cellular Stromal Vascular Faction (cSVF) elements and evolving applications of tissue Stromal Vascular Fraction (tSVF) component in repair and regeneration. Use of autologous stem/stromal components in human homeostasis and healing has shaped an evolving paradigm shift in many disciplines of Medicine & Surgery. Mass production or patentability of a patient’s own cell and stromal elements is a very unlikely possibility, making the use of these element supported by pharmaceutical companies unavailable.
As a very heterogenous mixture of cells in the cSVF, some actual stem/stromal cell mix offer unique uses which are complimented in the actual multipotent cells and their supportive cells felt to be important in optimizing their functions. Appreciation that the paracrine secretive factors play an integral role in the regenerative milieu, have led to examination of signaling via exosomal & micro vesicle communications.
Discussion of terms, who are providing these services, and the realm of applications evolving is discussed. Appreciation of the contribution of “biologicals” (in this context, meaning platelet-rich plasma concentrates) to enhance the repair and regenerative uses of stem/stromal cells are discussed as the provision of critical growth factors, cytokines, and signaling proteins. Evaluation of the safety and efficacy of these therapies are ongoing, often tracking with Institutional Review Board (IRB) oversight. This is considered of great value in bringing recognition and acceptance to both the cellular and biocellular applications in the future.
EVOLUTION OF REGENERATIVE MEDICINE IN PLASTIC SURGERY
Early in the 2000s, these bulky, and somewhat inefficient machines, were replaced by a number of medical device companies creating smaller, efficient, and more simple centrifugation systems which provided consistent and high concentration abilities. At the same time, this expanded delivery capabilities in a more cost effective and consistent protocols, for use in both the Operating Room and in the Outpatient Surgical Centers. With the advent of small footprint and affordable systems, the uses and advantages of utilizing these autologous elements expanded exponentially. The area of chronic wound healing, devascularizing injuries and a vast range of soft tissue repair became ubiquitous. As of the writing of this contribution, sophisticated understanding of optimization of exactly which product or content is most important in the various situations, the applications continue to grow and show their values.
Poorly understood is the fact that MANY products have rushed to market, each claiming capabilities and concentrations that are simply incorrect. During the evolution, many surgeons (especially Orthopaedic Surgery and related areas) were disappointed at the outcome improvement. A significant issue evolved with lessor understanding that, simply isolating some platelets did NOT yield high enough, or consistent enough, products which could deliver the most optimal outcomes. By accepted medical definition in the literature, a concentration ABOVE four to six (4-6X) times the individual measured concentration is considered High Density Platelet Rich Plasma. It has become very clear that there are those which provide a low concentration (single spin centrifugation yielding1.5 -2.5 times measured baselines) often can be of value in facial and dermal uses, whereas, much higher (>4-6 times baseline) concentrates are now consistently and easily obtained in point of care within outpatient or Operating Room settings.
Since it is now well established that there is a linear increase in available critical growth factors and signal proteins, most surgical and regenerative applications are favored with the higher concentrations. In addition, the level of understanding is being evolved where decisions of whether high hematocrit version, or a low hematocrit version have a clear indication tied to the specific locations where they may be utilized. In Aesthetic Plastic surgery, it is considered that the use of the highest concentrations that can be currently isolated is of significant clinical advantage over the lower concentrations.
Over the past 15+ years, many indications and uses have evolved. One of the most important related to the addition of these platelet concentrates to uses in autologous fat grafting (in small and large volumes) became more clear. The statistically significant reduction in lipid cysts and micro calcifications, coupled with more stable volume retention in use of autologous fat graft breast augmentation, has enhanced the use of the platelet products in such cases. In addition, observational findings of important skin vascularity and texture changes in the areas of the face/neck and the body areas has come to the forefront.
These concepts have made a major contribution that has spread well beyond the wound healing and aesthetic applications. For the past decade and one-half, uses in regenerative medical and surgical fields have become known as highly effective and safe alternatives to use of steroids, non-autologous products, and changed some indications for invasive surgical interventions. This contribution will expand on this background and evolving protocols aimed at skin, tissue supplementation and extensive applications using targeted guidance in chronic pain, musculoskeletal applications, and systemic cellular therapeutic options.
EVOLUTION OF CELL-BASED & BIOCELLULAR THERAPIES
Starting decades ago, use of an irritant solution (Dextrose) was injected to stimulate inflammatory reactions. This technique has been replaced in the past few years with transition to injecting various Platelet-Rich Plasma (PRP) concentrates for supporting an effective inflammatory reaction at damaged or degenerative sites. Use of the contained growth factors and signal proteins became recognized as offering a significant improvement in tissue healing responses, but seemed to be limited by incomplete repair while requiring a series (often 4-6 uses) to achieve long-term clinical improvement. Even then, if a site is severely depleted of cells and available growth factors often does not achieve a long-term improvement. Current evolution of combining these trophic growth factors and signal proteins PLUS concentrated undifferentiated cellular/stromal populations seemed like a logical and effective modality, moving into the forefront since 2000. Aesthetic and reconstructive applications led the way, as constant challenges of injury, loss of circulatory capabilities, degenerative changes, repair, etc. demanded an optimal approaches to structural augmentation and regenerative needs. In depth examination of how our body maintains itself, revealed that undesignated cells were integrally important to replace aging cells (such as skin, hair, bowel lining, etc.). Early on, fat was not thought of as undergoing such homeostatic mechanisms, since typical mitotic activities were not observed.
We now recognize that, rather than a static number of cells varying only in size, mature adipocytes do actually undergo total replacement at a rate of 10-20% per year, but do so in a different form of cell division, known as “asymmetric cell division”. The ability to have resident precursor cells which are capable to responding to local site signals, and the ability of providing a replacement cell of the needed type or participate in paracrine actions, resulting in potential replacement cell differentiation, while retaining a single, precursor cell type. At this time, the importance of the paracrine (secretory functions of exosomes and Micro Vesicles) are now thought to be a more important factor in activation, proliferation and functions of the resident un-desinganted cell groups. Without the asymmetric replenishment mechanisms and cellular signaling activations, we would eventually have an uncontrollable stem/stromal cell population [1].
With the advent of FDA approved tabletop programmable, centrifugal devices for custom High Density Platelet Concentrations (HD PRP) via closed system, use of a simple blood draw yielded more than 4-6 times patient’s own circulating baselines levels. It has been very well shown that the higher the achieved concentrations, the proportionally higher delivery of important factors intrinsically involved in all wound healing and repair (Figure 1).
