
A Short Review on MicroRNAs on the Proliferation and Apoptosis of Hair Follicle Stem Cells In superior-Pen-Hair Haimen Goat
*Corresponding Author(s):
Ji DejunCollege Of Animal Science And Technology, Yangzhou University, Yangzhou, China
Email:jidejun@qq.com
Abstract
The superior pen hair, famous for its pure whiteness, brilliant luster and fine elasticity characteristics, is exclusively produced by Yangtze River Delta white goat (Haimen goat) in China. The formation mechanism of the superior pen hair is limited to the role of androgen level and cold stress, which related to certain signals, including CMTM3, Hsp70, and some pathways, such as the Wnt/β-catenin pathway. The detailed regulatory pathway of the superior pen hair trait is still rarely reported. In this review, the role of MicroRNAs on the proliferation and apoptosis of hair follicle stem cells, especially in superior-pen-hair Haimen goat, was briefly discussed, hoping to illustrate the formation mechanism of the superior pen hair.
Keywords
Goat; Hair Follicle Stem Cells; Pen Hair; MicroRNA
Mechanism of the Formation of Superior-Quality Pen Hair in Goat
The superior pen hair, the finest raw material used for making Chinese calligraphy pens, is exclusively produced by Yangtze River Delta white goat (Haimen goat) in China. This goat breed has also been praised for this unique characteristic, resulting in a laudatory title: the pen hair goat. Pen hair is usually classified into three types: Type I refers to inferior-quality hair, Type II refers to normal-quality hair, and Type III refers to superior-quality hair. Superior pen hair (Type III) is famous for its pure whiteness, brilliant luster and fine elasticity characteristics [1]. The existing research revealed that, firstly, androgen secretion and cold stress can stimulate the formation of superior-quality pen hair in Yangtze River Delta white goat, which can further promote and activate the expression of proteins (such as β-fibrinogen and keratin) and related signaling pathways (such as the Wnt/β-catenin pathway) known to participate in hair growth; secondly, dual-specificity phosphatase 6 (DUSP6), S100 calcium-binding protein A (S100A), CKLF-like MARVEL transmembrane domain-containing 3 (CMTM3) and heat stress-associated genes, such as Hsp70, are important for regulating superior-quality pen hair traits; thirdly, CKLFSF3, a high methylation level of CMTM3 could promote the formation of superior-quality pen hair, and also could modulate the transcription level of androgen receptor (AR);moreover, CMTM3 gene is one of the target genes of miR-149-5p via the database analyzing [2,3].
The Regulatory Role Of MircoRNAs In Biological Process
MircoRNAs, referred to as miRNAs or "microRNAs" for short, are a class of short (about 22 NT) noncoding RNAs (ncRNAs), which are mainly encoded by endogenous genes (i.e., formed by the coding of genes or gene introns); The sequence of microRNAs is extremely conservative in different animals and plants. They play a very important role in the development and growth of animals and plants by participating in the post transcriptional regulation of genes [4]. MicroRNAs play a role in animals and plants mainly by regulating the biological functions of their target genes, that is, miRNAs bind to corresponding target genes through their 5’end seed sequence with a length of 7-9 NT, and then inhibit the expression of their target genes, such as inducing the degradation of target mRNA or inhibiting the translation of target genes [5]. The main mechanisms of action of microRNAs on target genes are as follows: 1) combine with the 3’UTR region sequence of target gene mRNA to exert its inhibitory effect on target gene transcription or translation [6]; 2) It combines with the 5 'UTR region sequence of the target gene mRNA to inhibit the normal protein translation process of the target gene [7]; 3) It can also combine with the coding sequence region of genes to improve the expression level of target gene proteins [8]. The biogenesis and action mechanism of microRNAs are shown in figure 1.
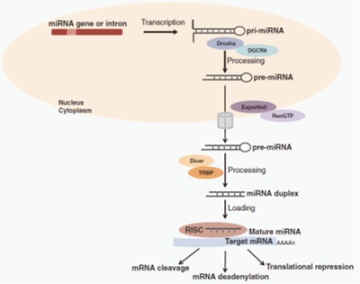 Figure 1: Schematic mechanism of microRNAs biogenesis and functions.
