
3D Bioprinting Technologies for Tissue Engineering: A Mini Review
*Corresponding Author(s):
Elizabeth G LoboaOffice Of The Provost, Southern Methodist University, PO Box 750221, Dallas, TX 75275, Texas, United States
Email:egloboa@smu.edu
Abstract
Tissue engineering aims to develop constructs that maintain, restore, or improve tissue function. Recent advancements in Three-Dimensional (3D) bioprinting have brought great potential for tissue engineering of many different tissues and organs, such as skin and heart. While advances in biomanufacturing organs and tissues have occurred, organs are highly complex and challenges in recapitulating the intricate structure and function of organs with current methods remain. Primary and most common bioprinting methods are described here; and, an overview of bioink formulations and cell sources used in 3D bioprinting is provided. Finally, current challenges and future needs are discussed.
INTRODUCTION
Recent advances in Computer-Aided Design/Computer-Aided Manufacturing (CAD/CAM) technology have enhanced the potential for Additive Manufacturing (AM), known as Three-Dimensional (3D) printing, for use in fabrication of 3D scaffolds for tissue engineering applications. 3D printing was initially introduced by Charles W Hull in 1986. Hull’s technique, termed stereolithography, utilizes ultraviolet light to cure thin layers of a material on top of existing layers, sequentially forming a three-dimensional structure [1]. 3D bioprinting is an interdisciplinary practice closely related to engineering and life sciences. It aims to develop 3D organ constructs that maintain, restore, or improve tissue function [2,3]. Layer-by-layer deposition allows for precisely and selectively deposited biological materials, biochemicals and, living cells.
The development of such 3D in vitro systems has attracted increasing attention in healthcare. This is predominantly driven by two needs: a limited supply of organs [4] and a demand for less expensive drug testing models [5]. The demand for organ transplantation has grown rapidly in recent years. Between 2006 and 2016, the number of patients in the United States on the organ transplant waiting list increased from 95,000 to 160,000 [6]. The substantial growth of the wait list illustrates the demand for new transplant solutions. In addition, lack of accurate 3D models for drug screening and medical mechanism studies is a niche that 3D bioprinting aims to fill.
Currently available Two-Dimensional (2D) cell culture validation techniques and animal testing models used for drug discovery and analyses of biochemical agents have a number of drawbacks. 2D culture methods fail to reproduce the in vivo microenvironment or recapitulate organ-level physiology properly, and animal models may poorly mimic the corresponding mechanisms in humans, tending to lead to ethical concerns [7]. For these reasons, 3D bioprinting technology holds great promise in the manufacture of engineered tissue constructs. This mini-review summarizes the primary and most common 3D bioprinting techniques used in tissue engineering. Fundamentals of these bioprinting methods and an overview of the formulations and properties of the bioinks and cell sources they use are provided. This article also provides commentary on the current limitations of 3D bioprinting technologies used for tissue engineering applications.
3D BIOPRINTING PROCESS
In general, the process for bioprinting 3D tissues can be divided into three primary steps:
Pre-bioprinting
The overarching goal of this step is to generate a 3D pin-point tissue or organ model that can be created using medical imaging technology or Computer-Aided Design (CAD). X-ray, Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are the most common imaging techniques utilized to provide information on the anatomical structure of the tissue or organ [8]. Design engineering software then “slices” a 3D model into horizontal cross-sectional layers creating stereolithography data that is then utilized in 3D-bioprinting for layer-by-layer stereolithographic accumulation to fabricate a 3D physical model [9].
Bioprinting
The next step is development of bioink for bioprinting of the tissue construct. Bioink refers to a cell-laden fluid material that may include biomaterials, cells, growth factors, microcarriers, etc. Development of appropriate bioink is a critical step for successful bioprinting. Properties of the bioink such as printability, biocompatibility, cell viability and mechanical properties strongly influence the printed tissue construct [10,11]. Likewise, it is crucial to choose an appropriate printing method and determine the optimal processing parameters as both directly impact the final bioprinted tissue construct.
Post-bioprinting
The post-bioprinting maturation process, which usually takes place in bioreactors, is a critical step for developing functional bioprinted constructs via both physical and chemical stimulation [8].
3D BIOPRINTING TECHNOLOGIES
The primary 3D bioprinting techniques utilized for tissue engineering applications are classified as inkjet, microextrusion and laser- assisted printing. Prototyping principles, features, and applications of each of these techniques (Figure 1) are discussed next.
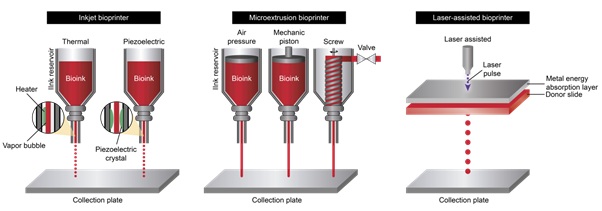 Figure 1: a) Inkjet bioprinter: A pulse thermal or piezoelectric stimulus causes fine droplets of bioink to be emitted from the nozzle. b) Microextrusion bioprinter: A stream of bioink is deposited in response to pressure exerted on the reservoir. c) Laser assisted bioprinter: A pulsed laser beam is directed at a band of metal which absorbs and transmits the energy to the bioink present underneath.
Figure 1: a) Inkjet bioprinter: A pulse thermal or piezoelectric stimulus causes fine droplets of bioink to be emitted from the nozzle. b) Microextrusion bioprinter: A stream of bioink is deposited in response to pressure exerted on the reservoir. c) Laser assisted bioprinter: A pulsed laser beam is directed at a band of metal which absorbs and transmits the energy to the bioink present underneath.
