
Artificial Nerve Conduits with Stem Cell Transplantation used for Bridging a Long Nerve Gap: A Review of Experimental Studies in Rats
*Corresponding Author(s):
Ryosuke KakinokiDepartment Of Orthopedic Surgery, Kindai University Hospital 377-2 Oono-higashi, Osaka-sayama, Osaka, 589-8511, Japan
Tel:+81 753660221,
Fax:+81 753660206
Email:rkakinoki@med.kindai.ac.jp
Abstract
Cells, scaffolds, growth factors and vascularity are four pillars in regenerative medicine. In the field of peripheral nerve regeneration, studies have been performed based on these four pillars. We have created an artificial nerve conduit model in rats, which can be applied to clinical settings in near future. Our artificial conduit is made of a polyglycolic acid filament-meshed tube containing a sural vascular pedicle and decellularized allogenic nerve basal lamellae implanted by bone marrow derived mesenchymal stem cells. In this article, we describe essential factors and their application to fabrication of artificial nerve from the perspective of regenerative medicine.
Keywords
Allogenic basal lamellae; Artificial nerve; Bone Marrow Derived-Mesenchymal Stem Cell (BMSC); Nerve conduit; Vascularity
Introduction
Artificial nerves developed from the concept of tubulation. Tubulation is a phenomenon in which peripheral nerve fibers extend through a tube-like material when the nerve stumps are joined to either end of the material [1,2]. Williams et al., investigated the process of nerve regeneration through a tube [3]. In the first stage, a fibrin matrix is formed between the stumps and the chamber space is filled with neurochemical factors that facilitate axon regeneration. In the second stage, capillaries extend into the fibrin matrix from the nerve stumps at either end of the tube. Together with the capillary extension into the fibrin matrix, Schwann cells also migrate into the fibrin matrix from both nerve stumps followed by axon extension from the proximal stumps.
Autologous nerve transplantation has been the gold standard for repair of nerve injury involving an interstump gap. This maneuver is associated with morbidity in the nerve donor sites and a limitation of the amount of donor nerves. It has been reported that fewer axons with smaller diameters regenerate for a shorter distance in tubulation compared with an autologous nerve graft [4,5]. A significant advance of tubulation aims to extend the distance across which axons can regenerate and increase the number and diameter of the regenerated axons. Toward achieving this goal, considerable research is being conducted on stem cell transplantation around the world.
Creation Of A Vessel-Containing Tube (VCT)
Silicone tubes were used as nerve guides in experimental studies of peripheral nerve regeneration [1,6]. Nerve tissue regenerated through a silicone tube is vascularized by capillaries that extend from the nerve stumps joined to either end of the tube. However, capillary extension from the nerve stumps is limited, which may restrict the distance across which nerve fibers can regenerate. Several authors have noted that the newly formed capillary wall is the direct target of Schwann cell migration [7-10] or that a new capillary network is formed before Schwann cell distribution in the process of formation of Bunguner’s bands [11,12]. It is known that the maximum interstump distance across which peripheral nerves can regenerate is about 10 mm through a silicone tube using a rat sciatic nerve [6]. Several studies reported that addition of Vascular Endothelial Growth Factor (VEGF) in intratubular transplantation led to vascularization of the tubular lumen [12,13] or that the use of porous tubes allowed capillaries to enter the chamber space [14]. However, the optimal amount and timing of VEGF application to the tubular lumen is unknown. Furthermore, the optimal size of the pores in the tubular wall has not been defined. Previous authors reported that tubes having pores that allowed 105 Da-particles to pass demonstrated better nerve regeneration than those having pore sizes passing 106 Da-particles. The pore size should be large enough to permit capillary infiltration while preventing scar invasion into the tubular lumen.