Figure 1: Schematic mechanism of microRNAs biogenesis and functions.
Mir-let-7 is the first mammalian microRNA discovered and characterized. In addition, as early as 1993, studies have confirmed that microRNA (mir-lin-4) exists in Caenorhabditis elegans and has the function of regulating its larval development [9]. So far, 38589 mature miRNAs have been annotated and included in the miRBase database. The biological functions of a large number of miRNAs have been widely revealed and characterized through the establishment of microRNAs knockout model and transgenic overexpression model [10]. Further studies have shown that miRNAs play an important role in multiple biological processes (regulating cell proliferation and differentiation, embryo formation, muscle development, organogenesis, tumorigenesis, etc.) [11]. For example, miR-1, miR-133, mir-487b-3p and other miRNAs can be used as key regulators to regulate the proliferation and differentiation of myoblasts and the development of skeletal muscle [12-14]; Mir-370 exerts its inhibitory effect on tumors by targeting different oncogenes in a variety of tumors and inhibiting the expression of oncogenes (inhibiting the proliferation and colony formation of osteosarcoma tumor cells) [15]; Contrary to the effect of mir-370, miR-155 inhibits transforming growth factor β Receptor 2 (TGF β R2) to promote the proliferation and migration of gastric cancer cells [16]. In addition, the deletion or abnormal expression of miRNAs can also lead to abnormal proliferation of germ cells and increase the rate of gamete deformity. Therefore, miRNAs are indispensable in the pre fertilization and post fertilization stages (such as sex differentiation, gametogenesis, embryo implantation, etc.) [17].
The Regulatory Role Of MircoRNAs In Hair Growth
Hair growth is closely associated with hair follicle development, and hair follicle stem cell proliferation, apoptosis and differentiation are critical for hair follicle development. However, the potential regulatory mechanism of ncRNAs (especially in circRNAs and miRNAs) involved in the development of goat hair follicle and the formation of superior-quality pen hair have not been reported. A recent study combining with RT-qPCR, Western blotting, cell cycle and cell apoptosis with flow cytometry assay, Cell immunofluorescence, Immuno- histochemistry-paraffin sections, RNA binding protein immunoprecipitation, Nuclear and cytoplasmic RNA extraction, Dual-luciferase assays and technics, to determine the functions and regulatory mechanism of miR-149-5p and circCOL1A1 in the formation of superior-quality pen hair traits in Yangtze River delta white goat [18]. The research reported that CMTM3 is the target gene of miR-149-5p, and miR-149-5p expression was obviously higher in skin tissues from the cervical spine in superior-quality pen hair goats than in those from normal-quality pen hair goats. The function and regulatory mechanism were illustrated in hair follicle stem cells by overexpressing or silencing miR-149-5p, showing that miR-149-5p directly targets the 3’-UTR of goat CMTM3 and upregulates AR expression, miR-149-5p promotes proliferation and inhibits apoptosis of goat hair follicle stem cells via targeting the CMTM3/AR axis; miR-149-5p facilitates β-catenin-induced hair follicle stem cell differentiation by upregulating the differentiation marker genes (β-catenin, C-myc and KRT6) expression. Otherwise, circCOL1A1 suppresses goat hair follicle stem cell proliferation by sponging miR-149-5p and circCOL1A1 facilitates goat hair follicle stem cell apoptosis by sponging miR-149-5p, which means miR-149-5p could weaken the inhibition role of circCOL1A1 in hair follicle stem cell proliferation and relieve the promotion role of circCOL1A1 in hair follicle stem cell apoptosis. Furthermore, circCOL1A1 controls goat hair follicle stem cell proliferation, apoptosis and differentiation to further inhibit the development of hair follicle and the formation of superior-quality pen hair traits through the circCOL1A1-miR-149-5p-CMTM3/AR axis (figure 2). These research results could provide some new ideas on the function and interactive role of non-coding RNAs (miRNAs and circRNAs) and key genes in superior-quality pen hair traits.