Inkjet 3D bioprinting
3D bioprinting initiated with researchers altering standard 2D inkjet printers in order to print bioink in successive layers. Inkjet printers work by depositing droplets of ink at precise points on a substrate. The droplets can be emitted from the reservoir nozzle using thermal, piezoelectric, or electromagnetic forces. Although these forces generate local extreme conditions, the transient nature of the pressure allows the cells to maintain viability with minimal stress [12]. Bioprinting with inkjet printers can be advantageous due to their ability to print at a relatively high-speed while being a commonly available, relatively low-cost technology. A drawback with use of inkjet printers is that special considerations need to be made in bioink selection. Since the ink has to be emitted from a small diameter nozzle at a high rate, low viscosity is paramount [13]. Even with the ideal bioink for an inkjet printer, clogging can still be an issue. Further, passing cells through a high-pressure bottle neck may have some effect on cellular function, including possible pressure to differentiate into a specific lineage when using stem cells [14].
Microextrusion 3D bioprinting
Microextrusion based 3D bioprinting, also known as Fused Deposition Modelling (FDM), is an additive manufacturing process that revolves around the deposition of a single near-continuous stream of material in successive layers to form the desired three-dimensional structure. The pressure on the reservoir can be supplied from a variety of mechanical apparatuses, with pneumatic / mechanical pistons and screw drive mechanisms being the most common. The biggest difference between microextrusion and inkjet deposition is the bioink that must be used. Microextrusion bioprinters have the advantage of being able to work with a wider range of viscous bioinks relative to inkjet and laser-assisted bioprinters [15,16]. However, although more viscous inks may be used, the high pressures generated when printing these inks can lead to effects on the cells. Even when cells can withstand the high shear forces of extrusion-based printing, they may have decreased viability or be mechanically stimulated to differentiate aberrantly [17]. Similar to inkjet bioprinting, nozzle clogging can be an issue since they both of these technologies utilize small diameter nozzles. Certain techniques, such as frequent cleaning and capping of the nozzle when not in use, can help prevent this issue.
Laser-assisted 3D bioprinting
Laser-Assisted 3D Bioprinting (LAB) utilizes a technique known as Laser Induced Forward Transfer (LIFT). In this process a laser beam is focused onto a precise point on a photo absorptive metal sheet (often gold or titanium). The energy absorbed by the metal is then transferred to a biologic solution underneath, which is then ejected from the reservoir onto a substrate. This method is advantageous because there is no nozzle to clog, and also because a relatively high printing resolution can be achieved [18]. LAB systems have a higher barrier to entry however, with some systems costing orders of magnitude more than inkjet bioprinters [19]. Even though a high energy laser delivers the pulse to emit the bioink droplets, cell viability remains high with this technique (95% on average) [20].
BIOINKS
The term bioink refers to any of the various combinations of biocompatible materials that are used in the 3D bioprinting process. They include both the biological component (cells and biopolymers) and synthetic materials that are present in some scaffolds. Developing an ideal bioink has consistently been a paramount goal for 3D bioprinting. The diverse properties and functionalities of various tissues that exist in the human body make bioink properties more specific and complex. The diversity and complexity of modern bioink formulations reflect the corresponding variety and intricacy of the milieu found within the human body. An ideal bioink should generally have the properties of excellent printability, biocompatibility and mechanical integrity. Printability refers to the capability of a bioink to deposit precisely and accurately in order to fabricate a 3D tissue construct with high structural integrity and fidelity, whereas biocompatibility indicates that the bioink is cell friendly without eliciting cell death or excessive immune response. Biocompatible materials support cell adhesion, migration, proliferation and differentiation [21]. A gelled bioink must have sufficient strength and stiffness to preserve structural integrity, internal architecture and interconnectivity following in vitro and in vivo culturing. In order to meet specific, desired properties of a given bioink, the rational design of bioink is highly dependent on the bioprinting modality and cell type (Table 1). For example, inkjet 3D bioprinting requires low viscosities and low thermal conductivity to prevent nozzle clogging and heat damage, while extrusion 3D bioprinting requires higher viscosities that may negatively affect cell viability [22]. Therefore, a vital step in bioprinting is to try and achieve an optimal balance between these bioink properties in order to meet specific needs of the target tissue. Typical materials utilized in bioprinting are comprised of naturally derived sources (including collagen [23,24], gelatin [25-27], fibrinogen [28,29], alginate [30-32], chitosan [33-35], silk [20,36,37], hyaluronic acid [27,38]), and/or synthetic materials (including polyethylene glycol (PEG)-based materials such as PEG diacrylate (PEGDA) and polyacrylamide (PAAm)-based gels [38,39]). An advantage of naturally derived polymers for 3D bioprinting applications is their inherently high biocompatibility. An advantage of synthetic polymers is that their physical properties can be modified to suit particular applications. However, there are challenges in using synthetic polymers including poor cell attachment, non-immunogenicity and loss of mechanical properties during degradation.