 Figure 1: Left: Adipose tissue complex (Mature Adipocytes provide approximately 90% by volume; 10 % of nuclei in tSVF); Right: Closed system for centrifugation, cell isolation/concentration at point of care (CC1000 Healeon Medical, Newbury Park, CA, USA).
Figure 1: Left: Adipose tissue complex (Mature Adipocytes provide approximately 90% by volume; 10 % of nuclei in tSVF); Right: Closed system for centrifugation, cell isolation/concentration at point of care (CC1000 Healeon Medical, Newbury Park, CA, USA).It has become clear that certain tissue cellular characteristics are most favorable for use in cell-based therapies including easy and safe access coupled with plentiful autologous stores of a group of cells possessing multi-potent potential. Multi-potency is important in that such cells have the capability of responding to local signals and possess the ability to transform or replenish signals needed at damaged or diseased site for repair or regeneration. Researchers are understanding that the participation in the regenerative properties offered by these cells are not limited to the cell components, but also due to active paracrine secretive activities participating in the chemo tactic and signaling processes.
Research has confirmed that the vast majority of such undesignated cells are associated and stored in proximity to the microvascular capillary system and adventitia (Figure 2). Essentially all tissues (with blood supply) have some of these multi-potent cells available to deal with local and isolated demands. The body retains the ability to chemotactically attract and mobilize cells from local and remote storage points in response to chemical and physical signaling in the body. Approximately 15 years ago, an important scientific advance was made by researchers in finding that adipose tissue (fat) contained high numbers of such cells with multiple cellular potentials of differentiation [2,3]. This finding correlates to with understanding that multipotent, undesignated cell populations are local on or near perivascular tissues, and is not surprising once it was confirmed that adipose tissue complex represents the largest micro vascular organ in the body.
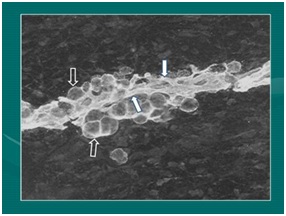 Figure 2: Micrograph demonstrating relationship of intimate relationship of microcapillaries and attached adipocyte during development (Photo: Fetal pig during extremity formation).
Figure 2: Micrograph demonstrating relationship of intimate relationship of microcapillaries and attached adipocyte during development (Photo: Fetal pig during extremity formation).Enhancement of cellular and biologic (in this context referring to growth factors, signal molecules such as contained in platelets) therapies comes directly with the ability for providers to be able to identify, target and guide the Cellular Vascular Fraction (cSVF) or cellular-biologic combination to areas of injury or degeneration. Management of protocols have led to improved structural grafting successes in addition to the contribution in orthopedic and sports medicine applications using the same cellular and platelet-derived growth factors & related components. In that regard, ultrasonography has clearly become a MAJOR feature to clinical responses and success. As example, in medium and deep targets, or those difficult to access, guided MSK ultrasound capabilities offers the optimal integral part of successful responses. Over the past decade, thousands of treatments using Biocellular Regenerative Medicine© techniques have proven safe and remarkably effective. The information provided in this chapter is intended as an introduction to important concepts and describe the current logic believed to be involved. Major steps have been taken, moving from the laboratory to the bedside. Today, musculoskeletal (MSK) and aesthetic-plastic surgical patients are routinely treated with this combination of Cellular or Biocellular elements [4-6].
WHAT IS BIOCELLULAR MEDICINE?
 Figure 3: General goals in regenerative medical applications.
Figure 3: General goals in regenerative medical applications.Cellular components
 Figure 4: Adipose tissue complex: Optimal cell source.
Figure 4: Adipose tissue complex: Optimal cell source.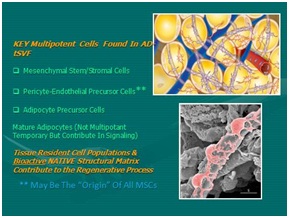 Figure 5: Multipotent cells found in Tissue Stromal Vascular Fraction (tSVF).
Figure 5: Multipotent cells found in Tissue Stromal Vascular Fraction (tSVF).Efforts at using mechanical separation (ultrasound, nutational, emulsification efforts) have not proved able to separate the very complex chemical binding of stem-capable group due to the cell-to-cell, or cell-to-matrix (ECM and periadventitial) attachments. The complex multifaceted connectivity necessary for cells to act on area signaling that exists with the ATC simply will not yield optimal separation and cellular purity needed to create a cSVF pellet. At this point in time, use of incubation, agitation, and digestive processes (blends of collagenase and neutral proteases) remain the optimal way to isolate and concentrate the desired cellular groups (Figures 6 and 7) [8].
 Figure 6: SEM Image of pericytes relationship to microcapillary. NOTE: See many complex cell-to-cell and cell-to-matrix attachments.
Figure 6: SEM Image of pericytes relationship to microcapillary. NOTE: See many complex cell-to-cell and cell-to-matrix attachments.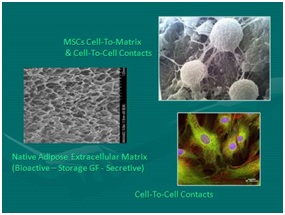 Figure 7: Left: Native scaffolding adipose tissue complex after cellular removal; Right Top: SEM mesenchymal stem cells intimate relationship with extracellular matrix and cell-to-cell elements; Right Bottom: Mesenchymal stem cells micrograph with NAPI nuclear stain.
Figure 7: Left: Native scaffolding adipose tissue complex after cellular removal; Right Top: SEM mesenchymal stem cells intimate relationship with extracellular matrix and cell-to-cell elements; Right Bottom: Mesenchymal stem cells micrograph with NAPI nuclear stain.The small nucleated cells found closely associated within the vascular tissues are now recognized as serving important roles in maintaining normal tissue content (homeostasis) PLUS have the ability to respond to injury or disease processes in a constant effort to maintain, heal, or repair damaged cells (as in aging, arthritis, musculoskeletal tissues, neurological disorders, etc.) the body. The remarkable design of the human body uses these reservoirs of available, non-differentiated, multipotent cells as the tissue “first responders” in the situations of major or micro-trauma and aging. By secretion and signaling of certain chemicals from a degenerating or injured site, these multipotent cells (i.e. can become various types of cells) can be called upon to participate in the repairs needed to restore tissues and functions. At first, it was felt that the cellular elements were the critical, and perhaps only, importance, experience now show that the paracrine secretory capabilities intrinsically contribute in critical roles (thought via exosomes, microvesicles, etc.). There are many peer-reviewed publications which provide examples of how the cells involved in this process can be enhanced by combined provision of the cellular, native scaffolding, and biologically active components.