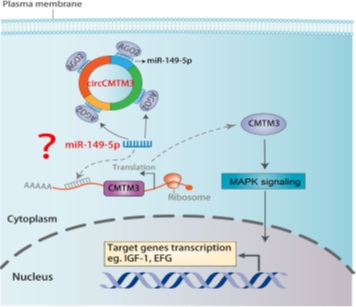 Figure 2: Hypothetical regulatory mechanism of circCMTM3s/miR-149-5p/CMTM3 in superior-quality pen hair traits.
Figure 2: Hypothetical regulatory mechanism of circCMTM3s/miR-149-5p/CMTM3 in superior-quality pen hair traits.
There are also many reports on the regulation of hair follicle development. Zhu et al., used high-throughput transcriptome sequencing method to study the gene expression difference between primary hair follicle and secondary hair follicle dermal papilla cells of cashmere goats, and reported that many differentially expressed genes focus on angiogenesis, extracellular matrix receptor interaction, wnt/catenin/lef1 and other signal pathways. These expression characteristics are closely related to the formation of hair follicle morphogenesis and fiber characteristics [19]. Li qinqun, inferred the role of mir-148b and mir-320 in hair follicle development and the related signal pathways that may be involved in the mechanism of action [20]. Wu et al., also used the miRNAs method to study the developmental characteristics of white goat and black goat wool follicle cells. The results show that miR-10b and miR-211 may play an important role in the formation of wool fibers with specific traits [21]. Bai et al., revealed the important regulatory role of miRNA in the development of cashmere goat hair follicles by studying the differentially expressed miRNAs at different stages of hair follicle development and constructing a differential miRNA regulatory network [22]. Ma et al., found that mir-let-7a plays a key regulatory role in two different stages of hair follicle development: growth stage and degradation stage by acting on its target genes c-myc and FGF5 [23].
With regard to the research on the regulating genes related to wool traits and their functions, di Jiang, screened the differentially expressed genes in the skin tissues of fine wool sheep with different wool fiber diameters by using expression profiling chip and other technologies, and thought that these genes might affect the wool fiber diameter by changing the process of skin hair follicle and wool differentiation [24]. Yang et al., identified the expression profile of coat colored miRNA in brown alpaca and white alpaca, and found that lpa-mirna-nov-66 directly or indirectly acts on guanylate cyclase (SGC) in melanocytes, thereby affecting the cyclic adenosine monophosphate (cAMP) pathway regulating melanin production [25]. In addition to the above research fields on gene regulation of hair follicle, wool growth and hair fiber traits, some researchers have found some growth factors such as insulin-like growth factor I (IGF-I), Epidermal Growth Factor (EGF) and transforming growth factor (TGF- α, TGF- β), Fibroblast growth factor (FGF) and some hormones such as prolactin (PRL), growth hormone (GH) and melatonin (MT) are closely related to the growth of wool fiber and the formation of wool fiber characteristics. Li et al., found that overexpression of fgf5s could stimulate the growth of sheep wool, resulting in the increase of wool length and fat wool weight [26] in the study on the formation of transgenic sheep by constructing ectopic expression of fgf5s recombinant lentivirus vector and injecting it into sheep fertilized eggs. Zhao et al., found that FGFs and TGF- β, IGFs and vascular endothelial growth factor (VEGF) family participate in the regulation of hair growth [27]. Lichun et al., analyzed the effect of MT on gene expression in cashmere goat skin by using RNA SEQ technology, and found that the differentially expressed genes are mainly involved in unsaturated fatty acid biosynthesis, fatty acid metabolism, melanin synthesis, Wnt signaling pathway, complement and coagulation cascade reaction, etc. MT may activate the activity of skin hair follicles in advance by regulating the expression changes of these genes, so as to promote the secondary cashmere of cashmere goats [28]. Zhao et al., found that during the "catagen" and "telogen" periods of hair growth, mir-218-5p and its target gene curl related protein 2 (SFRP2) showed opposite expression trends, and mir-218-5p regulated Wnt by targeting SFRP2/ β- Catenin signaling pathway, and then participate in the development of skin and hair follicles [29].
