
Are “Sartans” the Common Treatment for COVID-19 and Parkinson’s Disease?
*Corresponding Author(s):
Adonis SferaPatton State Hospital, Patton, CA, United States
Email:dr.sfera@gmail.com
Abstract
COVID-19 disease is caused by the SARS-CoV-2 virus that, after originating in Wuhan, China, spread rapidly throughout the world. As this pathogen enters host cells through the angiotensin in converting enzyme 2 protein, angiotensin in II hydrolysis may be impaired, contributing to COVID-19 pathology. Indeed, angiotensin in II serum levels have been positively corelated with both the SARS-CoV-2 viral load and the severity of lung injuries. Moreover, angiotensin in II binding toits type 1 receptors has been associated with host immune dysfunction and the subsequent COVID-19 critical illness. On the other hand, disruption of this signaling pathway in the brain substantia nigra has been associated with Parkinson’s disease, suggesting that SARS-CoV-2 infection may share a common pathophysiology with movement disorders. The connection between COVID-19 and Parkinson’s disease is further supported by the fact that antiviral natural killer cells and cytotoxic T cells express both renin-angiotensin and dopamine systems that the virus can exploit for immune evasion.
In this perspective article, we look at angiotens in II receptor blockers, “sartans”, alone or in combination with angiotens in (1-7) agonists, as potential treatment modalities for both COVID-19 and Parkinson’s disease.
Keywords
Sartans; COVID-19; Delirium
INTRODUCTION
COVID-19 pandemic is caused by the SARS-CoV-2 virus that originated in China and spread rapidly around the globe, infecting, at the time of this writing, over 3 million people and causing more than 300,000 fatalities. It is believed that 40-50% of COVID-19 patients present with neuropsychiatric symptoms, including strokes, cognitive dysfunction, depression, psychosis and delirium, suggesting that SARS-CoV-2 may be a neurotropic virus [1-4]. Although, Parkinson’s disease (PD) or secondary parkinsonism have not been described in association with COVID-19, movement disorders have accompanied prior pandemics, suggesting that they could follow [5,6].
SARS-CoV-2 enters host cells through the angiotensin converting enzyme 2 (ACE-2), likely impairing angiotensin II (ANG II) hydrolysis into angiotensin [1-7]. This may lead to the unopposed accumulation of ANG II and COVID-19 pathology. Indeed, several studies have associated ANG II serum levels with SARS-CoV-2 viral load and the severity of lung injuries [7-9]. In addition, ANGII activation of its type 1 receptors (AT-1Rs) was found to facilitate viral infection by disabling mitochondria-mediated immunity [10-12].
Aside from the traditional or circulating renin-angiotensin system (RAS), the brain and immune cells express a local RAS, essential for the integrity of antiviral defense and the substantia nigra (SN) dopaminergic neurons [13]. RAS homeostasis is maintained by balancing its two opposing branches, the vertical, with ANG II as its main representative, and the horizontal, comprised of angiotens in [1-7], alamandine and their receptors [14]. Together these pathways regulate the production of reactive oxygen species (ROS) and nitric oxide (NO), limiting the generation of toxic peroxynitrite [15]. When SARS-CoV-2 binds ACE-2, the horizontal RAS branch may be disabled, and the vertical enhanced, theoretically predisposing to both COVID-19 and parkinsonism (Figure 1). Indeed, the vertical branch inhibitors or angiotensin receptor blockers (ARBs) were found beneficial in PD animal models and are currently in clinical trials for COVID-19, suggesting therapeutic properties for both conditions [16-21] (NCT04335123)( NCT04312009)( NCT04311177). According to this model, ARBs combined with horizontal RAS agonists may completely reverse the SARS-CoV-2 immune suppression, ameliorating both the infectious and neuropsychiatric consequences of this virus.
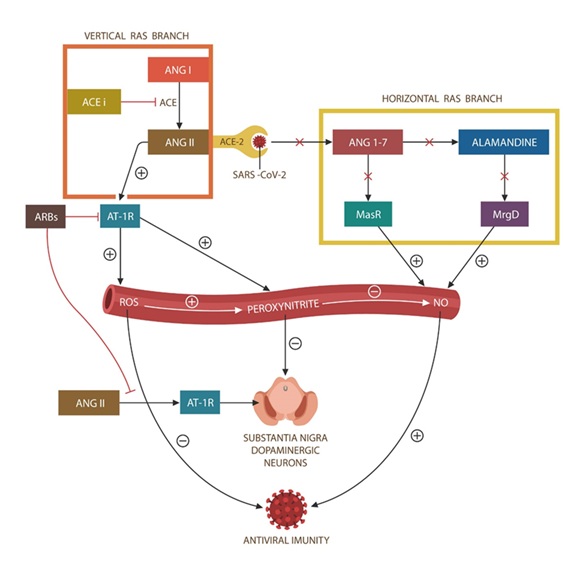 Figure 1: The vertical and horizontal RAS branches maintain redox homeostasis by regulating the generation of ROS and NO. ACE-2/SARS-CoV-2 binding disables the horizontal branch, disrupting the REDOX homeostasis with resultant NO depletion and upregulation of ROS and peroxynitrate. Since a local RAS is operational in immune cells and SN, the homeostasis of antiviral immunity and the dopaminergic neurons is impaired. ARBs block the vertical RAS pathway (although they do not affect the virus-blocked horizontal branch), rebalancing the system. Combining ARBs with ANG [1-7] agonists would likely reverse SARS-CoV-2 actions better than ARBs alone.