Biologic components
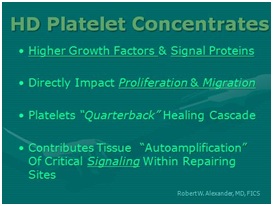 Figure 8: Why use of High Density Platelet Concentrates (HD-PRP)?
Figure 8: Why use of High Density Platelet Concentrates (HD-PRP)?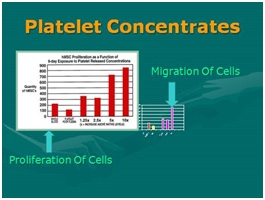 Figure 9: Importance of HD-PRP on proliferation of cells & migration of cells. [NOTE: Linear response as concentration increases.
Figure 9: Importance of HD-PRP on proliferation of cells & migration of cells. [NOTE: Linear response as concentration increases.The second source of biological contributors is found in bone marrow aspirates. Bone marrow has been used for many decades, and it is common use in blood related disorders. Although bone marrow does demonstrate microvasculature and, therefore, does have some undesignated reparative cells (stem/stromal cells of the reparative types). That said, the vast majority of “stem” cells located in marrow belong to the “hematopoietic” stem cell group are found there, and not considered extremely valuable in the case of regeneration or reparative cellular group. However, the desired stem/stromal cells are found in relatively very low numbers, compared to adipose tissues and its microvasculature. Therefore, many now consider bone marrow as useful primarily as a valuable biologic and platelet source. In order to become a valuable primary cell contributor for such reparative group, it is required that bone marrow aspirates be isolated, concentrated, and culture-expanded to achieve meaningful numbers needed in regenerative and healing applications. This source is technically a bit more invasive to obtain, pose higher complication-sequelae rates, and are significantly more expensive to the patients. In addition to the undesignated stem/stromal cells content considered of value (such as mesenchymal, peri-adventitial and endothelial), are MUCH more scarce compared to millions of resident hematopoietic cells. Many consider the primary difference between concentrated platelets from peripheral blood versus marrow concentrates, is a very large store of hematopoietic stem cells (HSCs) while offering almost identical platelet content. At this time there is very little evidence of significant contribution to the regeneration process of MSK tissues derived from the HSC group.
Concentrates primary importance and value is the ability to provide important growth factors and cytokines/chemokines to optimize earlier healing conditions and abilities. Of even more importance in the cellular therapeutic-based effects appears to be their important paracrine secretory influences, rather than contributions of individual cellular components and physical engraftment. Further, it well established that the mesenchymal group (MSC) of multipotent cells may actually originate from the pericyte-endothelial cell groups [10]. These offer a great amount of overlapping capabilities, in vitro, suggesting that all tissues having some microvasculature, have resident stem/stromal elements capable of providing “first responders” to sites of damage or degenerative effects. It is suggested that the multi-tissue MSC-like groups overlap at greater than 95% in their differential capabilities, at least in vitroconditions. Host site interaction with these stem/stromal cells, growth factors, and signal proteins seem to create a complex, heterogeneous precursor population that is considered “site specific” in many of their responses [11].
HOW DID BIOLOGIC & CELLULAR THERAPEUTIC CONCEPTS EVOLVE?
Once the complex processes of repair and regeneration were examined closely, it became apparent that determination of specific interactions of any single cell or chemical is not able to be determined at this time. At this time, the ability to create an in vitro architecture and situation that can duplicate the in vivomicroenvironment, making selection of “optimal” components impossible at this point in time.
Key adult multipotent cells are found in essentially every tissue and organ in the body. Determination that some of the highest concentrations of these adult stem/stromal cell populations were found within Adipose Tissue Complex (ATC), have led to a major trend shift to more closely evaluate the activities of such tissues, and how they can be easily and safely acquired, and concentrated, for uses in wound healing and repair. Early on, since adipocytes within the ATC were determined not to undergo mitosis, it was assumed that these were relatively static in number, and only changed in size according to lipid storage droplets. It is now clear that adipocytes do have a life cycle, replacing all mature adipocytes every 5-10 years [16]. Examination of how they accomplished this replacement, via a process of “asymmetrical cell division”, was found to be the mechanism, thereby preserving one stem cell during the process. This process results in activation of a precursor cell population is capable of reacting to secretions from a senescing adipocyte, beginning process of the precursor replacement cell to occur, while preserving the precursor cell. This replication by the asymmetric cell division results in a replacement immature adipocyte, while the other portion retaining its precursor forms and abilities. This is logical, in that, if otherwise, we would accumulate a massive number of precursor cells in our tissues (Figure 10).
 Figure 10: Adipose tissue complex: Advantages for use.
Figure 10: Adipose tissue complex: Advantages for use.It was not until Zuk et al., identified the multipotent capabilities of adipose-derived cellular stromal vascular fraction (AD-cSVF), with capabilities of differentiation to a variety of tissues, including bone, cartilage, tendon-ligament, muscle, fat, nerve, etc. (Figures 11 and 12). Once this capability was identified, efforts to isolate specific cell types started. This has proven somewhat difficult to interpret, in that it is currently impossible to imitate the in vivo microenvironment in the laboratory. Over the past decade, exploration of concentrating the identified ATC undifferentiated cell population coupled with High Density Platelet concentrates (HD-PRP) has received a great deal of attention. Identifying stem/stromal cells which can participate in the processes, have revealed what an “ideal” cell-based therapy may represent (Figure13).
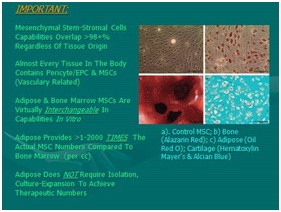 Figure 11: Mesenchymal Stem Cell differentiation (MSC) Potentials (Basic):
Figure 11: Mesenchymal Stem Cell differentiation (MSC) Potentials (Basic): a) Top Left, Micrograph Control MSC in vitro; b) Bottom Left, Bone; c) Top Right, Adipose; d) Bottom Right, Cartilage. [NOTE: Overlapping Differentiation Capabilities of All MSCs Is Extensive].
 Figure 12: Adipose tissue complex main cellular elements.
Figure 12: Adipose tissue complex main cellular elements. Figure 13: Ideal cell-based therapy advantages.
