
A Short Commentary Based on our Article Entitled “Capillary Networks for Bio-Artificial Three-Dimensional Tissues Fabricated Using Cell Sheet Based Tissue Engineering”
*Corresponding Author(s):
Hidekazu SekineInstitute Of Advanced Biomedical Engineering And Science, Tokyo Women’s Medical University, 8-1 Kawada-cho, Shinjuku-ku, Tokyo 162-8666, Japan
Email:sekine.hidekazu@twmu.ac.jp
Abstract
The development of organ-like tissues is the next major challenge facing regenerative medicine. Organ-like structures have been synthesized on a small scale, but overcoming current problems with cell sourcing and scalability will facilitate the creation of cardiac assist devices and organs for transplantation. A major obstacle to generating Three-Dimensional (3D) tissues is that diffusion is inadequate for the supply of oxygen/nutrients and removal of waste products, which limits the thickness of the structure to about 0.1 mm. The development of technologies that incorporate capillaries and lymphatic vessels within 3D tissue would extend the range of potential applications for regenerative medicine. Here, we describe a novel strategy that overcomes the limitation of passive diffusion by introducing functional capillaries into artificial tissues. This strategy utilizes bioengineered cell sheets containing vascular endothelial cells and a microvascular bed supplied by blood vessels that can be anastomosed to those of a host.
Keywords
Cell sheet; Myocardial tissue; Transplantation; Vascularization; Vascular bed
Introduction
Although cell-based therapies (injection of cell suspensions) and regenerative therapies (transplantation of bioengineered tissues) have already been applied clinically, the treatment of serious diseases will require the fabrication of artificial Three-Dimensional (3D) tissues and organs that can be transplanted instead of donor organs. The development of a novel method of introducing functional vascular networks into artificial tissues will facilitate the creation of thick 3D tissues/organs that are cell-dense and vascular-rich (e.g., cardiac, hepaticorrenal tissue).
Construction of viable 3D tissue is challenging because time is required for a vascular network to form after transplantation. Many currently used regenerative therapies rely on the development of new blood vessels from the host side to supply oxygen/nutrients and remove waste products. Although the slow release of angiogenic factors from a cell scaffold can promoteangiogenesis after transplantation, this approach cannot generate thick tissues because of the long time required for sufficient vascular network formation. Another method of promoting capillary angiogenesis involves co-culture with vascular endothelial cells that directly contribute to blood vessel formation. However, the vascular network produced with this technique is not continuous, and the thickness of the regenerated tissue is limited by ischemia during the early post-transplantation period. Other approaches have cultured vascular endothelial cells in scaffolds containing microfluidic channels or decellularized organs, but seeding cells within these ‘vascular’ structures is difficult, and this method cannot generate tissues with a high cell density.
Our group has investigated the use of cell sheet engineering to regenerate myocardial tissue. We have successfully laminated rat myocardial cell sheets to produce artificial tissue with a high cell density that beats autonomously after transplantation into the rat [1]. However, this technique can only produce myocardial sheets with a maximum of three layers because diffusion-mediated supply of oxygen/nutrients and removal of waste products are limited. Therefore, an important goal of our recent research has been to increase the thickness and functionality of bioengineered myocardial tissue through the addition of a functional vascular network. We have used a multifaceted approach to accelerate the vascularization of bioengineered tissue after transplantation. We are now able to fabricate cell sheets using cardiomyocytes and endothelial cells differentiated from human induced Pluripotent Stem (iPS) cells and are aiming to use our technology to create human 3D tissue with cardiac functionality.
Scaffold-Free Tissue Engineering Using Cell Sheet Technology
We have been developing cell sheet engineering as a regenerative medicine technique. Culture of a cell monolayer on a nanotechnology-enhanced temperature-responsive intelligent surface allows a cell sheet to be recovered through a reduction in temperature [1]. One way of generating 3D tissues is to stack cell sheets, but a maximum of only three cell sheets (equivalent to a thickness of 80 µm) can be stacked at one time due to limitations in the supply of oxygen/nutrients and removal of waste products through diffusion. Therefore, we established a new technique for introducing a vascular network into an artificial tissue constructed from multilayered sheets. Triple-layered myocardial cell sheets were transplanted at intervals of 1-2 days onto rat subcutaneous tissue, which acted as a vascular bed (Figure 1A). This multistep in vivo transplantation technique allowed sufficient time for the development of a functional vascular network in each newly transplanted cell sheet and enabled us to create synchronously beating cardiac tissue that was ~1 mm thick [2].