Figure 1: The vertical and horizontal RAS branches maintain redox homeostasis by regulating the generation of ROS and NO. ACE-2/SARS-CoV-2 binding disables the horizontal branch, disrupting the REDOX homeostasis with resultant NO depletion and upregulation of ROS and peroxynitrate. Since a local RAS is operational in immune cells and SN, the homeostasis of antiviral immunity and the dopaminergic neurons is impaired. ARBs block the vertical RAS pathway (although they do not affect the virus-blocked horizontal branch), rebalancing the system. Combining ARBs with ANG [1-7] agonists would likely reverse SARS-CoV-2 actions better than ARBs alone.
COVID-19 AND ANGIOTENSIN II
Numerous studies have connected COVID-19 pandemic with RAS and its component, ACE-2. As SARS-CoV-2 virus accesses host tissues via ACE-2 protein, it likely impairs ANG II hydrolysis, leading to the unopposed accumulation of this peptide. In addition, as RAS and ACE-2 are also expressed by the host immune cells, viral binding may disrupt immunity, predisposing to the COVID-19. Moreover, immune cells, including NK cells, also express a dopamine system (DAS) which interacts with RAS at the mitochondrial level, regulating antiviral responses [22,23]. Indeed, several earlier studies have shown that ANGII activation of its type 1 receptors (AT-1Rs) disrupts mitochondrial antiviral signaling (MAVS) proteins and the generation of type I interferon [10,12]. This is further supported by the studies that found a direct relationship between ANG II serum levels, SARS-CoV-2 viral load and the severity of lung injuries, linking this illness to mitochondria-mediated immunity [7-9].
Aside from regulating the arterial blood pressure, RAS is essential for NO homeostasis, peroxynitrite down regulation and lowering the oxidative and nitrosative stress [24]. Indeed, novel data have indicated that, contrary to prior beliefs, NO is a potent antioxidant, neuroprotector and inhibitor of SARS-CoV-2 replication [25]. However, in the presence of excessive oxidation, NO engenders peroxynitrite, a toxin that promotes both viral infection and SN damage [24,26,27]. Indeed, SARS-CoV-2 virus may facilitate its own replication by generating excessive peroxynitrite and ROS as well as by depleting NO [28,29]. As a result of these findings, not only has the US Food and Drug Administration (FDA)expanded the use of NO in COVID-19 but is currently evaluating it for its overall antiviral properties [30] (NCT04388683).
Aside from the immune cells, RAS and ACE-2 are also expressed by the SN dopaminergic neurons, therefore movement disorders could follow SARS-CoV-2 infection [13]. Indeed, dysfunctional ANG II/AT-1R signaling in SN was reported in PD animal models, revealing the RAS and DAS interconnectedness in this disorder [31]. Along these lines, recent studies have found that excessive SN peroxynitrite impairs dopamine (DA) synthesis, likely contributing to parkinsonism [32-34]. Others have demonstrated a direct relationship between the serum peroxynitrite level and scoring on the unified Parkinson’s disease rating scale (UPDRS), linking this disorder to RAS and DAS dysregulation [35,36].
RAS is comprised of two opposing pathways joined by the ANG II/ACE-2 interaction. The vertical branch, consisting of ANG II/AT-1Rs signaling, generates ROS which, aside from inducing oxidative stress, play a major signaling role [37]. The horizontal RAS branch, represented by ANG [1-7], alamandine and their respective receptors MasR and MrgD, generates NO, optimizing antiviral immunity and the homeostasis of SN dopaminergic neurons [38,39]. When SARS-CoV-2 binds ACE-2, the signaling in the vertical RAS branch may be enhanced, while the horizontal inhibited, favoring both viral infection and movement disorders (Figure 1).
Taken together, COVID-19 disrupts antiviral immunity by up regulating the vertical RAS and inhibiting the protective horizontal branch. This results in increased oxidative and nitrosative stress, peroxynitrite upregulation and lower NO, predisposing to COVID-19 and secondary parkinsonism [28].
COVID-19 AND CELLULAR SENESCENCE
Novel studies have connected the excessive activation of vertical RAS branch with p21-mediated cellular senescence in several tissues, including the brain, endothelia and immunity [40-42]. This is significant as a recent study reported that dopaminergic neurons in PD may not undergo apoptosis, as previously believed, but p21-induced cellular senescence [43,44]. As senescence may be potentially reversible, this is good news for patients with PD providing a safe modality for p21 inhibition.