Research Status of MicroRNAs Regulating Hair Follicle Development
In addition to the above different levels of regulation, miRNAs also play a very important role in regulating hair follicle development. So far, many miRNAs have been identified in the skin tissue of cashmere goats, and some miRNAs have been found to play a key role in regulating the genetic traits of cashmere goat hair color [30]. In addition, the study found that mir-202 family, miR-181a family and mir-let-7 family are differentially expressed in the skin tissues of mice, goats and sheep respectively, and participate in the formation of melanin in skin tissues as key regulatory factors, thus producing the characteristics of different coat colors in skin tissues [31,32]. Some miRNAs in skin tissue have also been revealed to have the function of regulating skin hair follicle development and hair regeneration. For example, miR-21 regulates hair follicle development in mouse skin tissue by acting on BMP (bone morphogenetic protein) signal pathway [33]; MiR-214 regulates Wnt by targeting/ β- Catenin signaling pathway down regulates the expression level of zeste homolog enhancer of zestehomolog 2 (EZH2), thereby inhibiting the proliferation and differentiation of human hair follicle stem cells [34].
However, the mechanism of miRNAs regulating the development of white goat hair follicles and the formation of high-quality pen hair traits in the Yangtze River Delta is rarely reported. Therefore, based on the research results that "cmtm3 gene is one of the key genes for the formation of high-quality pen hair traits in white goats in the Yangtze River Delta" and further combined with the database (targetscan, Starbase, pictar, etc.), The research group predicted that cmtm3 gene is the target gene of miRNAs such as chi-mir-149-5p, chi-mir-23a-3p, chi-mir-23b-3p, chi-mir-365-3p; Their bioinformatics analysis showed that the binding site between mir-149-5p and cmtm3 gene was the most conservative. mir-149-5p may regulate the growth of white goat hair follicle stem cells and the formation of high-quality pen hair traits in the Yangtze River Delta by targeting cmtm3 [3].
Another important miRNA, MiR-101, encoded by two precursor transcripts (miR-101-1 and miR-101-2), is considered a tumor suppressor, as it targets mRNAs of critical oncogenes or anti-oncogenes, and its loss is associated with the occurrence and progression of various diseases [35]. miR-101 is associated with the metastasis of the tumor. For example, inhibiting miR-101 can promote colorectal cancer metastasis.
Huang exposed a direct target of miR-101, the enhancer of zeste homolog 2 (EZH2), a histone methyltransferase. Overexpression of EZH2 promoted colorectal cancer cell line migration and this effect was inhibited by forcing the expression of miR-101 [36]. Such effect of miR-101 is also manifested in lung cancer, bladder transitional cell carcinoma [37] and embryonal rhabdomyosarcoma [38]. Furthermore, Wang et al., revealed that the upregulation of miR-101-3p promotes apoptosis and inhibits the viability of oral cancer cells [39]. However, the function and mechanism of miR-101 in animals are rarely reported. In a recent study, Qu et al., explored the relationship between miR-101 and its target DUSP1, particularly focusing on their role in the production of superior-quality pen hair in Yangtze River Delta white goats [40]. This study identifies miR-101 as a positive regulator in the proliferation of hair follicle stem cells related to the production of high-quality pen hair, and downregulating miR-101 could inhibit the proliferation and promotion of apoptosis of hair follicle stem cells.
Acknowledgments
This research is supported by National natural science foundation of china (32172690, 31572355), Jiangsu higher education key natural science research program (21KJA230002). We also express great gratitude to Professor Li yongjun for his management and crucial role in completing this report.
References
- National livestock and Poultry Genetic Resources Committee. Chinese Livestock and Poultry Genetic Resources; China Agriculture Press: Beijing, China, 2011.
- Qiang W, Guo H, Li Y, Shi J, Yin X, et al. (2018) Methylation analysis of CMTM3 and DUSP1 gene promoters in high-quality brush hair in the Yangtze River delta white goat. Gene 668: 166-173.
- Wang J, Qu J, Li Y, Feng Y, Ma J, et al. (2020) miR-149-5p Regulates Goat Hair Follicle Stem Cell Proliferation and Apoptosis by Targeting the CMTM3/AR Axis During Superior-Quality Brush Hair Formation. Front Genet 11: 529757.