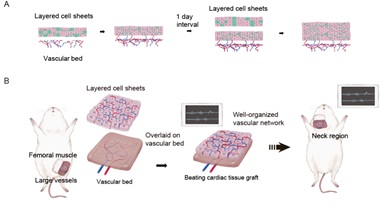 Figure 1: Construction of thick cardiac tissue grafts with blood vessels by the sequential implantation of multiple cardiomyocyte sheets containing vascular endothelial cells onto the vascular bed (A).Creation of structures with access to blood vessels and their transplantation by vascular anastomosis (B).
Figure 1: Construction of thick cardiac tissue grafts with blood vessels by the sequential implantation of multiple cardiomyocyte sheets containing vascular endothelial cells onto the vascular bed (A).Creation of structures with access to blood vessels and their transplantation by vascular anastomosis (B).
Subsequently, we demonstrated that neovascularization of the bioengineered tissue was improved if vascular endothelial cells were introduced into the cell sheets so that an endothelial cell network formed in vitro [3]. Transplantation of grafts constructed from myocardial/endothelial cell sheets was found to enhance cardiac function in a rat model of myocardial infarction, and blood vessels in the graft were observed to form connections with host vasculature.
Creation Of Vascularized 3D Tissues With Blood Vessels That Can Be Anastomosed To Those Of A Host
Although stepwise stacking of myocardial sheets can facilitate the development of a capillary network, multi-stage transplantation of cardiomyocyte sheets directly onto a failing heart is impractical because it would require frequent open heart surgery. Therefore, we devised an in vivo tissue culture method that enabled the creation of artificial myocardial tissue with a vascular bed that could be anastomosed with host blood vessels [2]. Triple-layered cardiomyocyte sheets were transplanted onto branches of the rat femoral artery and veinat 1-day intervals to allow time for capillary network formation. After two weeks, the vascularized myocardial tissue was resected together with the host vessels and re-transplanted into the neck, and the graft was provided with a blood supply by anastomosing the femoral artery and vein to the carotid artery and jugular vein, respectively (Figure 1B).
Based on the success of the in vivo culture technique, we developed an in vitro method for generating vascularized cardiac tissue involving the sequential stacking of cell sheets onto a free vascular bed in a tissue perfusion bioreactor that mimicked the in vivo environment (Figure 2A). Triple-layered myocardial/endothelial cell sheets were laminated in stages onto the vascular bed, which consisted of rat femoral muscle tissue with branches of the femoral artery and vein (Figure 2B). The perfusion culture solution contained basic fibroblast growth factor to promote angiogenesis. We confirmed that capillaries generated in the myocardial tissue had connected with capillaries in the vascular bed after 3 days of perfusion culture (Figure 3). By stacking cell sheets four times in a stepwise manner, we were able to construct myocardial tissue with a thickness of ~0.2 mm in vitro. The artificial tissue maintained its beating two weeks after implantation in vivo with anastomoses of graft and host blood vessels. This achievement attracted worldwide attention as a breakthrough in tissue engineering technology [4].
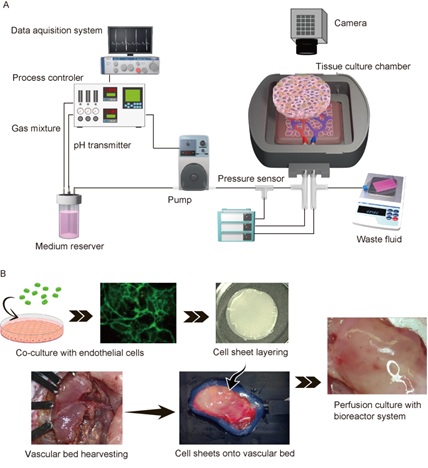 Figure 2: Tissue perfusion bioreactor system (A) Ex vivo perfusion culture of cardiomyocyte sheets co-cultured with endothelial cells and resected vascular beds with arteries and veins (B).