Many studies have reported that viruses replicate more effectively in senescent cells and can induce this phenotype in host tissues [45-47]. For example, the H7N9 influenza virus triggers endothelial cell senescence to disrupt the blood-brain barrier (BBB) and enter the brain [48]. Other examples of virus-induced cellular senescence include the human immunodeficiency virus (HIV) and cytomegalovirus (CMV) that thrive by promoting host immune senescence [49]. To induce senescence and activate p21, viruses often attack mitochondria, releasing excessive ROS and mitochondrial DNA (mtDNA) [50]. Interestingly, PD was associated with abnormal “immune aging”, involving NK cells and CD4 lymphocytes, linking senescence to the pathogenesis of this movement disorder [51-53]. Along these lines, a recent study has found that COVID-19 patients age and die prematurely, losing on average a decade in years of life lost (YLL), linking this virus to aging [54]. Moreover, since COVID-19 appears to target older individuals, this pathogen may promote premature senescence in addition to accelerating the normal aging process [55]. Taken together, this data is in line with prior findings, linking virus-induced senescence to neurodegenerative disorders [45,56,57].
A FRESH LOOK AT VIRAL PARKINSONISM
Viral parkinsonism is a topic closely related to cellular senescence as viruses were demonstrated to induce this phenotype in many cell types, including the neurons and lymphocytes, promoting neurodegeneration [58]. Primary PD is a progressive neurodegenerative disorder marked by motor and nonmotor symptoms, caused by an impairment of SN dopaminergic neurons [59]. Secondary parkinsonism or PD-like symptoms can be induced by numerous agents, including viral infections along with the oxidative or nitrosative stress triggered by these pathogens [60-62].
Many viruses, probably including SARS-CoV-2, are neurotropic and capable of inducing SN damage. Indeed, around half of COVID-19 patients, were found to experience DAS-related symptoms, including inattention, depression, psychosis and delirium [1-4]. Similar neuropsychiatric manifestations were described following the 1918 influenza pandemic that may have caused encephalitis lethargica (EL), a condition marked by various central nervous system (CNS) symptoms, including movement disorders [63-65]. Contemporary cases of virus induced EL have been accompanied by secondary parkinsonism, linking viruses to DAS disruption [64]. Interestingly, viruses were demonstrated to lower immune responses by altering host DA levels which in return may induce parkinsonism [23,66]. For example, Japanese encephalitis virus (JEV) was shown to enter host cells via DA receptors and target SN, resulting in movement disorders [67]. Another example is HIV which lowers host immune responses by down regulating human dopamine transporters (hDATs), an action that often triggers parkinsonism [68,69]. The link between DA and immunity is further substantiated by the fact that PD patients treated with L-DOPA or DA agonists frequently develop immune adverse effects [70,71]. Interestingly, peroxynitrate was found to inhibit hDAT, suggesting that SARS-CoV-2-upregulation of this toxin may suppress immunity [72,73]. Indeed, as low hDAT levels characterize PD, COVID-19 peroxynitrite upregulation may lower these proteins further, exacerbating SN damage [74]. On the other hand, DA blockers, including chlorpromazine, have been demonstrated to augment host antiviral immunity and were proposed as COVID-19 treatments [75-77] (NCT04366739). As a result of these findings, we speculate that patients taking antipsychotic medications may be somewhat protected against SARS-CoV-2 infection as suggested by the emerging research on forensic inpatients (unpublished data).Additionally, methamphetamine (METH) use disorder has been associated with both PD and impaired immunity, linking this drug to COVID-19 vulnerability (see the next section).
ARE NK CELLS THE MISSING LINK BETWEEN COVID-19 AND PD?
NK cells are innate immune lymphocytes that play a major role in clearing malignant, virus-infected and senescent cells, preventing their accumulation and disease progression [78]. These lymphocytes, expressing both RAS and DAS, maintain the integrity of both antiviral immunity and the SN dopaminergic neurons [22,40,78,79]. Lately, NK cells have been under intense scrutiny by the research community as their dysfunction was associated with viral infections, cancer and PD [80]. Indeed, it was recently reported that, under normal circumstances, NK cells lower the progression of PD by clearing α-synuclein [81]. Others have reported an inverse relationship between α-synuclein and hDATs in NK cells and SN, emphasizing the role of these transporters in antiviral immunity and the SN integrity [82]. As hDATs regulate the extra and intracellular DA levels, viral exploitation of these proteins may lower immune responses and predispose to PD. Indeed, as human NK cells express higher levels of DA receptors compared to other immune cells, they are more susceptible to viral manipulation [23]. Indeed, earlier studies have reported that up regulated DA inhibits NK cell function via the immune checkpoint, NKG2A [79]. A recent study found that SARS-CoV-2 can disrupt NK cells DAS by up regulating NKG2A [83]. For this reason, active NK cells are currently in clinical trials for COVID-19 (NCT04324996) (NCT04375176). Furthermore, a sunder normal circumstances, NK cells also function to eliminate senescent cells, they may directly link PD to p21-induced cellular senescence [10,43]. Indeed, it has been reported that senescent cells over expressing NKG2A immune checkpoint are resistant to NK cells elimination, associating once more viruses with senescence [83,84].