- Wang WX, Wilfred BR, Xie K, Jennings MH, Hu YH, et al. (2010) Individual microRNAs (miRNAs) display distinct mRNA targeting “rules”. RNA Biol 7: 373-380.
- Huntzinger E, Izaurralde E (2011) Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nature Reviews Genetics 12: 99-110.
- Xu L, Yang BF, Ai J (2013) MicroRNA transport: a new way in cell communication. J Cell Physiol 228: 1713-1719.
- Mizuno H, Nakamura A, Aoki Y, Ito N, Kishi S, et al. (2011) Identification of muscle-specific microRNAs in serum of muscular dystrophy animal models: promising novel blood-based markers for muscular dystrophy. PLoS One 6: 18388.
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136: 215-233.
- Roush S, Slack FJ (2008) The let-7 family of microRNAs. Trends Cell Biol 18: 505-516.
- Hammond SM (2015) An overview of microRNAs. Adv Drug Deliv Rev 87: 3-14.
- Ha M, Kim VN (2014) Regulation of microRNA biogenesis. Nature Reviews Molecular Cell Biology 15: 509-524.
- Diniz GP, Wang DZ (2016) Regulation of Skeletal Muscle by microRNAs. Compr Physiol 6: 1279-1294.
- Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, et al. (2013) MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab 17: 210-224.
- Wang J, Tan J, Qi Q, Yang L, Wang Y, et al. (2018) miR-487b-3p Suppresses the Proliferation and Differentiation of Myoblasts by Targeting IRS1 in Skeletal Muscle Myogenesis. Int J Biol Sci 14: 760-774.
- Zhang W, Duan N, Zhang Q, Song T, Li Z, et al. (2017) DNA methylation mediated down-regulation of miR-370 regulates cell growth through activation of the Wnt/β-Catenin signaling pathway in human osteosarcoma cells. Int J Biol Sci 13: 561-573.
- Qu Y, Zhang H, Sun W, Han Y, Li S, et al. (2018) MicroRNA-155 promotes gastric cancer growth and invasion by negatively regulating transforming growth factor-β receptor 2. Cancer Sci 109: 618-628.
- Reza AMMT, Choi YJ, Han SG, Song H, Park C, et al. (2019) Roles of microRNAs in mammalian reproduction: from the commitment of germ cells to peri-implantation embryos. Biol Rev Camb Philos Soc 94: 415-438.
- Wang J, Wu X, Sun X, Zhang L, Wang Q, et al. (2022) The Circular RNA CircCOL1A1 Functions as a miR-149-5p Sponge to Regulate the Formation of Superior-Quality Brush Hair via the CMTM3/AR Axis. Front Cell Dev Biol 10: 760466.
- Zhu B, Xu T, Yuan J, Guo X, Liu D (2013) Transcriptome sequencing reveals differences between primary and secondary hair follicle-derived dermal papilla cells of the Cashmere goat (Capra hircus). PLoS One 8: 76282.
- Qinqun L (2014) The regulation of hair follicle development by MIR-148b and MIR-320 in sheep. Wuhan: Huazhong agricultural university, China.
- Wu Z, Fu Y, Cao J, Yu M, Tang X, et al. (2014) Identification of Differentially Expressed miRNAs between White and Black Hair Follicles by RNA-Sequencing in the Goat (Capra hircus). Int J Mol Sci 15: 9531-9545.
- Bai WL, Dang YL, Yin RH, Jiang WQ, Wang ZY, et al. (2016) Differential Expression of microRNAs and their Regulatory Networks in Skin Tissue of Liaoning Cashmere Goat during Hair Follicle Cycles. Anim Biotechnol 27: 104-112.
- Ma T, Li J, Jiang Q, Wu S, Jiang H, et al. (2018) Differential expression of miR-let7a in hair follicle cycle of Liaoning cashmere goats and identification of its targets. Funct Integr Genomics 18: 701-707.
- Jiang D, Ainiwaer L, Xin-mini X, Yan-hua Z, Ke-chuan T, et al. (2013) Genome Array on Differentially Expressed Genes of Skin Tissue in Fine Wool Sheep with Different Fiber Diameter. Acta Veterinaria Et Zootechnica Sinica 44: 681-689.