Figure 13: Ideal cell-based therapy advantages.In the early 1990s, a method of closed syringe microcannula lipoaspiration was patented, permitting less traumatic and efficient means of acquiring ATC for use as a small particle structural graft [17]. This has since evolved to a disposable, microcannula option which permits safe and efficacious low pressure acquisition of AD-tSVF, without the potential contamination associated with difficult cleaning and sterilization. In the past 15 years, clinicians and laboratory researchers have identified several important cell types which interact to provide remarkable contributions in tissue repair and regeneration. These have been identified as a very complex and heterogeneous population, closely related to cellular, adventitial areas, and extracellular matrix contacts. At first, mesenchymal cell group (MSC or ADSC) was thought to be the most important multipotent “stem” cell. Further examination, however, now suggest that these may serve a “sentry” capacity, and that the actual cell group known as pericyte/endothelial stem/stromal cells (Figures 14, 15) [10].
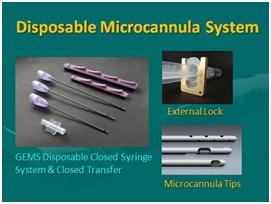 Figure 14: Closed, Sterile Disposable, Microcannula System (GEMS) (Tulip Medical, San Diego, CA, USA). Left Top: Internal lock sample (Purple), Microcannulas (2.11 mm OD) showing multiport infiltrator, spiral cannula (2.11mm), and, single port injector(1.65 mm), sterile clear luer-to-luer tranfer; Top Right: External universal non-disposable lock; Bottom Right: Microcannula tips.
Figure 14: Closed, Sterile Disposable, Microcannula System (GEMS) (Tulip Medical, San Diego, CA, USA). Left Top: Internal lock sample (Purple), Microcannulas (2.11 mm OD) showing multiport infiltrator, spiral cannula (2.11mm), and, single port injector(1.65 mm), sterile clear luer-to-luer tranfer; Top Right: External universal non-disposable lock; Bottom Right: Microcannula tips. Figure 15: Comparative cellular recovery: Closed syringe microcannula harvest versus machine vacuum on mesenchymal cells in ATC.
Figure 15: Comparative cellular recovery: Closed syringe microcannula harvest versus machine vacuum on mesenchymal cells in ATC.There is much confusion in interpretation of the scientific and clinical published materials caused by a lack of explanation of the difference between tissue Stromal Vascular Fraction (tSVF) and cellular Stromal Vascular Fraction (cSVF) (Figure 16). For clarification, cSVF is the isolated cellular elements in the ATC created via use of certain collagenase-enzyme blends to separate the complex and multiple attachments comprising the cell-to-cell or cell-to-matrix connections (Figure 7). The utilization of such cSVF is currently the subject of multiple clinical trial applications, and is heavily utilized in cell isolation, culture expansion, and cell characterization studies. This creates an “information gap” between clinical applications and those strictly of research value. If clinicians read only the peer-reviewed clinical journals, they will miss more than 85% of the pertinent information and data evolving on almost a daily basis, as the important advances appear with basic scientific and engineering publications. It is said that these information channels are currently doubling the existing knowledge base every 4-6 months, making keeping current with scientific and the clinical use information difficult to assimilate.
 Figure 16: Important understanding of terms: Tissue Stromal Vascular Fraction (tSVF) and Cellular Stromal Vascular Fraction (cSVF).
Figure 16: Important understanding of terms: Tissue Stromal Vascular Fraction (tSVF) and Cellular Stromal Vascular Fraction (cSVF).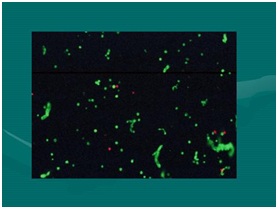 Figure 17: Flow cytometry (Live/Dead) stain (Acridine Orange A/O & Propidium Iodide (PI). NOTE: The freen “Strings” are viable cells with extracellular matrix residual attachments. Effectively transplantation of living Microenvironments (tSVF).
Figure 17: Flow cytometry (Live/Dead) stain (Acridine Orange A/O & Propidium Iodide (PI). NOTE: The freen “Strings” are viable cells with extracellular matrix residual attachments. Effectively transplantation of living Microenvironments (tSVF).In biological aspects, it is important to clearly recognize that not all platelet-rich plasma preparations and concentrates are the same. It is clearly shown that, the amount of growth factors, signal proteins, and important chemical agents have a DIRECT, linear relationship to the concentration of platelets actually achieved. It is confusing to follow the variety of processes used in creating what is being called PRP, particularly since most practitioners do not have the capability of confirming actual patient measured baselines to compare with achieved concentrations. To qualify as a true “high-density” PRP, we utilize the minimal concentrations to be 4-6 times an actual MEASURED baseline, not a calculated extrapolation. This is a very important concept to understand, based on the correlation of such concentration to cellular proliferation and migration capabilities. This confirms the importance of choosing the optimal platelet concentrates for the use, and understanding that NOT ALL PRP CONCENTRATES are equal. Refinements in HD-PRP options are now recognized as many subdivisions, such as low and high hematocrit solutions which have definite clinical implications to tissue tolerance and reactivity [18].
The value of use of centrifugation has increased in Biocellular applications, as it creates a very effective “gravity density separation”, important to avoid cellular debris, unwanted fluids and local anesthetics, and isolation of the unwanted free lipid layer from the upper portions of the lipoaspirate. In addition, it permits decrease of the interstitial fluid load, a factor requiring “overcorrection” of grafts or small joint placements. This unneeded load is felt to potentially impact site perfusion (as potentially can appear in “overfilled” target may negatively impact perfusion and healing in sites with small potential spaces. This is a factor of importance in many plastic surgical, reconstructive poor perfusion wounds, and Musculoskeletal (MSK) applications, such as seen in small joints and limited perfusion sites [19].
The final area of importance in MSK applications relates to the ability of optimal targeting of areas of damage, degeneration or inflammation. Without use of high definition ultrasonography, it is virtually impossible to assure accurate placement of the Biocellular therapeutic modality. With the use of ultrasonography, coupled with compressed and thoroughly mixed biocellular components, patients respond more rapidly, show metrics of responses, and achieve earlier final outcome than when placed via palpation only [20].
Within the past 4 years, an interesting option of removing the unwanted mature adipocytes from the AD-tSVF has become available. It is well documented that the large, mature adipocytes do not contribute significant value to an injection site (including when performing structural fat grafting in aesthetic surgery) as they are gradually lost and removed following their anoxic exposure. It is likewise clear that the stem/stromal cells in the ATC are not as susceptible to those conditions, and in fact, may be stimulated in low oxygen tension environments [21]. Recent publication of viability and numbers of stem/stromal cells remaining after emulsification process confirm that the relative numbers of such cells remain statistically the same as those not submitted for emulsification. One of the advantages of this process is that the AD-tSVF not only retains valuable stromal tissue, but the entire specimen (mixed with HD PRP) can be easily injected through small bore needles (25-30 gauge). This facilitates uses in scars, radiated damage skin, hair loss plus permits more patient comfort in MSK injections (including small joint targets) [22].