The NK cells RAS and DAS and their link to antiviral immunity and PD is further supported by methamphetamine (METH) studies. Several reports have been published, linking chronic METH exposure to lower NK cells number and function, suggesting that users may beat higher risk of both PD and COVID-19 illness [85-89]. Indeed, METH-upregulated peroxinitrite and hDAT downregulation were reported to exacerbate movement disorders [87,88]. Interestingly, like COVID-19, METH was demonstrated to augment ANG II/AT-1R signaling, linking DA to vertical RAS activation [90]. Moreover, preclinical studies have reported that candesartan treatment decreases METH self-administration, linking RAS and DAS and indicating that this ARB may be the drug of choice for COVID-19 METH users [91]. Furthermore, aside from directly inhibiting hDAT, METH may promote PD indirectly by disrupting the function of NK cells leading to α-synuclein accumulation [92-95].
Several studies have connected METH with mitochondrial disruption and susceptibility to COVID-19, suggesting once more the centrality of these organelles in antiviral immunity [96-98]. Indeed, under normal circumstances, viral RNA activates mitochondrial MAVS (via RIG-1), inducing the synthesis of type 1 interferon to eliminate the virus (Figure 2) [99]. With the same token, α-synuclein accumulation may predispose to COVID-19 by impairing NK cells [100].
Taken together, this data suggests that ARBs may benefit both COVID-19 and PD patients as these agents may augment immunity and protect SN. Moreover, adding ANG [1-7] agonists may optimize the antiviral and PD treatment by reactivating the horizontal RAS branch.
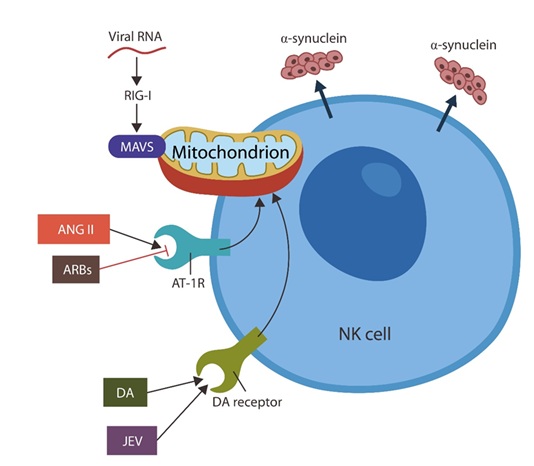 Figure 2: Natural killer cells play a crucial role in both COVID-19 (by eliminating virus-infected cells) and PD (by clearing α-synuclein). Since these lymphocytes express both RAS and DAS their disruption alters mitochondrial function, impairing antiviral immunity. Under physiological circumstances, SARS-CoV-2 RNA activates mitochondrial MAVS (via RIG-1), promoting the synthesis of type 1 interferon (not shown). The unopposed activation of the vertical RAS branch induces mitochondrial damage via ANG II. This action is inhibited by ARBs. DAS overactivation by viruses, including JAV and possibly SARS-CoV-2, disrupts the mitochondrion. This action is blocked by DA blockers, such as chlorpromazine.
Figure 2: Natural killer cells play a crucial role in both COVID-19 (by eliminating virus-infected cells) and PD (by clearing α-synuclein). Since these lymphocytes express both RAS and DAS their disruption alters mitochondrial function, impairing antiviral immunity. Under physiological circumstances, SARS-CoV-2 RNA activates mitochondrial MAVS (via RIG-1), promoting the synthesis of type 1 interferon (not shown). The unopposed activation of the vertical RAS branch induces mitochondrial damage via ANG II. This action is inhibited by ARBs. DAS overactivation by viruses, including JAV and possibly SARS-CoV-2, disrupts the mitochondrion. This action is blocked by DA blockers, such as chlorpromazine.
CONCLUSION
Severe illness and neuropsychiatric manifestations associated with COVID-19 may have a common denominator: over activation of the vertical RAS branch and blocking the horizontal. Excess generation of ANG II and peroxynitrite likely disrupts the RAS and DAS in both NK cells and SN, damaging antiviral immunity and the dopaminergic neurons.
This model predicts both, that movement sequelae may follow COVID-19 and that “sartans”, alone or in combination with ANG [1-7] agonists, could be therapeutic for both.
REFERENCES
- Mao L, Wang M, Chen S (2020) Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: A retrospective case series study. JAMA Neurol 77: 683-690.
- Jin H, Hong C, Chen S, Zhou Y, Wang Y, et al. (2020) Consensus for prevention and management of coronavirus disease 2019 (COVID-19) for neurologists. Stroke and Vascular Neurology 5: 146-151.