We inserted a blood pedicle into a silicone tube to provide vascularity within the tube. We made a Vessel-Containing Tube (VCT) model using a rat hindlimb. A pedicled sural vascular bundle was elevated from the lower leg, turned proximally and inserted into a silicone tube through a longitudinal slit that was sealed with silicone liquid after the insertion [15-17] (Figure 1). Gaps of 10 mm in rat sciatic nerves were bridged using VCTs and simple empty silicone tubes were used as a control. Nerve regeneration in VCTs was significantly greater than that seen in the empty silicone tubes histomorphometrically and electrophysiologically at 12 weeks, but no significant difference was found between the two types of tubes at 24 weeks [15]. The distance across which axons can regenerate was extended up to 25 mm using VCTs in a rat sciatic nerve model [17]. Intrachamber vascularity promoted the rate of nerve regeneration and extended the distance across which axons can regenerate [15-17] but did not increase the axon diameters or total axon numbers in the regenerated nerves in the VCTs [15].
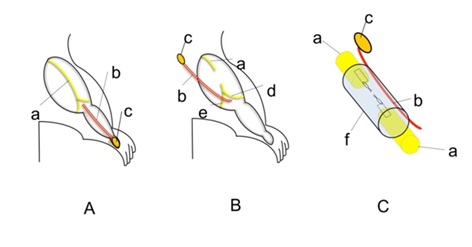 Figure 1: Creation of a VCT.
Figure 1: Creation of a VCT.
A: A myocutaneous flap (c) vascularized by the sural vessels (b) was elevated from the posterior surface of the ankle.
B: The flap was turned proximally.
C: The sural vascular pedicle was inserted into a silicone tube (f). The nerve stumps of the sciatic nerve (a) were joined to either end of the tube.
a) sciatic nerve; b) sural vessels; c) a myocutaneous monitor flap; d) tibial nerve; e) peroneal nerve.
BMSC Transplantation in a VCT
In a rat sciatic nerve, VCTs can provide vascularity in the intrachamber space even if the interstump gap extends more than 10 mm. The intratubular space is filled with neurochemical factors that can drive stem cells to form glial cells. This indicates that a VCT creates an environment where stem cells can survive with blood supplied from the intratubular transplanted blood vessels and differentiate into glial cells, which may facilitate nerve regeneration through the tube [18,19].
Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs) are popular stem cells transplanted into the chamber of nerve conduits to accelerate nerve regeneration. BMSCs transplanted in a tubular lumen were reported to have several functions that facilitate nerve regeneration within the conduit, such as homing ability [20-22], production of neurotrophic factors [23-26], and differentiation into Schwann cell-like cells [18,19,27-32].
A VCT model with a 15-mm interstump gap was created using a Lewis rat sciatic nerve. BMSCs harvested from the femur and tibia of Lewis rats were cultured in vivo for 6-7 passages. Ten million BMSCs were then transplanted into the chamber of a VCT [18]. VCTs with BMSC transplantation demonstrated an increased number and diameter of regenerated axons compared with VCTs without BMSC transplantation. The model also revealed that approximately 30% of the transplanted BMSCs differentiated into glial cell marker-positive cells [18]. Axons were shown to extend along the vascular bundle in VCTs [15-18]. These results indicated that a sural vascular bundle inserted in a silicone tube acted as a nutrient supplier to cells including transplanted BMSCs as well as a scaffold of axon regeneration within the VCT. We also created a VCT model using a canine ulnar nerve with a 30-mm interstump gap. Successful nerve regeneration occurred through a silicone tube containing the ulnar vessels. Electrophysiologic and histomorphometric studies demonstrated that nerve regeneration through a VCT with a 30-mm interstump gap was almost equal to that of a 30-mm autologous ulnar nerve segment 24 weeks after transplantation [19].
Schwann cell transplantation may be an option. However, the cooperative and organized contribution of fibroblasts and macrophages as well as Schwann cells is important in the process of nerve regeneration in tubulation. We used immature BMSCs, predicting that they would differentiate not only into Schwann cells but also into fibroblasts, macrophages and some other cells, depending on the environment in which they were transplanted. Tohill et al., reported that BMSCs expressing glial cell markers promote nerve regeneration [27]. In our pilot study, we compared the nerve regeneration between VCTs seeded with immature BMSCs and those with BMSCs having been differentiated to cells having neural cell characteristics [27]. There were no significant differences between the two tubes. Immature BMSCs might have differentiated into several different cell types in the tubular lumen, which may promote nerve regeneration.
