
Antibody-Mediated Immune Response and COVID-19
*Corresponding Author(s):
Zhaleh NajafiDepartment Of Nursing, Islamic Azad University, Firoozabad, Iran, Islamic Republic Of
Email:najafizh44@gmail.com
Abstract
This work presents a Mini- review of the literature on COVID-19 antibody tests. Severe Acute Respiratory Syndrome of Corona Virus 2 (SARS-CoV-2) and the resulting COVID-19 pandemic are important diagnostic challenges. There are several diagnostic tools available to identify a current infection, rule out an infection, identify people in need of intensive care, or test for a previous infection and immune response. Serological tests to detect the presence of anti-SARS-CoV-2 antibodies are performed to detect a previous SARS-CoV-2 infection and may help confirm the presence of a current infection. Antibodies that strengthen the body's immune system against the corona virus may only stay in a person's body for a few months. Infection with the corona virus will not lead to lifelong resistance. The immune system will learn how to make new antibodies to a disease it has had in the past.
Keywords
Antibody; Corona virus; Immune System
INTRODUCTION
Serological testing or corona antibodies look for antibodies that are produced by the immune system in response to a threatening agent, such as a specific type of virus. Antibodies help fight infection, are made a few days or weeks after the infection, and remain in the body for several weeks after recovery. For this reason, antibody testing should not be used to detect active corona virus infection. Researchers do not know at present what the presence of antibodies means for the body's immunity to the corona virus in the future or not [1,2]. People develop antibodies when their body’s immune system responds to an infection. For this test, a blood sample is collected from the arm, finger stick and the serologic test is done for detecting IgG antibody, it indicates that you may have had COVID-19 in the recent past and have developed an immune response against the virus.
COVID-19 TESTS DETECT TWO TYPES OF ANTIBODIES
IgM, which the body makes for two weeks and then its levels drop IgG, which the body builds up more slowly (about 4 weeks) but usually lasts longer. Because IgM antibodies may not be detected in the first few days of the disease and may not be detectable, the IgG antibody test is more specific for COVID-19, and is more commonly used to check for infection or immunity in a person [3-5].
The antibody test can show if you have ever been exposed to or infected with the Covid-19 virus, and if your body has made antibodies in an effort to defend itself. It takes at least twelve days after exposure to the virus for the body to make enough antibodies to detect in the test. This test helps scientists gather information on how the immune system fights Covid-19 in recovered patients [6-8].
We do not yet know whether a person with a positive antibody test, against re- infection whether it is protected by a virus or not, and if protected, how long this protection lasts. Of course, this does not mean that the body cannot be resistant to Covid 19 or that an effective vaccine cannot be obtained. In a study of 30 patients with coronary artery disease, the lifespan of antibodies was examined and it was found that half of these antibodies have a lifespan of only 73 days and then disappear. The immune system of people with COVID-19 can build up proteins in the blood called antibodies that can attack the virus and respond to infection. Antibody tests in a person's blood may indicate that they already have COVID-19 [3,7,9,10]. Antibody levels increase and decrease at different times after infection. IgG is the last to increase but remains the highest. Antibody levels are usually at their highest a few weeks after infection. Some antibody tests require special laboratory equipment. Others use disposable devices, similar to pregnancy tests. These tests can be done in laboratories or wherever the patient is present (point-of-care), in the hospital or at home. It takes about 4 weeks for your body to produce IgM antibodies. But scientists are not sure how long it will take for this to happen in the case of Corona.
More experiments are needed to understand this. Keep in mind that current antibody tests cannot tell you if you are safe with Corona. Because we do not know how long these antibodies can protect you against the Corona virus [5,10,11].
Antibody tests detected only 30% of people with COVID-19 one week after the first symptoms. Test accuracy increased in the second week with 70% of diagnoses and was at its highest in the third week (more than 90% of diagnoses). Little evidence was available for the time after the third week. In 2% of people without COVID-19, the test results were false positive [3,4,7].
It is hoped that people with COVID-19 antibodies will be able to return to work and normal life as quickly as possible. These tests may also help with an experimental corona-specific treatment called improved plasma. Plasma is the fluid part of your blood. Researchers are studying how donating plasma antibodies to people who have recovered from the corona may help those infected with the virus. One theory is that this plasma may help sick people recover faster. But more research is needed [12,13].
If you test positive for coronavirus-19 antibodies, it usually means you have corona virus. But if you only have the virus for a short time, you may get a negative result. You may have been exposed to the virus and not produced antibodies. You may also receive a “false positive” answer. This means that you have antibodies but another type of corona virus [1,14].
We need a set of T cells to perform the complex interactions that lead to the production of antibodies. T cells play an important role in creating immunity to Covid-19. Helper T cells, also called CD4 T cells or CD4 helper T cells (because they carry a protein Called Differentiation set 4 (CD4) on their surface) protect the body against pathogens. When a virus infects a cell, there are two ways to alert the immune system to an external intruder. B cell-assisted T cell, another type of white blood cell and antibody-specific producer, allows a specific type of G antibody to produce Immunoglobulin (Ig) in response to a viral peptide.
The B cell then transforms into a plasma cell or a B-memory cell in response to the interaction with the helper T cell. Plasma cells produce antibodies for several weeks after moving to the bone marrow and remain in the bone marrow for long-term protection.
Memory cells remain in circulation or settle in important parts of the body as part of the body’s immune system. If the body is exposed to the same virus again, the memory less cells detect the virus antigen, process it, and return it to the donor T cell [12,14].