- Varatharaj A, Thomas N, Ellul M, Davies NWS, Pollak T, et al. (2020) UK-wide surveillance of neurological and neuropsychiatric complications of COVID-19: The first 153 patients. The Lancet Psychiatry 7: 875-882.
- Rogers JP, Chesney E, Oliver D, Pollak AP, McGuire PP, et al. (2020) Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 7: 611-627.
- Troyer EA, Kohn JN, Hong S (2020) Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun 87: 34-39.
- Tipton PW, Wszolek ZK (2020) What can Parkinson's disease teach us about COVID-19? Neurol Neurochir Pol 54: 204-206.
- Liu Y, Yang Y, Zhang C, Huang F, Wang F, et al. (2020) Clinical and biochemical indexes from 2019?nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63: 364-374.
- Reddy R, Asante I, Liu S, Parikh P, Liebler J, et al. (2019) Circulating angiotensin peptides levels in Acute Respiratory Distress Syndrome correlate with clinical outcomes: A pilot study. PLoS ONE 14: e0213096.
- Kuba K, Imai Y, Rao S, Gao H, Guo F, et al. (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus?induced lung injury. Nat Med 11: 875-879.
- Song P, Kim JH (2019) Benefits of Angiotensin Receptor Blockade: Preventing Smooth Muscle Cell Senescence and Beyond. Korean Circ J 49: 627-628.
- Saavedra JM (2020) Angiotensin receptor blockers and COVID-19. Pharmacol Res 156: 104832.
- Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, et al. (2017) A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care 21: 234.
- Angajala A, Lim S, Phillips JB, Kim JH, Yates C, et al. (2018) Diverse Roles of Mitochondria in Immune Responses: Novel Insights Into Immuno-Metabolism. Front Immunol 9: 1605.
- Schleifenbaum J (2019) Alamandine and Its Receptor MrgD Pair Up to Join the Protective Arm of the Renin-Angiotensin System. Front Med (Lausanne) 6: 107.
- Dibo P, Marañón RO, Chandrashekar K, Mazzuferi F, Silva GB, et al. (2019) Angiotensin-(1-7) inhibits sodium transport via Mas receptor by increasing nitric oxide production in thick ascending limb. Physiol Rep 7: e14015.
- Lee YC, Lin CH, Wu RM, Lin JW, Chang CH, et al. (2014) Antihypertensive agents and risk of Parkinson's disease: A nationwide cohort study. PLoS One 9: e98961.
- Perez-Lloret S, Otero-Losada M, Toblli JE, Capani F (2017) Renin-angiotensin system as a potential target for new therapeutic approaches in Parkinson's disease. Expert Opin Investig Drugs 26: 1163-1173.
- Rodriguez-Pallares J, Parga JA, Muñoz A, Rey P, Guerra MJ, et al. (2007) Mechanism of 6-hydroxydopamine neurotoxicity: The role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J Neurochem 103: 145-156.
- Mertens B, Varcin M, Michotte Y, Sarre S (2011) The neuroprotective action of candesartan is related to interference with the early stages of 6-hydroxydopamine-induced dopaminergic cell death. European Journal of Neuroscience 34: 1141-1148.
- Villapol S, Saavedra JM (2015) Neuroprotective effects of angiotensin receptor blockers. Am J Hypertens 28: 289-299
- Reardon KA, Mendelsohn FA, Chai SY, Horne MK (2000) The angiotensin converting enzyme (ACE) inhibitor, perindopril, modifies the clinical features of Parkinson's disease. Aust N Z J Med 30: 48-53.
- Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takukara A, et al. (2007) Human T and natural killer cells possess a functional renin-angiotensin system: Further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol 18: 1093-1102.
- Arreola R, Alvarez-Herrera S, Pérez-Sánchez G, Villanueva EB, Cruz-Fuentes C, et al. (2016) Immunomodulatory Effects Mediated by Dopamine. J Immunol Res 2016: 3160486.
- Hummel SG, Fischer AJ, Martin SM, Schafer FQ, Buettner GR (2006) Nitric oxide as a cellular antioxidant: A little goes a long way. Free Radic Biol Med 40: 501-506
- Akerström S, Mousavi-Jazi M, Klingström J, Leijon M, Lundkvist A, et al. (2005) Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol 79: 1966-1969
- Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315-424.
- Mohanakumar KP, Thomas B, Sharma SM, Muralikrishnan D, Chowdhury R, et al. (2002) Nitric oxide: An antioxidant and neuroprotector. Ann N Y Acad Sci 962: 389-401.
- Green SJ (2020) Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microbes Infect 22: 149-150.
- Delgado-Roche L, Mesta F (2020) Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) infection. Arch Med Res 51: 384-387.
- Kobayashi J, Murata I (2020) Nitric oxide inhalation as an interventional rescue therapy for COVID-19-induced acute respiratory distress syndrome. Ann Intensive Care 10: 61.