In regenerative medicine, the main goals are relatively well established (Figure 3). Likewise, description of “Optimal” features of cellular based therapy in both aesthetic and regenerative applications are becoming standardized. It is important to recognize that the combination of platelet concentrates and AD-tSVF appears to be more effective than either of the entities by themselves.
UNDERSTANDING THE “WORKERS AND BRICKS” ANALOGY
 Figure 18: “Workers and bricks” analogy.
Figure 18: “Workers and bricks” analogy.If you have a brick wall that is beginning to break down, some of the mortar holding the bricks together is lost or crumbling. What is needed to repair the wall would be hiring “WORKERS” to come in, clean up the site, and repair and replace the damaged mortar. Once completed, the wall is repaired and functions as originally intended. These workers are found in great quantities in platelet concentrates, and comprise the “biological” contribution of the Biocellular Regenerative treatments.
In the event, however, that your wall is not only losing mortar holding the bricks in place, imagine if you have lost or broken many of the bricks in the wall. This would require NOT ONLY the ‘workers”, but would also require “BRICKS” to replace the lost and damaged ones. The “bricks” in this analogy come from the cellular source (cSVF). Combining biologics + cell sources have proved more successful than use of EITHER of the agents by themselves.
It is well established that there are many more of these undifferentiated cells located in the largest micro-vascular organ of your body, within the adipose (fat) matrix. Therefore, the readily available and safely accessible “cellular” contributor of choice has become adipose tissue retrieved from subdermal fat deposits in the abdomen and thigh areas. These are gently removed via closed syringe lipoaspiration, compressed by centrifugation, and mixed the platelet concentrates (>4-6 times patient measured circulating platelets) to form the therapeutic mixture known as “Biocellular Regenerative Matrix”.
This mixture is in current use in aesthetic (plastic), reconstructive& wound healing, sports & pain medicine, orthopedic medicine and surgery, neurological disorders, musculoskeletal and arthritic applications, and a wide area of overlapping disorders.
WHAT ARE “ADULT STEM/STROMAL CELLS”?
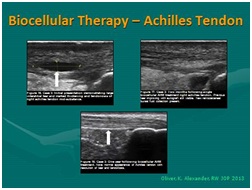 Figure 19: Ultrasound guided; biocellular therapy in Torn Achilles Tendon. Top Left: Tendon tear pre-treatment [NOTE: Rest of tendon showing tendinosis]; Top Right: Post-treatment tendon (At 6-8 Weeks); Post-treatment one year (1 yr) [NOTE: Resolution of tear without scarring, rest of tendon returning to more normal fibrillar tendon echotexture].
Figure 19: Ultrasound guided; biocellular therapy in Torn Achilles Tendon. Top Left: Tendon tear pre-treatment [NOTE: Rest of tendon showing tendinosis]; Top Right: Post-treatment tendon (At 6-8 Weeks); Post-treatment one year (1 yr) [NOTE: Resolution of tear without scarring, rest of tendon returning to more normal fibrillar tendon echotexture].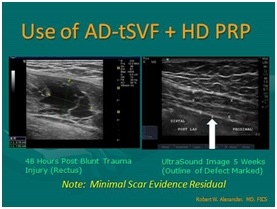 Figure 20: Use of tSVF + high density platelet concentrate (Biocellular Therapy) in torn rectus abdominus trauma. Left: Pre-Treatment image; Right: Area of trauma at 5 weeks post-trauma. [NOTE: Return of muscle echotexture without scarring].
Figure 20: Use of tSVF + high density platelet concentrate (Biocellular Therapy) in torn rectus abdominus trauma. Left: Pre-Treatment image; Right: Area of trauma at 5 weeks post-trauma. [NOTE: Return of muscle echotexture without scarring].There are many experiences in such cases over the past 15years in musculoskeletal area, and for more than30 years in aesthetic surgical practice. These are often reported on small case series or case reports of treatment& outcomes, and are now being further studied in many clinical trials [26]. Evolving clinical trials include both guided placement of stem/stromal elements and biological agents in orthopedic medicine and surgery, but also intravenous and central nervous system placement in a variety of complex disorders which do minimally or not respond to conventional therapy (such as Diabetes, Multiple Sclerosis, Alzheimer’s disease, Parkinson’s disease, Severe Limb Ischemia, Traumatic Brain Injuries, etc.) [27,28]. Early reports of improvement in chronic conditions, including pain, arthritis, damaged tendons-ligaments, low back & facet degeneration, etc., are driving many to select this option to improve surgical outcomes or avoid surgical interventions and shorten the demands for physical therapy.
Many remained confused about the potentials or best source of stem/stromal cells, often believing this only refers to use of embryonic tissues or non-autologous sources such as placental amniotic or umbilical cord-derived cells. In the past 15 plus years, much evidence has led us to understand that our on fat may be a much more plentiful and optimal cell source, avoiding the need to destroy fetal or embryonic tissues, or undergo more invasive marrow access in order to acquire cells and culture-expand them to achieve optimal potential. Further, the stem cells found in bone are heavily weighted to the blood forming side, rather the reparative group of cells.
Considering ready availability of fat, minimally invasive access (using disposable, microcannula (< 2.4 mm) closed syringe liposuction for example), adipose now has become an optimal source for these cells with a high safety profile for patients. As previously described, ATC is the largest micro-vascular organ in the body, and as such, have become well recognized as the largest depositories of undifferentiated stem/stromal cells in the entire body. The ease of gathering fat tissues on an outpatient basis and local anesthetic has led to evolution of “Biocellular Therapy” and “Cell-Based Therapy”) for a wide variety of disorders and conditions. With the advent of closed cell isolation/concentration systems (incubation, agitative digestion of cellular contact points) at Point-of-Care (POC) availability, the opportunity of cell enrichment in grafting procedures PLUS the availability of parenteral intravascular deployments can be achieved. It is most common for these procedures to be performed in outpatient Ambulatory Surgical Centers (ASC) or dedicated clinic procedural facilities.