|
|
Bioprinter type |
References |
||
|
Inkjet 3D bioprinting |
Microextrusion 3D bioprinting |
Laser-assisted 3D bioprinting (LAB) |
||
|
Viscosity (mPa/s |
3.5-12 |
30-6×107 |
1-300 |
[15,16,40,41] |
|
Gelation methods |
Chemical, photo-crosslinking |
Chemical, photo-crosslinking, sheer thinning, temperature |
Chemical, photo-crosslinking |
[42,43-45] |
|
Resolution |
50-300 µm wide droplets |
100 µm to 1 mm wide |
50µm |
[46-49] |
|
Accuracy |
Medium |
Medium-to-low |
High |
[50] |
|
Print speed |
Fast |
Slow |
Medium-to-fast |
[46,49,51,52] |
|
Nozzle size |
20-150 µm |
20 µm-millimeter |
Nozzle-less |
16,40,53,54] |
|
Purchase cost |
Low |
Medium |
High |
[55] |
|
Preparation time |
Low |
Low-to-medium |
Medium-to-high |
[76,56-58] |
|
Cell density |
106 - 107 cell/mL |
High, cell spheroids |
106 - 108 cell/mL |
[46,59,60] |
|
Cell viability |
>85% |
40%-80% |
95% |
[12,58,61,62] |
|
Biomaterials used |
Hydrogels, fibrin, agar, collagen, alginate |
Hyaluronic acid, gelatin, alginate, collagen, fibrin |
Hydrogels, nano-hydroxyapatite |
[50] |
|
Examples applications |
Skin, vascular, cartilage |
Trachea, cardiac valve |
Skin |
[18,63-67] |
Table 1: 3D bioprinter methods in tissue engineering.
CELL SOURCES
The selection and utilization of an appropriate bioink for printing a 3D tissue construct requires consideration of cell types and tissue sources. The cell source used for tissue or organ bioprinting must take into account the function and composition of the tissue to be replaced. Transplanted tissues or organs must be able to restore the original function of the tissues or organs they are meant to replace; therefore, the bioprinting process must utilize a cell type that provides support for, at a minimum, the primary cell type in the tissue. Precise cell proliferation, regeneration and differentiation are the primary processes involved during vascularization and deposition of multifunctional layers in the bioprinting process. Cells used in the scaffold must mimic the primary cell type functions and structures in vivo and in vitro under optimum conditions (Table 2) [8]. Transplantation of bioprinted tissue requires analogous and patient-specific cell components, of which the former can be obtained by biopsy or by differentiation of the patient’s stem cells. Further, most tissues are multilayered, with tissue function varying by layer, and often require specific cell differentiation to mimic the functions of the various layers. Because stem cells can differentiate into a multitude of cells with specific functions, they can serve as an excellent candidate cell source for synthesis of analogous cells in the bioprinting scaffold. There are several types of stem cells including embryonic, pluripotent, and adult stem cells [68].
|
Organ Systems |
Cells types used in 3D Bioprinting |
|
Cardiovascular Tissue |
Embryonic stem cells, Mesenchymal stem cells, Cardiac progenitor cells, Adipose derived stromal vascular fraction cells, Myoblasts |
|
Musculoskeletal Tissue |
Mesenchymal stem cells, Myeloid-derived suppressor cells, Myoblasts |
|
Neural Tissue |
Embryonic stem cells, Mesenchymal stem cells, Glioma stem cells, Neural stem cells |
|
Hepatic Tissue |
Human induced pluripotent stem cells, Embryonic stem cells, hepatocyte like cells, iPSC derived hepatic progenitor cells |
|
Adipose Tissue |
Adipose derived stem cells |
|
Skin Tissue |
Amniotic fluid stem cells, Mesenchymal stem cells, Adipose derived stem cells, Epithelial progenitor cells |
Table 2: Cells type used in 3D bioprinting [19].
Embryonic Stem Cells (ESCs) are pluripotent stem cells isolated from the blastocyst stage of in vitro fertilized embryos [69]. To grow ESCs, cells from the blastocyst stage are usually cultured on a feeder layer of irradiated mouse fibroblasts with growth factors; however, newer methods have been developed to culture cells without the mouse feeder layer so as to decrease the risk of viral transfer [70]. Many ethical debates were sparked by the use of fertilized embryos; therefore, other researchers began using dead embryos and single cell biopsy [71]. ESCs proliferating in culture for at least 6 months without differentiating, that appear karyotypically normal, are considered an ESC line and can be frozen and sent to other laboratories for use. They can then undergo directed differentiation into various cell types [19]. However, ESC use in research in the U.S. is currently limited due to ethical concerns [72].
Induced Pluripotent Stem Cells (IPSCs) are somatic cells that can be reprogrammed into stem cells. For the development and generation of induced pluripotent stem cells, four transcriptional factors (present in embryonic stem cells; Oct3/4, Sox2, c -Myc, Klf4) are introduced into fibroblasts using viruses as a host [73]. The inner cell mass destiny is monitored by expression levels of Oct3/4. The interaction of Sox2 with Oct3/4 develops and controls gene expression levels and maintenance of pluripotency. c-Myc controls differentiation and growth while the regeneration of stem cells and maintenance of pluripotency is regulated by Klf4 [74]. Pluripotent or immature cells at the stage of primed pluripotent stem cells do not have more capacity for differentiation as compared to embryonic stem cells but can develop enhanced risk of teratoma formation [69].