- Perez-Lloret S, Otero-Losada M, Toblli JE, Capani F (2017) Renin-angiotensin system as a potential target for new therapeutic approaches in Parkinson's disease. Expert Opin Investig Drugs 26: 1163-1173.
- Park SU, Ferrer JV, Javitch JA, Kuhn DM (2002) Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: Potential mechanism of neurotoxicity in dopamine neurons. J Neurosci 22: 4399-4405.
- Ara J, Przedborski S, Naini AB, Lewis VJ, Trifelti RR, et al. (1998) Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proc Natl Acad Sci U S A 95: 7659-7663.
- Szabó C, Ischiropoulos H, Radi R (2007) Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6: 662-668.
- Girouard H, Park L, Anrather J, Zhou P, Iadecola C (2007) Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol 27: 303-309.
- Kouti L, Noroozian M, Akhondzadeh S, Abdollahi M, Javadi MR, et al. (2013) Nitric oxide and peroxynitrite serum levels in Parkinson's disease: Correlation of oxidative stress and the severity of the disease. Eur Rev Med Pharmacol Sci 17: 964-970.
- Welch WJ (2008) Angiotensin II-dependent superoxide: Effects on hypertension and vascular dysfunction. Hypertension 52: 51-56.
- Rabie MA, Abd El Fattah MA, Nassar NN, El-Abhar HS, Abdallah DM (2018) Angiotensin 1-7 ameliorates 6-hydroxydopamine lesions in hemiparkinsonian rats through activation of MAS receptor/PI3K/Akt/BDNF pathway and inhibition of angiotensin II type-1 receptor/NF-κB axis. Biochem Pharmacol 151: 126-134.
- Wright JW, Kawas LH, Harding JW (2013) A Role for the Brain RAS in Alzheimer's and Parkinson's Diseases. Front Endocrinol (Lausanne) 4: 158.
- Labandeira-García JL, Garrido-Gil P, Rodriguez-Pallares J, Valenzuela R, Borrajo A, et al. (2014) Brain renin-angiotensin system and dopaminergic cell vulnerability. Front Neuroanat 8: 67.
- Haynes LD, Verma S, McDonald B, Wu R, Tacke R, et al. (2015) Cardif (MAVS) Regulates the Maturation of NK Cells. J Immunol 195: 2157-2167.
- Martin-Ruiz C, Williams-Gray CH, Yarnall AJ, Boucher JJ, Lawson RA, et al. (2020) Senescence and Inflammatory Markers for Predicting Clinical Progression in Parkinson’s Disease: The ICICLE-PD Study. J Parkinsons Dis 10: 193-206.
- Riessland M, Kolisnyk B, Kim TW, Cheng J, Ni J, et al. (2019) Loss of SATB1 Induces p21-Dependent Cellular Senescence in Post-mitotic Dopaminergic Neurons. Cell Stem Cell 25: 514?e8.
- Im JH, Yeo IJ, Jeon SH, Lee DH, Ham HJ, et al. (2020). p21 inhibitor UC2288 ameliorates MPTP induced Parkinson’s disease progression through inhibition of oxidative stress and neuroinflammation. European PMC.
- Kim JA, Seong RK, Shin OS (2016) Enhanced Viral Replication by Cellular Replicative Senescence. Immune Netw 16: 286-295.
- Chuprin A, Gal H, Biron-Shental T, Biran A, Amiel A, et al. (2017) Cell fusion induced by ERVWE1 or measles virus causes cellular senescence. Genes Dev 27: 2356-2366.
- Kritsilis M, V Rizou S, Koutsoudaki PN, Evangelou K, Gorgoulis VG, et al. (2018) Ageing, Cellular Senescence and Neurodegenerative Disease. Int J Mol Sci 19: 2937.
- Yan Y, Du Y, Zheng H, Wang G, Li R, et al. (2017) NS1 of H7N9 Influenza A Virus Induces NO-Mediated Cellular Senescence in Neuro2a Cells. Cell Physiol Biochem 43: 1369-1380.
- Combs JA, Norton EB, Saifudeen ZR, Bentrup KHJ, Katakam PV, et al. (2020) Human Cytomegalovirus Alters Host Cell Mitochondrial Function during Acute Infection. J Virol 94: e01183-19.
- Passos JF, Nelson G, Wang C, Richter T, Simillion C, et al. (2010) Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6: 347.
- Williams-Gray CH, Wijeyekoon RS, Scott KM, Hayat S, Barker RA, et al. (2018) Abnormalities of age-related T cell senescence in Parkinson’s disease. J Neuroinflammation 15: 166.
- Costantini E, D'Angelo C, Reale M (2018) The Role of Immunosenescence in Neurodegenerative Diseases. Mediators Inflamm 2018: 6039171.
- Tansey MG, Romero-Ramos M (2019) Immune system responses in Parkinson's disease: Early and dynamic. Eur J Neurosci 49: 364-383.