- Yang S, Fan R, Shi Z, Ji K, Zhang J, et al. (2015) Identification of a novel microRNA important for melanogenesis in alpaca (Vicugna pacos). J Anim Sci 93: 1622-1631.
- Li WR, He SG, Liu CX, Zhang XM, Wang LQ, et al. (2017) Ectopic expression of FGF5s induces wool growth in Chinese merino sheep. Gene 627: 477-483.
- Zhao J, Liu N, Liu K, He J, Yu J, et al. (2017) Identification of genes and proteins associated with anagen wool growth. Anim Genet 48: 67-79.
- Chun L, Shao-yin F, Jian-meng W, Wei W (2018) Analysis on Effects of Melatonin on Genes Expression in Cashmere Goat Skin by RNA-Seq. Animal Husbandry and Feed Science 39: 26-30.
- Zhao B, Chen Y, Yang N, Chen Q, Bao Z, et al. (2019) miR-218-5p regulates skin and hair follicle development through Wnt/β-catenin signaling pathway by targeting SFRP2. J Cell Physiol 234: 20329-20341.
- Liu Z, Xiao H, Li H, Zhao Y, Lai S, et al. (2012) Identification of conserved and novel microRNAs in cashmere goat skin by deep sequencing. PloS One 7: 50001.
- Qu L, Li J, Zhao Z, Jiang H, Zhang Q (2017) Differential Expression of miR-202 and Validation of Predicted Target Genes in the Skin Tissue of C57BL/6 Black Mice and BALB/c White Mice. DNA Cell Biol 36: 443-450.
- Frucht CS, Santos-Sacchi J, Navaratnam DS (2011) MicroRNA181a plays a key role in hair cell regeneration in the avian auditory epithelium. Neurosci Lett 493: 44-48.
- Ahmed MI, Mardaryev AN, Lewis CJ, Sharov AA, Botchkareva NV (2011) MicroRNA-21 is an important downstream component of BMP signaling in epidermal keratinocytes. J Cell Sci 124: 3399-3404.
- Du KT, Deng JQ, He XG, Liu ZP, Peng C, et al. (2018) MiR-214 Regulates the Human Hair Follicle Stem Cell Proliferation and Differentiation by Targeting EZH2 and Wnt/β-Catenin Signaling Way In Vitro. Tissue Eng Regen Med 15: 341-350.
- Li J, Li Y, Wang Y, He X, Wang J, et al. (2021) Overexpression of miR-101 suppresses collagen synthesis by targeting EZH2 in hypertrophic scar fibroblasts. Burns Trauma 9: 038.
- Huang Z, Wu X, Li J (2021) miR-101 suppresses colon cancer cell migration through the regulation of EZH2. Rev Esp Enferm Dig 113: 255-260.
- Friedman JM, Liang G, Liu CC, Wolff EM, Tsai YC, et al. (2009) The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res 69: 2623-2629.
- Vella S, Pomella S, Leoncini PP, Colletti M, Conti B, et al. (2015) MicroRNA-101 is repressed by EZH2 and its restoration inhibits tumorigenic features in embryonal rhabdomyosarcoma. Clin Epigenetics 7: 82.
- Wang H, Guo Y, Mi N, Zhou L (2020) miR-101-3p and miR-199b-5p promote cell apoptosis in oral cancer by targeting BICC1. Mol Cell Probes 52: 101567.
- Qu J, Wu X, Wang Q, Wang J, Sun X, et al. (2022) Effect of miR-101 on the Proliferation and Apoptosis of Goat Hair Follicle Stem Cells. Genes 13: 1035.
Citation: Ubaid M, Dejun J, Hanzhe W, Jian W, Jian Q, et al. (2022) A Short Review on MicroRNAs on the Proliferation and Apoptosis of Hair Follicle Stem Cells In superior-Pen-Hair Haimen Goat. J Stem Cell Res Dev Ther 8: 097.
Copyright: © 2022 Muhammad Ubaid, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