Specific “Key” cells have been credited in promotion of healing and repairs reside in tissue micro-environments, where they comprise parts of tissues and organ systems identification of pathways, however, remains somewhat elusive. The complex components within the AD-tSVF may be considered to offer “smorgasbord” of elements which can become available to any site or tissue. Analyses of growth factors and signal chemicals would suggest that the intact AD-tSVF may offer contributions over and above those as isolated elements [9]. The cell groups participating in the healing or repair are subject to important contributions of native cell components in vascularized tissues, and by introduction of concentrates of cells and biologics appear to “auto-enhance” the site controls and effects. These native site cell groups are also called “niches”, and are the locations where injury or disease must be addressed to permit the body to repair or regenerate itself. It is believed that when that process is underway, addition of needed cell types and biological elements specifically targeted (via ultrasound guidance for example), can effectively utilize your own tissues to heal themselves in a more efficient and effective manner.
WHAT IS INVOLVED IN PROVIDING CELLULAR OR BIOCELLULAR REGENERATIVE THERAPY?
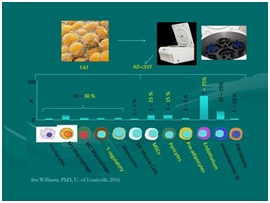 Figure 21: Main component cells in cellular stromal vascular fraction: [NOTE: Approximately one-third have multipotent capabilities, balance supportive cells of importance] Courtesy of Dr. Stu Williams, University of Louisville.
Figure 21: Main component cells in cellular stromal vascular fraction: [NOTE: Approximately one-third have multipotent capabilities, balance supportive cells of importance] Courtesy of Dr. Stu Williams, University of Louisville.At this point, cellular isolates/concentrates may be used in multiple applications, such as cell enrichment (where these isolates are added back to a tSVF portion) to provide higher stem/stromal cell numbers for potential graft and injection applications. The other option, is to re-suspend this cSVF group in 500 cc Normal Saline for delivery parenterally (IV, IA, Intrathecal, Intraperitoneal, or mucosal spray) depending on desired locations and uses. If IV or IA, typical very fine in line tubing filters (150 micron) ensure removal of any fibrin or other components in a manner common during blood product transfusions (which utilize a standard 170 micron filter).
In the case of parenteral deployment, most clinical researchers are using this within an FDA/NIH approved Clinical Trial situation, where safety and efficacy data is developed and reported.
The Biocellular Therapies refer to the use of tSVF (harvested via microcannula syringe systems) PLUS the mixed additive of HD PRP (or lower concentrations when used on radiation damaged, hair and dermal uses). The tSVF may be used after a centrifugation (typically 800-1200 g-Force for 3-4 minutes), mixed with an appropriate concentration (by volume percentage) of PRP, then guided or intradermally placed to targets. Plastic Surgery literature has many examples of improved structural grafting accomplished using cell-enriched tSVF as previously discussed (Figure 22).
 Figure 22: Value of centrifugation for layer separation compared to gravity decantation. NOTE: Density gradient separation at 800-1000 g-Force/3 min provides effective excess liquid/debris (Infranatant), compressed graft (tSVF), and well separated unwanted free lipid (Supranatant).
Figure 22: Value of centrifugation for layer separation compared to gravity decantation. NOTE: Density gradient separation at 800-1000 g-Force/3 min provides effective excess liquid/debris (Infranatant), compressed graft (tSVF), and well separated unwanted free lipid (Supranatant).An autologous tSVF sample is easily harvested from subdermal fat deposits under sterile protocols, using the patented, disposable closed syringe system. This is often referred to as microcannula lipoaspiration or lipoharvesting [17]. This Adipose Tissue Complex (ATC) may be cleaned, compressed (centrifuged) and unwanted liquid layers separated by centrifugation. This process not only helps with removal of unwanted liquids, but also compresses the adipose cellular components to provide a more effective cell and bioactive matrix with less intercellular fluid load (Figure 23). By effectively reducing the volume of injection materials, earlier recovery of comfort and ambulation is common. Biologicals such as High-Density Platelet Concentrates (HD-PRP) can then be added via closed, sterile luer-to-luer transfer to create a mixture of cells and the important growth factors/signal proteins provided from within the platelet alpha-granules. There is a direct correlation between concentration achieved and the delivery to targets [29].
 Figure 23: Left: showing centrifuged specimen; Right: Loading of compressed graft via clear luer-to-luer transfer, leaving supranatant layer (Not desired for injection).
Figure 23: Left: showing centrifuged specimen; Right: Loading of compressed graft via clear luer-to-luer transfer, leaving supranatant layer (Not desired for injection).The cellular isolates are currently being utilized for a wide variety of human clinical applications on a global basis. Following a myriad of basic research studies, animal models were tested and reported in the bioscientific literature, and gradually being reported in translational clinical Journals in the medical literature. As the Cellular Therapy group is well recognized as favoring immune privilege and pro “benign” inflammation (resulting healing with minimal scarring), those local, systemic and autoimmune issues are included in many clinical studies (Figures 24 and 25).

 Figure 25: Disposable system for emulsification (Micronization, “NanoFat”). Top: Screen (Offset 600/400 micron screen); Bottom: Shows “partial-emulisification luer-to-luer series of progressively smaller internal sizes (2.4, 1.4, and 1.2 Respectively). Photo Provided By Tulip Medical, San Diego, CA, USA.
Figure 25: Disposable system for emulsification (Micronization, “NanoFat”). Top: Screen (Offset 600/400 micron screen); Bottom: Shows “partial-emulisification luer-to-luer series of progressively smaller internal sizes (2.4, 1.4, and 1.2 Respectively). Photo Provided By Tulip Medical, San Diego, CA, USA.The importance of this understanding has led to appreciation of the paracrine capabilities via exosomes and microvesicles, and their roles in transferring signals via mitochondrial RNA (miRNA) and messenger RNA (mRNA). It is now believed that the exchanges of these elements are the means of communication and stimulation of nuclear change leading to the proliferative and trophic effects to guide the differentiation for cell activities or secretions in specific sites. There is currently important studies utilizing both autologous and non-autologous exosomes and microvesicles as an important contribution to the activation and guidance of cells to respond (Figures 26 and 27) [31,32].
 Figure 26: Paracrine secretions and inter-cellular communications: Exosomes and microvesicles as means of signaling for stem cell activation and proliferation.
Figure 26: Paracrine secretions and inter-cellular communications: Exosomes and microvesicles as means of signaling for stem cell activation and proliferation. Figure 27: Diagrammatic representation of exosomes and microvesicles.