- Hanlon P, Chadwick F, Shah A, Wood R, Minton J, et al. (2020) COVID-19-exploring the implications of long-term condition type and extent of multimorbidity on years of life lost: A modelling study. Wellcome Open Res 5: 75.
- Sargiacomo C, Sotgia F, Lisanti MP (2020) COVID-19 and chronological aging: Senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging (Albany NY) 12: 6511-6517.
- Malavolta M, Giacconi R, Brunetti D, Provinciali M, Maggi F (2020) Exploring the Relevance of Senotherapeutics for the Current SARS-CoV-2 Emergency and Similar Future Global Health Threats. Cells 9: 909.
- Delgado-Roche L, Mesta F (2020) Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch Med Res 51: 384-387.
- Zhou L, Miranda-Saksena M, Saksena NK (2013) Viruses and neurodegeneration. Virol J 10: 172.
- DeMaagd G, Philip A (2020) Parkinson's Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. PT 40: 504-532.
- Colosimo C, Morgante L, Antonini A, Barone P, Avarello TP, et al. (2010) Non-motor symptoms in atypical and secondary parkinsonism: The PRIAMO study. J Neurol 257: 5-14.
- Lee C (2018) Therapeutic Modulation of Virus-Induced Oxidative Stress via the Nrf2-Dependent Antioxidative Pathway. Oxid Med Cell Longev 2018: 6208067.
- Castro R, Pinzón HS, Alvis-Guzman N (2015) A systematic review of observational studies on oxidative/nitrosative stress involvement in dengue pathogenesis. Colomb Med (Cali) 46: 135-143.
- Jang H, Boltz DA, Webster RG, Smeyne RJ (2009) Viral parkinsonism. Biochim Biophys Acta 1792: 714-721.
- Dale RC, Webster R, Gill D (2007) Contemporary encephalitis lethargica presenting with agitated catatonia, stereotypy, and dystonia-parkinsonism. Mov Disord 22: 2281-2284.
- Von Economo C (1931) In: Encephalitis Lethargica: Its Sequelae and Treatment. Newman KO, translator. London: Oxford University Press.
- Rohr O, Sawaya BE, Lecestre D, Aunis D, Schaeffer E (1999) Dopamine stimulates expression of the human immunodeficiency virus type 1 via NF-kappaB in cells of the immune system. Nucleic Acids Res 27: 3291-3299.
- Simanjuntak Y, Liang JJ, Lee YL, Lin YL (2017) Japanese Encephalitis Virus Exploits Dopamine D2 Receptor-phospholipase C to Target Dopaminergic Human Neuronal Cells. Front Microbiol 8: 651.
- Cardoso F (2002) HIV-related movement disorders: Epidemiology, pathogenesis and management. CNS Drugs 16: 663-668.
- Zhu J, Ananthan S, Zhan CG (2018) The role of human dopamine transporter in NeuroAIDS. Pharmacol Ther 183: 78-89.
- Lieberknecht V, Junqueira SC, Cunha MP, Barbosa TA, de Souza LF, et al. (2017) Pramipexole, a Dopamine D2/D3 receptor-preferring agonist, prevents experimental autoimmune encephalomyelitis development in mice. Mol Neurobiol 54: 1033-1045.
- Breger LS, Kienle K, Smith GA, Dunnett SB, Lane EL (2017) Influence of chronic L-DOPA treatment on immune response following allogeneic and xenogeneic graft in a rat model of Parkinson’s disease. Brain Behav Immun 2017: 155-164.
- Park S, Geddes TJ, Javitch JA, Kuhn DM (2003) Dopamine prevents nitration of tyrosine hydroxylase by peroxynitrite and nitrogen dioxide: Is nitrotyrosine formation an early step in dopamine neuronal damage? J Biol Chem 278: 28736-28742.
- Park SU, Ferrer JV, Javitch JA, Kuhn DM (2002) Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: Potential mechanism of neurotoxicity in dopamine neurons. J Neurosci 22: 4399-4405.
- Mackie P, Lebowitz J, Saadatpour L, Nickoloff E, Gaskill P, et al. (2018) The dopamine transporter: An unrecognized nexus for dysfunctional peripheral immunity and signaling in Parkinson's Disease. Brain Behav Immun 70: 21-35.
- Bhattacharyya S, Warfield KL, Ruthel G, Bavari S, Aman MJ, et al. (2010) Ebola virus uses clathrin-mediated endocytosis as an entry pathway. Virology 401: 18-28.
- Plaze M, Attali D, Petit AC, et al. (2020) Repositionnement de la chlorpromazine dans le traitement du COVID-19: Etude recovery. Encephale 46: S35-S39.
- Smith JL, Stein DA, Shum D, Fischer MA, Radu C, et al. (2014) Inhibition of dengue virus replication by a class of small-molecule compounds that antagonize dopamine receptor d4 and downstream mitogen-activated protein kinase signaling. J Virol 88: 5533-5542.