ADULT STEM CELLS
Bone Marrow Stem Cells (BMSCs) are a type of adult stem cell found in bone marrow. Adult stem cells are multipotent and reside in an area called the “stem cell niche.” They usually remain quiescent until they are activated to maintain normal tissues or repair diseased and injured tissues. They typically exist in small quantities and have a limited capacity to divide in vitro. It is thought that BMSCs will not induce rejection after transplantation of differentiated cells, thereby eliminating the need for immunosuppressive drugs that have many harsh side effects [75]. Bone marrow contains both hematopoietic stem cells and stromal stem cells, also known as mesenchymal stem cells. The stromal stem cells make up a small portion of the bone marrow and can generate many tissue types [76]. They require less in vitro manipulation than ESCs and iPSCs, and have a much lower rate of malignant transformation than iPSCs [76]. However, their proliferation and differentiation potential changes with increasing age [77,78] and harvest of BMSCs requires a painful procedure [79].
Adipose Derived Stem Cells (ADSCs) are another type of adult stem cell which is abundant in white adipose (fat) tissues [80]. Adipose derived stem cells are present in larger numbers and have five times the lifespan compared to adult bone marrow stem cells [80,81]. Cartilage and bone engineering can be done by using adipose fat tissues although it has been published that more precise results may be obtained by use of an infrapatellar fat pad source [82,83]. Adipose derived stem cells are useful and helpful for the synthesis and fabrication of analogous tissue and have great potential for multiple tissue engineering applications [84].
In a 3D bioprinted construct, a high rate of cellular proliferation may be required to ensure appropriate ratios of functional and supporting cells. Ideally, proliferation should remain at a constant and appropriate rate in order to maintain tissue homeostasis without the formation of hypertrophic cells (an initial sign of tumor). Techniques have been developed to overcome this problem, such as viral transfection or utilization of small molecules to prompt cell proliferation. Irregular proliferation may cause disturbances in the construct cell type. Likewise, differentiation depends upon many different factors at play during the creation of bioprinted constructs. Differentiation is affected by mechanical properties and characteristics of bioink scaffolds, which vary depending on the tissue type and compatibility [85,86]. Stem cell differentiation within the bioprinted scaffold is primarily regulated by two main structural factors: scaffold density and elasticity. Strong and stiff scaffolds (9-31 kPa) have been shown to stimulate differentiation toward musculoskeletal lineages whereas elastic and soft scaffolds (0.1-5 kPa) may stimulate differentiation into adipose and neural lineages [87-90]. Re-creation of the native environment for any given tissue type involves recapitulating in vivo stresses and stimulation of stem cell differentiation [87]. Mechanical properties exhibit heterogeneity between morphology and variety of composition and internal organization. In general, the cytoskeleton in stem cells usually re-forms and rearranges during lineage specification and modulates mechanical characteristics by accumulation of intracellular metabolites. Growth factors and other chemical stimuli further the cell differentiation process across different cell lineages in a tissue specific manner for expression of the appropriate phenotype.
Cells at this stage are usually fully differentiated, multipotent and exist at a high density [91]. Additive factors (e.g. growth factors and chemicals in the bioink) are one of the primary and direct approaches that can affect stem cell differentiation [86]. These factors may be added before or after the printing process. They can alter cellular mechanics and affect the differentiation process. Fibroblast growth factor, platelet-derived growth factor, ascorbic acid, dexamethasone, and bone morphogenetic proteins are some common examples of bioactive molecules used to enhance bioprinted constructs. Microcarriers (small polymer spheres) are another class of additives that stimulate the differentiation of stem cells while acting as a source of adhesion and providing stiffness [92]. For bioprinting applications, the cells must be able to tolerate multiple mechanical and chemical stressors such as pressure, shear stress, aberrant pH, and the presence of toxins or enzymes once transplanted. Certain bioprinting technologies are designed to carefully deposit cell types that are more sensitive to shear stress during preparation of the construct. Hence, cellular proliferation and the differentiation processes are key players in the efficacy and functionality of cells for bioprinting applications [68].
OUTLOOK AND FUTURE CHALLENGES
The field of bioprinting is at an early developmental stage and has garnered some notable successes in creation of transplanted functional constructs for a variety of tissues [68]. One of the main challenges in 3D bioprinting is designing suitable bioinks for each tissue type that meet the required mechanical, biological and physiological properties. Development and engineering of innovative bioinks or biomaterial formulations remain major areas of interest and investigation. For this purpose, more work will be required in the creation of new matrices and models to evaluate and monitor the characteristics and processes of a variety of bioink materials. The field of bioprinting also strives for enhanced resolution, speed and biocompatibility. Bioprinting has been developing to expand the range of compatible materials and methods for deposition of materials with greater specificity and accuracy.
Vascularization remains another major challenge in the area of tissue engineering and bioprinting 3D tissues with appropriate functions. Having adequate vascularization in bioprinted constructs is a critical factor for long term functional 3D bioprinted tissue. Without adequate cellular perfusion, cells may die of hypoxia and exhibit stagnant growth due to waste and toxin accumulation. Effective construction of a multi-scale perfused vascular network, and subsequent promotion of its vascularization through mechanical or chemical stimulation, is a basis for biofabricating more voluminous tissues [93]. Traditional 3D bioprinting platforms have been predominantly used to design and engineer 3D bioprinted constructs in vitro prior to subsequent implantation of the construct into the body. However, in terms of clinical applicability, the in vitro bioprinting approach may have some logistical challenges including: i) 3D bioprinted constructs are often fragile, and internal micro-features may be disrupted during transport from the fabrication room to the operating room; ii) a highly sterile environment is required; and iii) necessity to modify and trim the bioprinted construct prior to implantation. This last challenge arises when the geometry of the bioprinted construct differs from the actual defect size as a result of limited resolution capability of the CT and MRI scans utilized to create the construct. Due to these challenges, in situ bioprinting directly in the body in the clinical setting has been proposed [94,95]. Two primary in situ technologies have been developed: i) a highly portable handheld printer [96-98] (Figure 2), and ii) a robotic arm carrying the bioprinting unit which is capable of performing real-time printing [99] (Figure 3). To date, various studies have shown the feasibility of the in situ bioprinting concept for regeneration of skin [42], cartilage [96], and bone [100]. While much progress has been made with in situ bioprinting technologies, numerous challenges remain, including but not limited to: i) requiring a large number of cells before surgery, ii) need for printers with high resolution, iii) creation and utilization of bioinks that can form the desired stable structure instantly and prompt tissue regeneration, iv) ethical dilemma, and v) high cost.