Figure 27: Diagrammatic representation of exosomes and microvesicles.A RECENT ADVANCE IN USE OF BIOCELLULAR USES: MICRONIZED &“NANOFATTM” (FULLY OR PARTIALLY EMULSIFIED AD-TSVF)
Recent advances now offer the opportunity to have a disposable system to achieve maximal lysis of adipocytes, while preserving the much smaller cSVF component (Figures 28 and 29). It is important to note that this emulsified (micronized) tSVF product is NOT ABLE to produce true cSVF and, therefore, CANNOT be safely used in any intravascular uses. Although not believed to be as effective as using larger fragment tSVF for structural grafting, can still be combined with PRP concentrates to provide improved dermal vascularity and configuration.
 Figure 28: Emulsified adipose tissue complex (tSVF). Left: Showing SEM of AD-tSVF prior to emulsification; Right: SEM of AD-tSVF after emulsification [NOTE: Essential Removal of All Intact Mature Adipocytes, Leaving Perivascular and Extracellular Matrix (ECM) Intact].
Figure 28: Emulsified adipose tissue complex (tSVF). Left: Showing SEM of AD-tSVF prior to emulsification; Right: SEM of AD-tSVF after emulsification [NOTE: Essential Removal of All Intact Mature Adipocytes, Leaving Perivascular and Extracellular Matrix (ECM) Intact]. 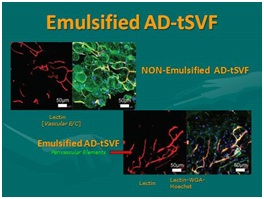 Figure 29: Emulsification of adipose tissue complex (tSVF). Top Left: Showing AD-tSVF prior to Emulsification (Stain showing vascularity on left, and intact adipocytes (Green) on right of micrograph); Bottom Right: showing AD-tSVF after emulsification of mature adipocytes. [NOTE: Multiple blue stained nuclei remaining intact (Small fragment tSVF, NOT a cSVF).
Figure 29: Emulsification of adipose tissue complex (tSVF). Top Left: Showing AD-tSVF prior to Emulsification (Stain showing vascularity on left, and intact adipocytes (Green) on right of micrograph); Bottom Right: showing AD-tSVF after emulsification of mature adipocytes. [NOTE: Multiple blue stained nuclei remaining intact (Small fragment tSVF, NOT a cSVF).With the ability to injection through small bore needles, patient comfort in dermal and hair injections is enhanced, plus offers a range of radiation/solar damaged skin and small joint targeted applications in orthopaedic medicine. The abilities of Biocellular modalities to promote wound healing and regenerative capabilities via intradermal placement has opened opportunities to permit improved skin circulation and texture, improve skin aging and radiation damage, hair regeneration, participate in chronic wound applications, as well as many small joint and superficial targets in musculoskeletal applications (Figure 30).
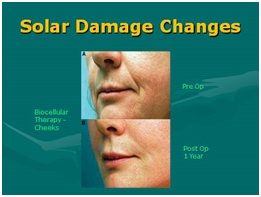 Figure 30: Sample of post-radiation skin aging/damage (Sun). Top: Pre-treatment biocellular mix; Bottom: Post-treatment biocellular grafting (1 Year) of cheeks, lips and nasolabial folds. [NOTE: To show true texture change as result of biocellular mix, placed subdermally].
Figure 30: Sample of post-radiation skin aging/damage (Sun). Top: Pre-treatment biocellular mix; Bottom: Post-treatment biocellular grafting (1 Year) of cheeks, lips and nasolabial folds. [NOTE: To show true texture change as result of biocellular mix, placed subdermally].WHAT IS THE FUTURE IN STEM/STROMAL CELLULAR AND BIOCELLULAR TREATMENTS?
With the changes in legislation and within the FDA, new and exciting possibilities of IND/RMAT designations are being considered and suggested guidelines evolving. Timing of these changes is optimal, as much of the safety and early efficacy opportunities will already be in place by the time such designations are better understood. The 21st Century Cures Act and the “Right to Try” 2018 Legislation are examples of some of these changes.
Isolation of these cells permits creation of what is termed “cell enriched” biocellular grafts. These grafts, higher in numbers of the heterogeneous undifferentiated cells, are believed to provide an even more potent guided injectable therapy. For example, there are many peer reviewed clinical articles providing strong evidence of enhanced outcomes within the aesthetic-plastic surgical literature. Over the past decade, there are estimated numbers of use of Biocellular therapies in musculoskeletal application exceeding 150,000 human clinical uses, with a remarkable efficacy and safety profile. These are reported in case series or reports, and should not be discarded out of hand, simply because they are not participating in specific trial settings. It remains a pivotal value to insure that very accurate diagnostics and guided placement to defined targets. Ultrasonography, with its dynamic abilities during examination, will remain as a needed core competency for those taking care of musculoskeletal and chronic wound cases.
In the future, it is very likely that such isolated cells will provide parenteral (intravenous, intra-arterial, intra-thecal, intra-peritoneal, etc.) pathways, and become effective for a very expansive treatment in such disorders as neurodegenerative diseases (MS, Alzheimer’s, ALS, Parkinson’s, brain injuries/stroke, etc.), diabetes, chronic lung disease, heart disease and damage, chronic wound healing, fibromyalgia/causalgia, ulcerative bowel disease, Crohn’s, colitis, and so forth.
PROVIDERS OF BIOCELLULAR TREATMENT?
Most times these procedures are completed on outpatient, ambulatory basis using local anesthesia, nitrous oxide, or occasionally light sedation depending on patient needs and desires. These cases are designed and planned to be completed within the same day. Providers handle tissues using standard aseptic protocols. Since the advent of using a variety of mechanical emulsification systems which permit guided injections through very small bore needles (25 gauge-30 gauge) without statistically reduced viabilities or clinical outcomes. The ability to provide improved patient comfort and access to skin, hair and small joints is rapidly becoming an appealing option.
CONCLUSION
There are many evolving clinical trials (NIH/FDA oversight) recognizing the very remarkable abilities of cellular components of adipose tissue complex (ATC). The number of active and evolving trials using ATC is surpassing the uses of bone marrow due mostly to the much higher numbers of reparative cells within the marrow. Marrow remains the gold standard for various blood-related diseases, but is not clear that it possess very few of the non-hematologic (reparative) stem cells, and it thought by many to be only useful as a biologic contributor to the wound healing and regenerative processes discussed in this chapter.
To date, researchers and bioengineers are not able to effectively reproduce or provide true 3-dimensional scaffolding at this time. This inability limits development of understanding how to mimic a true in vivo culture/expansion capability, and continues to delay understanding of how these cells act and are controlled at the local tissue level. Translation from tissue culture and the inherent changes potentially introduced in the process leaves us with the need to cautiously advance therapeutic modalities into clinical practices. Classic tissue culture/expansion remains problematic, in that it is known to introduce variables which are not able to be translated into how the body uses these undesignated cell populations.