- Antonangeli F, Zingoni A, Soriani A, Santoni A (2019) Senescent cells: Living or dying is a matter of NK cells. J Leukoc Biol 105: 1275-1283.
- Mikulak J, Bozzo L, Roberto A, Pontarini E, Tentorio P, et al. (2014) Dopamine inhibits the effector functions of activated NK cells via the upregulation of the D5 receptor. J Immunol 193: 2792-2800.
- Niwa F, Kuriyama N, Nakagawa M, Imanishi J (2012) Effects of peripheral lymphocyte subpopulations and the clinical correlation with Parkinson's disease. Geriatr Gerontol Int 12: 102-107.
- Earls RH, Menees KB, Chung J, Gutekunst C, Lee HJ, et al. (2020) NK cells clear α-synuclein and the depletion of NK cells exacerbates synuclein pathology in a mouse model of α-synucleinopathy. Proc Natl Acad Sci U S A 117: 1762-1771.
- Butler B, Saha K, Rana T, Becker JP, Sambo D, et al. (2015) Dopamine Transporter Activity Is Modulated by α-Synuclein. J Biol Chem 290: 29542-29554.
- Yaqinuddin A, Kashir J (2020) Innate immunity in COVID-19 patients mediated by NKG2A receptors, and potential treatment using Monalizumab, Cholroquine, and antiviral agents. Med Hypotheses 140: 109777.
- Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers CS, Subramanian P, et al. (2019) Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat Commun 10: 2387.
- Lappin JM, Darke S, Farrell M (2018) Methamphetamine use and future risk for Parkinson's disease: Evidence and clinical implications. Drug Alcohol Depend 187: 134-140.
- Volkow ND (2020) Collision of the COVID-19 and Addiction. Ann Intern Med 173: 61-62.
- Imam SZ, Crow JP, Newport GD, Islam F, Slikker W, et al. (1999) Methamphetamine generates peroxynitrite and produces dopaminergic neurotoxicity in mice: Protective effects of peroxynitrite decomposition catalyst. Brain Res 837: 15-21.
- Martel J, Ko YF, Young JD, Ojcius DM (2020) Could nasal nitric oxide help to mitigate the severity of COVID-19? Microbes Infect 22: 168-171.
- Harms R, Morsey B, Boyer CW, Fox HS, Sarvetnick N (2012) Methamphetamine administration targets multiple immune subsets and induces phenotypic alterations suggestive of immunosuppression. PLoS One 7: e49897.
- Marchese NA, Artur de laVillarmois E, Basmadjian OM, Perez MF, Baiardi G, et al. (2016) Brain Angiotensin II AT1 receptors are involved in the acute and long-term amphetamine-induced neurocognitive alterations. Psychopharmacology (Berl) 233: 795-807.
- Xu X, Pan J, Li X, Cui Y, Mao Z, et al. (2019) Inhibition of Methamphetamine Self-Administration and Reinstatement by Central Blockade of Angiotensin II Receptor in Rats. J Pharmacol Exp Ther 369: 244-258.
- Potula R, Haldar B, Cenna JM, Sriram U, Fan S (2018) Methamphetamine alters T cell cycle entry and progression: Role in immune dysfunction. Cell Death Discovery 4: 44.
- Sriram U, Haldar B, Cenna JM, Gofman L, Potula R (2015) Methamphetamine mediates immune dysregulation in a murine model of chronic viral infection. Front Microbiol 6: 793.
- Jiang L, Zhu R, Bu Q, Li Y, Shao X, et al. (2018) Brain Renin-Angiotensin System Blockade Attenuates Methamphetamine-Induced Hyperlocomotion and Neurotoxicity. Neurotherapeutics 15: 500-510.
- Callaghan RC, Cunningham JK, Sykes S, Kish SJ (2012) Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug and Alcohol Dependence 120: 35-40.
- Liu XL, Wang YD, Yu XM, Li DW, Li GR (2018) Mitochondria-mediated damage to dopaminergic neurons in Parkinson's disease (Review). Int J Mol Med 41: 615-623.
- Doughan AK, Harrison DG, Dikalov SI (2008) Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488-496.
- Koshiba T, Bashiruddin N, Kawabata S (2011) Mitochondria and antiviral innate immunity. Int J Biochem Mol Biol 2: 257-262.
- Wu W, Wang X, Zhang W (2018) RIG-I Signaling via MAVS Is Dispensable for Survival in Lethal Influenza Infection In Vivo. Mediators Inflamm 2018: 6808934.
- Ganjam GK, Bolte K, Matschke LA, Neitemeier S, Dolgaet AM, et al. (2019) Mitochondrial damage by α-synuclein causes cell death in human dopaminergic neurons. Cell Death Dis 10: 865.
Citation: Sfera A (2020) Are “Sartans” the Common Treatment for COVID-19 and Parkinson’s Disease? J Alzheimer’s Neurodegener Dis 6: 048.
Copyright: © 2020 Adonis Sfera, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