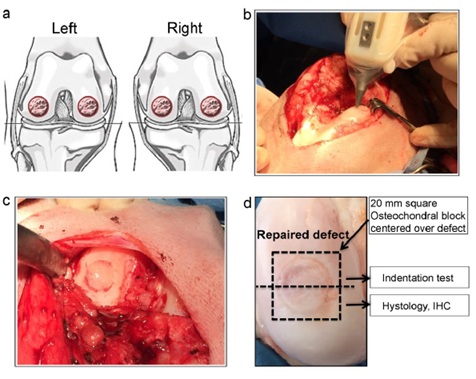 Figure 2: a) Schematic drawing of full thickness chondral defects in weight bearing areas of the medial and lateral femoral condyles of both stifle joints in sheep. b) Intra-operative image of handheld printer. c) Defect filled with handheld in situ 3D printed HA-GelMA scaffold and coated with fibrin glue spray. d) Macroscopic image of repaired cartilage defect. Figure reproduced with permission from Di Bella et al. [96].
Figure 2: a) Schematic drawing of full thickness chondral defects in weight bearing areas of the medial and lateral femoral condyles of both stifle joints in sheep. b) Intra-operative image of handheld printer. c) Defect filled with handheld in situ 3D printed HA-GelMA scaffold and coated with fibrin glue spray. d) Macroscopic image of repaired cartilage defect. Figure reproduced with permission from Di Bella et al. [96].
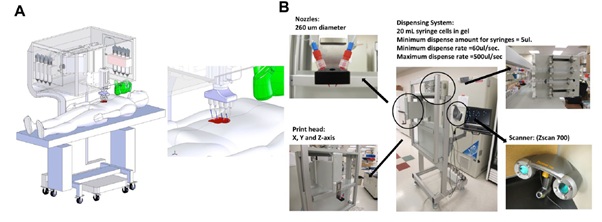 Figure 3: A) Schematic illustrating scale, design and component of in situ bioprinter for wound regeneration. B) Primary components of an in situ bioprinter, including 260 µm diameter nozzles, driven by up to 8 independent dispensers connected to a print head with XYZ movement system, and 3D wound scanner. Reproduced with permission [99]. Copyright 2019, Springer Nature.
Figure 3: A) Schematic illustrating scale, design and component of in situ bioprinter for wound regeneration. B) Primary components of an in situ bioprinter, including 260 µm diameter nozzles, driven by up to 8 independent dispensers connected to a print head with XYZ movement system, and 3D wound scanner. Reproduced with permission [99]. Copyright 2019, Springer Nature.
In summary, 3D bioprinting is a promising technique for the generation of functional engineered tissues. Different methods including inkjet, extrusion, laser assisted and subtractive techniques are common technologies used for bioprinting, and every method has its own advantages and disadvantages (Table 1). Ideally, bioprinting approaches would have high resolution and speed and utilize an optimal bioink that supports necessary mechanical and biological needs as well as a robust vascularization process. With these goals in mind, choosing the appropriate bioprinting technique and developing an appropriate bioink with a cell type that supports the primary cells of interest are vital steps toward successful fabrication of a printed tissue construct.
CONCLUSION
Three-dimensional bioprinting techniques have garnered great interest and exhibited significant advancements for tissue engineering applications during the last decade. These methods have led to hope for improvement in regenerative medicine and the ability to move patients off years-long transplant lists. 3D bioprinting is currently experiencing rapid development. While many challenges remain, initial studies have shown promise toward fabrication of functional printed tissues and organs; however, more time and effort in multidisciplinary research is needed to surpass these challenges so that engineered tissues can be fully utilized in the clinical setting.
ACKNOWLEDGEMENTS
The authors particularly wish to acknowledge the contribution of enthusiastic graduate students Blake Darkow and Michal Juda. This research was supported by NSF CBET (1702841; EGL).
REFERENCES
- Hull CW (1986) Apparatus for production of three-dimensional objects by stereolithography, USA.
- Seyedmahmoud R, Rainer A, Mozetic P, Giannitelli SM, Trombetta M, et al. (2015) A primer of statistical methods for correlating parameters and properties of electrospun poly(l-lactide) scaffolds for tissue engineering-PART 1: Design of experiments. J Biomed Mater Res A 103: 91-102.
- Langer R, Vacanti JP (1993) Tissue engineering. Science 260: 920-926.
- Huang J, Mao Y, Millis JM (2008) Government policy and organ transplantation in China. Lancet 372: 1937-1938.
- Shafiee A, Atala A (2016) Printing Technologies for Medical Applications. Trends Mol Med 22: 254-265.
- Abouna GM (2008) Organ Shortage Crisis: Problems and Possible Solutions. Transplant Proc 40: 34-38.