Figure 2: Tissue perfusion bioreactor system (A) Ex vivo perfusion culture of cardiomyocyte sheets co-cultured with endothelial cells and resected vascular beds with arteries and veins (B).
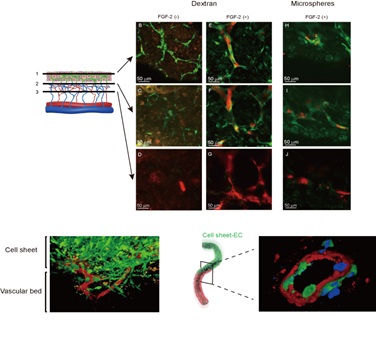 Figure 3: Functional vascularization of the myocardial tissue. Endothelial cells in the cell sheet tissue undergo luminalization and migrate to the vascular bed, resulting in vascular coupling between them.
Figure 3: Functional vascularization of the myocardial tissue. Endothelial cells in the cell sheet tissue undergo luminalization and migrate to the vascular bed, resulting in vascular coupling between them.
The results summarized above illustrate three important concepts: 1) thick artificial tissues with a high cell density can be constructed by multistage layering of cell sheets in vitro or in vivo without the need for an artificial scaffold; 2) functional capillaries can be induced in bioengineered tissue in an ex vivo environment; and 3) angiogenesis can be enhanced by co-culturing myocardial cells with vascular endothelial cells. Furthermore, stacking cell sheets on a vascular bed in a bioreactor can generate 3D artificial tissues that are beyond conventional tissue engineering technology. Our techniques have potential for development into a new method of fabricating perfusable, functional 3D tissues or even entire organs. A future aim is to optimize the vascular bed and bioreactor system to enable the construction of organs with complex structures and high vascular requirements such as the heart, kidney and liver.
Conclusion
We have described one of the current state-of-the-art tissue engineering techniques that potentially could be used to build functional 3D tissues and organs. In particular, we have focused on ways of introducing a vascular network into cell sheet-derived myocardial tissue. Cell sheets cultured on temperature-responsive dishes have several advantages over other available methods including more efficient transplantation, construction of tissues that cannot be produced by existing techniques, and the ability to layer cells. Furthermore, cell sheet-based therapies have shown promising results in human clinical trials. Challenges that will need to be overcome to allow the construction of functional tissues and organs include the development of cells suitable for transplantation into humans, the generation of larger-sized tissues, and the preservation of grafts prior to transplantation. Tissue engineering has developed through cooperation between biology-related and engineering-related fields, and we believe that further multidisciplinary cooperation will lead to novel discoveries and new therapies for diseases that are currently difficult to treat. If more progress is made in the development of vascular beds and bioreactors for in vitro vascularization of cell-sheet derived tissues, the ultimate goal of creating highly-vascularized, complex organs such as the heart, kidney and liver can be achieved. The establishment of a technology for generating artificial 3D tissues that contain capillaries will advance the field of regenerative medicine and contribute to novel treatments for patients. Our techniques could form the basis of a new technology to generate large artificial 3D tissues that could be used to replace damaged/missing structures or as an alternative to conventional organ transplantation.
Acknowledgements
This research was supported by JSPS KAKENHI grant number 19H04453. We thank OxMedComms (www.oxmedcomms.com) for writing assistance. We would like to thank Reika Okuda of CyLinks, Inc. for drawing the diagram.
References
- Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, et al. (2002) Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res 90: e40.
- Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, et al. (2006) Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J 20: 708-710.
- Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, et al. (2008) Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation 118: S145-S152.
- Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, et al. (2013) In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun 4: 1399.
Citation: Sekine H, Okano T (2021) A Short Commentary Based on our Article Entitled “Capillary Networks for Bio-Artificial Three-Dimensional Tissues Fabricated Using Cell Sheet Based Tissue Engineering”. J Stem Cell Res Dev Ther 7: 070.
Copyright: © 2021 Hidekazu Sekine, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