There are different types of T cells. But both are very popular. Cytotoxic cells, or eight positive CDs, attach to a foreign agent in the body and destroy it, and one is helper T cells, also called CD4 positives. These CD4-positive cells activate B lymphocytes, and after activation, it is B lymphocytes that produce antibodies or antibodies against the invading virus; therefore, if we want to develop a vaccine for coronary heart disease in the future, this vaccine should be able to activate CD4-positive or helper cells helper T cells can identify a part of the virus we call protein S. It may be through this S protein that T cells are activated, especially helper T, and if donor T cells are activated, then B cells are activated, which themselves produce antibodies and create immunity for our body [10,12,15,16].
In recognizing the important role of T cells, it is enough to know that, for example, in patients whose work is transferred to the ICU, the number of these cells is extremely low. Severe patients with COVID- 19 also do not have T cells in their blood and are equally weak against certain viruses, such as AIDS or an increase in inflammatory factors in the body, which can lead to death and lower levels of these cells in the body [14]. When T cells are activated, they activate B cells and make antibodies against the virus for us.
CD4 helper T cell and CD8 T cell both play an important role in Covid-19. CD8 T cell response is not as effective in people with severe Covid-19 disease as in those with mild disease. In particular, they may have fewer CD8 T cells in their bodies and those CD8 T cells in their bodies may not be able to convert to memory CD8 T cells. The researchers found that in some substances, the CD8 T cell response was very high in patients with Covid-19.
Most hospitalized patients appear to have CD4 + and CD8 + T cell responses, and evidence suggests that T cell responses to this disease are severe, excessive, or inappropriate in severe conditions [11,12,15,17] (Figure 1).
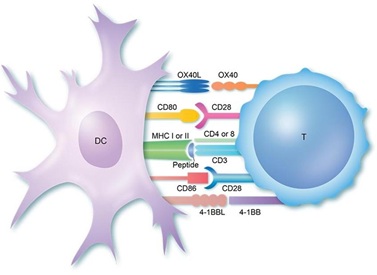 Figure 1: CD4 helper T cell and CD8 T cell both play an important role in Covid-19. CD8 T cell response was very high in patients with Covid-19.
Figure 1: CD4 helper T cell and CD8 T cell both play an important role in Covid-19. CD8 T cell response was very high in patients with Covid-19.
CONCLUSION
The sensitivity of antibody tests in the first week after the onset of symptoms is very low and may not play a major role in the diagnosis of COVID-19, but it may still complement other tests in people who later develop the disease when the RT-PCR test is negative. Or not done, have. If antibody tests are used on the 15th day or more after the onset of symptoms, they can play an important role in diagnosing a previous SARS-CoV-2 infection.
However, the duration of antibody enhancement is currently unknown, and we found very little data for more than 35 days from the onset of symptoms.
Therefore, we are unsure about the use of these tests for serological outbreaks for general health management purposes. Concerns about the high risk of bias and its application suggest that the accuracy of tests; when used in clinical care may be lower than reported in studies. Sensitivity was mainly evaluated in hospitalized patients, so it is unclear whether these tests will be able to detect lower levels of antibodies that are likely to be milder and asymptomatic with COVID-19.
REFERENCES
- FDA (2020) Antibody (Serology) Testing for COVID-19: Information for Patients and Consumers. FDA, USA.
- mayoclinic.org › about ›pac-20489696 Mayo Clinic COVID-19 antibodytestingAug 28, 2020 - Overview · Binding antibodies. These widely available antibody tests detect whether you've developed any antibodies in response to a COVID- 19
- CliniSciences (2019) SARS-CoV-2 (Covid-19): Diagnosis by IgG/IgM Rapid Test. CliniSciences, France.
- National Jewish Health (2020) The Difference between Tests for COVID-19(Coronavirus). ational Jewish Health, USA.
- Liu X, Wang J, Xu X, Liao G, Chen Y (2020) Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg Microbes Infect 9: 1269-1274.
- https://www.youtube.com/watch?v=NOK6drNPNck
- Cochrane (2020) What is the diagnostic accuracy of antibody tests for the detection of infection with the COVID-19 virus?Cochrane, United Kingdom.
- gov.im›about-coronavirus›igg-antibody-test...IgG Antibody Testing - Covid-19.gov.imThis test is designed to detect antibodies against the virus that causes COVID-19. Antibodies are proteins produced by the immune system in response to an
- CDC (2020) Interim Guidelines for COVID-19 Antibody Testing. CDC, USA.
- Webmd (2020) Coronavirus antibody testing. Webmd, USA.
- ECDC (2020) Immune responses and immunity to SARS-CoV-2. ECDC, Sweden.
- FDA (2020) Coronavirus (COVID-19) Update: FDA Authorizes First Point-of-Care Antibody Test for COVID-19. FDA, USA.
- Hurwitz JL (2020) B Cells, Viruses, and the SARS-CoV-2/COVID-19 Pandemic of 2020. Viral Immunology 33: 251-252.
- Chowdhury MA, Hossain N, Kashem MA, Shahid A, Alam A (2020) Immune response in COVID-19: A review. Journal of Infection and Public Health 13: 1619-1629.
- NIH (2020) Immune cells for common cold may recognizeSARS-CoV-2. NIH, USA.
- WHO (2020)COVID-19 Immunity & Clinical Manifestations. WHO, Switzerland.
- Mayo Clinic (2020) Convalescent plasma therapy. Mayo clinic, USA.
Citation: Najafi Z (2020) Antibody-Mediated Immune Response and COVID-19.J Diabetes Metab Disord 7: 035.
Copyright: © 2020 Zhaleh Najafi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