- Jang J, Yi H-G, Cho DW (2016) 3D Printed Tissue Models: Present and Future. ACS Biomaterials Science & Engineering 2: 1722-1731.
- Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32: 773-785.
- Mironov V, Kasyanov V, Drake C, Markwald RR (2007) Organ printing: promises and challenges. Regen Med 3: 93-103.
- Pati F, Jang J, Ha DH, Kim SW, Rhie JW, et al. (2014) Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5: 3935.
- Guvendiren M, Molde J, Soares RMD, Kohn J (2016) Designing Biomaterials for 3D Printing. ACS Biomater Sci Eng 2: 1679-1693.
- Xu T, Jin J, Gregory C, Hickman JJ, Boland T (2005) Inkjet printing of viable mammalian cells. Biomaterials 26: 93-99.
- Nishiyama Y, Nakamura M, Henmi C, Yamaguchi K, Mochizuki S, et al. (2009) Development of a three-dimensional bioprinter: construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J Biomech Eng 131: 035001.
- Ong CS, Yesantharao P, Huang CY, Mattson G, Boktor J, et al. (2018) 3D bioprinting using stem cells. Pediatr Res 83: 223-231.
- Cui X, Boland T, D'Lima DD, Lotz MK (2012) Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul 6: 149-155.
- Chang CC, Boland ED, Williams SK, Hoying JB (2011) Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater 98: 160-70.
- Shav D, Einav S (2010) The effect of mechanical loads in the differentiation of precursor cells into mature cells. Ann N Y Acad Sci 1188: 25-31.
- Cui X, Breitenkamp K, Finn MG, Lotz M, D'Lima DD (2012) Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 18: 1304-1312.
- Mitchell MG (2017) Chapter 5 - Materials for Use in Bioprinting, in Bioprinting. Academic Press Pg No’s 81-94.
- Costa JB, Silva-Correia J, Oliveira JM, Reis RL (2017) Fast Setting Silk Fibroin Bioink for Bioprinting of Patient-Specific Memory-Shape Adv Healthc Mater 6.
- Kyle S, Jessop ZM, Al-Sabah A, Whitaker IS (2017) ‘Printability' of Candidate Biomaterials for Extrusion Based 3D Printing: State-of- the-Art. Adv Healthc Mater 6: 1700264.
- Chimene D, Lennox KK, Kaunax RR, Gaharwar (2016) Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann Biomed Eng 44: 2090-102.
- Yeo MG, Kim GH (2017) A cell-printing approach for obtaining hASC-laden scaffolds by using a collagen/polyphenol bioink. Biofabrication 9: 025004.
- Diamantides N, Wang L, Pruiksma T, Siemiatkoski J, Dugopolski C, et al. (2017) Correlating rheological properties and printability of collagen bioinks: the effects of riboflavin photocrosslinking and pH. Biofabrication 9: 034102.
- Bertassoni LE, Cardoso JC, Manoharan V, Cristino AL, Bhise NS, et al. (2014) Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 6: 024105.
- Liu W, Heinrich MA, Zhou Y, Akpek A, Hu N, et al. (2017) Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Adv Healthc Mater 6.
- Seyedmahmoud R, Çelebi-Saltik B, Barros N, Nasiri R, Banton E, et al. (2019) Three-Dimensional Bioprinting of Functional Skeletal Muscle Tissue Using Gelatin Methacryloyl-Alginate Bioinks. Micromachines (Basel) 10: 679.
- Hakam MS, Imani R, Abolfathi N, Fakhrzadeh H, Sharifi AM (20160 Evaluation of fibrin-gelatin hydrogel as biopaper for application in skin bioprinting: An in-vitro study. Biomed Mater Eng 27: 669-682.
- England S, Rajaram A, Schreyer DJ, Chen X (20107) Bioprinted fibrin-factor XIII-hyaluronate hydrogel scaffolds with encapsulated Schwann cells and their in vitro characterization for use in nerve regeneration. Bioprinting 5: 1-9.
- Shang W, Liu Y, Wan W, Hu C, Liu X, et al. (2017) Hybrid 3D printing and electrodeposition approach for controllable 3D alginate hydrogel formation. Biofabrication 9: 025032.
- Do AV, Akkouch A, Green B, Ozbolat I, Debabneh A, et al. (2017) Controlled and Sequential Delivery of Fluorophores from 3D Printed Alginate-PLGA Tubes. Ann Biomed Eng 45: 297-305.
- Heo EY, Ko NR, Bae MS, Lee SJ, Choi B-J, et al. (2017) Novel 3D printed alginate–BFP1 hybrid scaffolds for enhanced bone Journal of Industrial and Engineering Chemistry 45: 61-67.
- Cheng YL, Chen F (2017) Preparation and characterization of photocured poly (ε-caprolactone) diacrylate/poly (ethylene glycol) diacrylate/chitosan for photopolymerization-type 3D printing tissue engineering scaffold application. Mater Sci Eng C Mater Biol Appl 81: 66-73.
- Lee CM, Yang SW, Jung SC, Kim BH (2017) Oxygen Plasma Treatment on 3D-Printed Chitosan/Gelatin/Hydroxyapatite Scaffolds for Bone Tissue Engineering. J Nanosci Nanotechnol 17: 2747-750.
- Elviri L, Foresti R, Bergonzi C, Zimetti F, Marchi C, et al. (2017) Highly defined 3D printed chitosan scaffolds featuring improved cell growth. Biomed Mater 12: 045009.
- Yetiskin B, Okay O (2017) High-strength silk fibroin scaffolds with anisotropic mechanical Polymer 112: 61-70.