ACKNOWLEDGEMENT
CONFLICT OF INTEREST
REFERENCES
- Alexander RW (2012) Understanding adipose-derived stromal vascular fraction (AD-SVF) cell biology and use on the basis of cellular, chemical, structural and paracrine components: a concise review. Journal of Prolotherapy 4: 855-869.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211-228.
- Zuk P (2013) Adipose-Derived Stem Cells in Tissue Regeneration: A Review. Stem Cells Pg no: 35.
- Alexander RW (2013) Understanding Adipose-Derived Stromal Vascular Fraction (SVF) Cell Biology In Reconstructive and Regenerative Applications On the Basis of Mononucleated Cell Components. J of Prolo 10: 15-29.
- Alderman DD, Alexander RW, Harris GR, Astourian PC (2011) Stem Cell Prolotherapy in Regenerative Medicine: Background, Theory and Protocols. J. of Prolotherapy 3: 689-708.
- Sadati KS, Corrado AC, Alexander RW (2006) Platelet-rich plasma (PRP) utilized to promote greater graft volume retention in autologous fat grafting. American Journal of Cosmetic Surgery 23: 203-211.
- Alexander, Robert W (2010) Author Textbook, Use of PRP in Autologous Fat Grafting, in Autologous Fat Grafting, Textbook, Shiffman, M. ed., Springer, Berlin 14: 87-112.
- Alexander RW (2016) Biocellular Regenerative Medicine: Use of Adipose-Derived Stem/Stromal Cells and It's Native Bioactive Matrix. Phys Med Rehabil Clin N Am 27: 871-891.
- Blaber SP, Webster RA, Hill CJ, Breen EJ, Kuah D, et al. (2012) Analysis of In Vitro Secretion Profiles from Adipose-Derived Cell Populations. J Transl Med 10: 172.
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, et al. (2008) A Perivascular Origin For Mesenchymal Stem Cells In Multiple Human Organs. Cell Stem Cell 3: 301-313.
- Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, et al. (2009) Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5: 17-26.
- Brizzi MF, Tarone G, Defilippi P (2012) Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol 24: 645-651.
- Kim SH, Turnbull J, Guimond S (2011) Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 209: 139-151.
- Alexander RW (2009) Fat Transfer with Platelet-Rich Plasma for Breast Augmentation. Breast Augmentation Pg no: 451-469.
- Kato H (2011) Short- and long-term cellular events in adipose tissue remodel¬ing after non-vascularized grafting. Paper presented at the International Federation for Adipose Therapeutics and Science Miami, 9th Annual Symposium on Adipose Stem Cells and Clinical Applications of Adipose Tissue; Nov 4-6; Miami, Florida, USA.
- Alexander RW (1994) Liposculpture in the Superficial Plane: Closed Syringe System for Improvements in Fat Removal and Free Fat Transfer. Am J Cosm Surg 11: 127-134.
- Alexander RW, Harrell DB (2013) Autologous fat grafting: use of closed syringe microcannula system for enhanced autologous structural grafting. Clin Cosmet Investig Dermatol 6: 91-102.
- Alexander, Robert. 2019: Unpublished Data
- Kurita M, Matsumoto D, Shigeura T, Sato K, Gonda K, et al. (2008) Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg 121: 1033-1041.
- Alexander RW (2015) Contributing Author,Textbook “Introduction To Biocellular Medicine”, in Sonography of the Extremities: Techniques & Protocols 4th Ed., Moore, R., Editor, General Musculoskeletal Imaging Inc., Cincinnati Pg no: 97-104.
- Eto H, Suga H, Inoue K, Aoi N, Kato H, et al. (2011) Adipose injury-associated factors mitigate hypoxia in ischemic tissues through activation of adipose-derived stem/progenitor/stromal cells and induction of angiogenesis. Am J Path 178: 2322-2332.
- Alexander RW (2016) Understanding Mechanical Emulsification (Nanofat) Versus Enzymatic Isolation of Tissue Stromal Vascular Fraction (tSVF) Cells from Adipose Tissue: Potential Uses in Biocellular Regenerative Medicine. Journal of Prolotherapy 8: 947-960.
- Tate-Oliver K, Alexander RW (2013) Combination of Autologous Adipose-Derived Tissue Stromal Vascular Fraction Plus High Density Platelet-Rich Plasma or Bone Marrow Concentrates in Achilles Tendon Tears. J of Prolotherapy 5: 895-912.
- Albano JJ, Alexander RW (2011) Autologous Fat Grafting As a Mesenchymal Stem Cell Source and Living Bioscaffold In a Patellar Tendon Tear. Clin J Sport Med 21: 359-361.
- Alderman D, Alexander RW (2011) Advances in Regenerative Medicine: High-density Platelet-rich Plasma and Stem Cell Prolotherapy for Musculoskeletal Pain. Journal of Pract Pain Management (PPM) 10: 49-90.
- Alexander RW (2011) Autologous Fat Grafts as Mesenchymal Stromal Stem Cell Source For Use in Prolotherapy: A Simple Technique to Acquire Lipoaspirants. J of Prolo 3: 680-688.
- Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, et al. (2016) Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells 34: 2210-2223.
- Alexander RW (2017) Use of Software Analytics of Brain MRI (With & Without Contrast) As Objective Metric in Neurological Disorders and Degenerative Diseases. Int Phys Med Rehab J 2: 203-206.
- Tobita M, Tajima S, Mizuno H (2015) Adipose Tissue-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma: Stem Cell Transplantation Methods That Enhance Stemness. Stem Cell Res Ther 6: 215.
- Alexander RW (2017) Use of PIXYL software analysis of brain MRI (with & without contrast) as valuable metric in clinical trial tracking in study of multiple sclerosis (MS) and related neurodegenerative processes. Clin Trials Degener Dis 2: 1-6.
- Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, et al. (2013) Nanofat Grafting: Basic Research and Clinical Applications. Plast Reconstr Surg 152: 1017-1026.
- Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW, et al. (2015) Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev 21: 45-54.
Citation: Alexander RW (2019) Overview of Cellular Stromal Vascular Fraction (cSVF) & Biocellular Uses of Stem/Stromal Cells & Matrix (tSVF + HD-PRP) in Regenerative Medicine, Aesthetic Medicine and Plastic Surgery. J Stem Cell Res Dev Ther: S1003.
Copyright: © 2019 Robert W Alexander, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