- Compaan AM, Christensen K, Huang Y (2017) Inkjet Bioprinting of 3D Silk Fibroin Cellular Constructs Using Sacrificial Alginate. ACS Biomaterials Science & Engineering 3: 1519-1526.
- Heinrich MA, Liu W, Jimenez A, Yang J, Akpek A, et al. (2019) 3D Bioprinting: from Benches to Translational Applications. Small 15: 1805510.
- Reece TB, Maxey TS, Kron IL (2001) A prospectus on tissue adhesives. Am J Surg 182: S40-S44.
- Kim JD, Choi JS, Kim BS, Choi YC, Cho YW (2010) Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer Polymer 51: 2147-2154.
- Guillotin B, Guillemot F (2011) Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol 29: 183-190.
- Michael S, Sorg H, Peck CT, Koch L, Deiwick A, et al. (2013) Tissue Engineered Skin Substitutes Created by Laser-Assisted Bioprinting Form Skin-Like Structures in the Dorsal Skin Fold Chamber in Mice. Plos One 8: e57741.
- Murphy SV, Skardal A, Atala A (2013) Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A 101: 272-284.
- Smith CM, Christian JJ, Warren WL, Williams SK (2007) Characterizing environmental factors that impact the viability of tissue-engineered constructs fabricated by a direct-write bioassembly tool. Tissue Eng 13: 373-383.
- Koch L, Kuhn S, Sorg H, Gruene M, Schlie S, et al. (2009) Laser Printing of Skin Cells and Human Stem Cells. Tissue Eng Part C Methods 16: 847-854.
- Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, et al. (2010) Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 31: 7250-7256.
- Campbell PG, Miller ED, Fisher GW, Walker LM, Weiss LE (2005) Engineered spatial patterns of FGF-2 immobilized on fibrin direct cell Biomaterials 26: 6762-6770.
- Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, et al. (2008) Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells 26: 127-134.
- Smith CM, Stone AL, Parkhill RL, Stewart RL, Simpkins MW, et al. (2004) Three-dimensional bioassembly tool for generating viable tissue-engineered Tissue Eng 10: 1566-1576.
- Lee VK, Dai G (2015) Three-dimensional bioprinting and tissue fabrication: prospects for drug discovery and regenerative medicine. Advanced Health Care Technologies 1: 23-25.
- Demirci U, Montesano G (2007) Single cell epitaxy by acoustic picolitre droplets. Lab Chip 7: 1139-1145.
- Nair K, Gandhi M, Khalil S, Yan KC, Marcolongo M, et al. (2009) Characterization of cell viability during bioprinting processes. Biotechnol J 4: 1168-1177.
- Duan B (2017) State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann Biomed Eng 45: 195-209.
- Wüst S, Müller R, Hofmann S (2011) Controlled Positioning of Cells in Biomaterials-Approaches Towards 3D Tissue Printing. J Funct Biomater 2: 119-154.
- Jones N (2012) Science in three dimensions: the print revolution. Nature 487: 22-23.
- Peltola SM, Melchels FPW, Grijpma DW, Kellomäki M (2008) A review of rapid prototyping techniques for tissue engineering purposes. Ann Med 40: 268-280.
- Norotte C, Marga FS, Niklason LE, Forgacs G (2009) Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30: 5910-5917.
- Hopp B, Smausz T, Kresz N, Barna N, Bor Z, et al. (2005) Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng 11: 1817-23.
- Mironov V, Kasyanov W, Markwald RR (2011) Organ printing: from bioprinter to organ biofabrication line. Curr Opin Biotechnol 22: 667-673.
- Marga F, Jakab K, Khatiwala C, Shepherd B, Dorfman S, et al. (2012) Toward engineering functional organ modules by additive manufacturing. Biofabrication 4: 022001.
- Xu T, Gregory CA, Molnar P, Cui X, Jalota S, et al. (2006) Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 27: 3580-3588.
- Chang R, Nam J, Sun W (2008) Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng Part A 14: p. 41-48.
- Lee W, Debasitis JC, Lee VK, Lee JH, Fischer K, et al. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 30: 1587-1595.
- Lee VK, Kim DY, Ngo H, Lee Y, Seo L, et al. (2014) Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 35: 8092-8102.
- Park JH, Jung JW, Kang HW, Joo YH, Lee JS, et al. (2012) Development of a 3D bellows tracheal graft: mechanical behavior analysis, fabrication and an in vivo feasibility study. Biofabrication 4: 035004.
- Duan B, Hockaday LA, Kang KH, Butcher JT (2013) 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin J Biomed Mater Res A 101: 1255-1264.
- Koch L, Deiwick A, Schlie S, Michael S, Gruene M, et al. (2012) Skin tissue generation by laser cell printing. Biotechnol Bioeng 109: 1855-1863.
- Leberfinger AN, Ravnic DJ, Dhawan A, Ozbolat IT (2017) Concise Review: Bioprinting of Stem Cells for Transplantable Tissue Stem Cells Transl Med 6: 1940-1948.
- Lewandowski J, Kurpisz M (2016) Techniques of Human Embryonic Stem Cell and Induced Pluripotent Stem Cell Derivation. Arch Immunol Ther Exp (Warsz) 64: 349-370.
- Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, et al. (2014) A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep 4: 3594.
- Mehta RH (2014) Sourcing human embryos for embryonic stem cell lines: problems & perspectives. Indian J Med Res 140: S106-S111.
- Lo B, Parham L (2009) Ethical issues in stem cell research. Endocr Rev 30: 204-213.
- Zhang S, Cui W (2014) Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells 6: 305-311.
- Zhang P, Andrianakos R, Yang Y, Liu C, Lu W (2010) Kruppel-like factor 4 (Klf4) prevents embryonic stem (ES) cell differentiation by regulating Nanog gene expression. J Biol Chem 285: 9180-9189.
- Pittenger MF (2008) Mesenchymal stem cells from adult bone marrow. Methods Mol Biol 449: 27-44.
- Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG (1999) Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med 10: 165-81.
- Sreejit P, Dilip KB, Verma RS (2012) Generation of mesenchymal stem cell lines from murine bone marrow. Cell Tissue Res 350: 55-68.
- Bodle JC, Teeter SD, Hluck BH, Hardin JW, Bernacki SH et al. (2014) Age-related effects on the potency of human adipose-derived stem cells: creation and evaluation of superlots and implications for musculoskeletal tissue engineering applications. Tissue Eng Part C Methods 20: 972-983.
- Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JAW (2015) Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 71: 181-97.
- Bodle J, Hamouda MS, Cai S, Williams RB, Bernacki SH, et al. (2019) Primary Cilia Exhibit Mechanosensitivity to Cyclic Tensile Strain and Lineage-Dependent Expression in Adipose-Derived Stem Cells. Sci Rep 9: 8009.
- Locke M, Windsor J, Dunbar PR (2009) Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ J Surg 79: 235-244.
- Storti G, Scioli MG, Kim BS, Orlandi A, Cervelli V (2019)Adipose-Derived Stem Cells in Bone Tissue Engineering: Useful Tools with New Stem Cells Int 2019: 3673857.
- Veronesi F, Maglio M, Tschon M, Aldini NN, Fini M (2014) Adipose-derived mesenchymal stem cells for cartilage tissue engineering: State-of-The-Art in in vivo studies. J Biomed Mater Res A 102: 2448-2466.
- Tangchitphisut P, Srikaew N, Numhom S, Tangprasittipap A, Woratanarat P, et al. (2016) Infrapatellar Fat Pad: An Alternative Source of Adipose-Derived Mesenchymal Stem Cells. Arthritis 2016: 4019873.
- Nordberg RC, Bodle JC, Loboa EG (2018) Mechanical Stimulation of Adipose-Derived Stem Cells for Functional Tissue Engineering of the Musculoskeletal System via Cyclic Hydrostatic Pressure, Simulated Microgravity, and Cyclic Tensile Strain. Methods Mol Biol 1773: 215-230.
- Mellor LF, Nordberg RC, Huebner P, Mohiti-Asli M, Taylor MA, et al., (2020) Investigation of multiphasic 3D-bioplotted scaffolds for site-specific chondrogenic and osteogenic differentiation of human adipose-derived stem cells for osteochondral tissue engineering applications. J Biomed Mater Res B Appl Biomater 108: 2017-2030.
- Gao G, Schilling AF, Hubbell K, Yonezawa T, Truong D, et al. (2015) Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol Lett 37: 2349-2355.
- Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, et al. (2010) Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 9: 518-526.
- Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 126: 677-689.
- Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE (2007) Extracellular matrix elasticity directs stem cell differentiation. J Musculoskelet Neuronal Interact 7: 335.
- González-Cruz RD, Fonseca VC, Darling EM (2012) Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. Proc Natl Acad Sci U S A 109: E1523-E1529.
- Phadke A, Chang CW, Varghese S (2010) Functional Biomaterials for Controlling Stem Cell Differentiation. Biomaterials as Stem Cell Niche. Studies in Mechanobiology, Tissue Engineering and Biomaterials.
- Gu Z, Fu J, Lin H, He Y (2019) Development of 3D bioprinting: From printing methods to biomedical applications. Asian Journal of Pharmaceutical Sciences.
- Campbell PG, Weiss LE (2007) Tissue engineering with the aid of inkjet printers. Expert Opin Biol Ther 7: 1123-1127.
- Li Q, Ma L, Gao C (2015) Biomaterials for in situ tissue regeneration: development and Journal of Materials Chemistry B 3: 8921-8938.
- Di Bella C, Duchi S, O'Connell CD, Blanchard R, Augustine C, et al. (2018) In situ handheld three-dimensional bioprinting for cartilage regeneration. J Tissue Eng Regen Med 12: 611-621.
- Duchi S, Onofrillo C, O'Connell CD, Blanchard R, Augustine C, et al. (2017) Handheld Co-Axial Bioprinting: Application to in situ surgical cartilage repair. Sci Rep 7: 5837.
- Hakimi N, Cheng R, Leng L, Sotoudehfar M, Ba PQ, et al. (2018) Handheld skin printer: in situ formation of planar biomaterials and tissues. Lab Chip 18: 1440-1451.
- Albanna M, Binder KW, Murphy SV, Kim J, Qasem SA, et al. (2019) In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci Rep 9: 1856.
- Keriquel V, Guillemot F, Arnault I, Guillotin B, Miraux S, et al. (2010) In vivo bioprinting for computer- and robotic-assisted medical intervention: preliminary study in mice. Biofabrication 2: 014101.
Citation: Seyedmahmoud R, Messler MJ, Loboa EG (2020) 3D Bioprinting Technologies for Tissue Engineering: A Mini Review. J Stem Cell Res Dev Ther 6: 046.
Copyright: © 2020 Rasoul Seyedmahmoud, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