Transplantation Of Decellularized Allogenic Nerve Lamellae In A VCT
When BMSCs were transplanted to the chamber, the transplanted cells might have leaked from the nerve conduit from both ends of the tube. The percentage of transplanted cells that remained in the chamber is unknown.
We transplanted Decellularized Allogenic Nerve Basal Lamellae (DABLs) [33] seeded with BMSCs along the sural vessel pedicle in the VCT, expecting that the DABLs would retain the transplanted BMSCs inside the tube as well as act as a scaffold for axon regeneration.
Nerve regeneration within a VCT with a 20-mm interstump gap containing BMSCs (3 × 106) and DABLs was significantly greater than that containing DABLs without BMSCs and reached a similar level to that of a 20-mm-long autologous nerve graft 24 weeks after transplantation in rats (Figure 2). Successful nerve regeneration across a 20-mm gap in a rat sciatic nerve corresponds to regeneration across a 6-cm interstump gap in human or primate peripheral nerves [34].
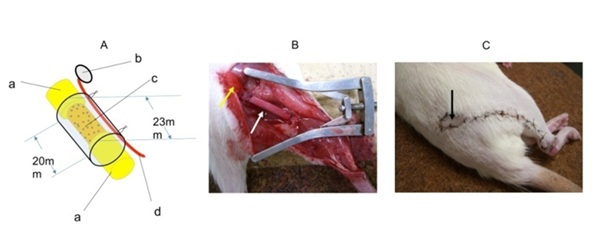 Figure 2: A) A VCT with DABLs seeded with BMSCs. B) The transplanted VCT with DABLs seeded with BMSCs. The yellow arrow indicates the myocutaneous flap. The white arrow indicates a silicone tube. C) After the transplanted surgery. The black arrow indicates the myocutaneous flap that was used as a monitor of the sural vessel patency.
Figure 2: A) A VCT with DABLs seeded with BMSCs. B) The transplanted VCT with DABLs seeded with BMSCs. The yellow arrow indicates the myocutaneous flap. The white arrow indicates a silicone tube. C) After the transplanted surgery. The black arrow indicates the myocutaneous flap that was used as a monitor of the sural vessel patency.
DABLs can be created using thermal [33], irradiation [35] or chemical techniques [36-38]. We used thermally created DABLs because of the ease of their creation. We found that a small amount of genomic DNA (0.91-2.88 µg/mg) was retained in thermally created DABLs, suggesting that thermally created DABL transplantation opens the possibility of organism contamination.
We were also concerned about immunological rejection of the DABLs because of residual DNA. DABLs were made from sciatic nerves of Dark Agouti (DA) rats, which have major histocompatibility mismatch with Lewis rats. We created three surgical groups bridging a 20-mm-long sciatic nerve gap in Lewis rats, a VCT containing DABLs and BMSCs, a 20-mm-long fresh autologous nerve graft, and a 20-mm-long fresh allogenic nerve graft (harvested from DA rats). The number of CD8 positive cells remaining in the DABL segments was almost equal to that found in fresh autologous nerve segments and significantly smaller than that in fresh allogenic nerve segments at 4 weeks after surgery [34]. This indicated that the DABL transplantation was not associated with immunological rejection. Immunostaining demonstrated retention of the extracellular matrix, including the laminin within the DABLs [34]. We also showed capillary infiltration into the DABLs from the transplanted sural vessels by using Rat Endothelial Cell Antigen-1 (RECA-1) specific immunostaining [34]. A VCT containing DABLs seeded with BMSCs is thus considered to be an optimal nerve-inducing conduit that retains the transplanted BMSCs, supplies vascularity to the intratubular- transplanted BMSCs and nerve fibers, and provides the nerve fibers with a scaffold containing extracellular matrix without inducing immunological rejection during the transplantation. Nerve regeneration through this guide tube bridging a 20-mm interstump gap was similar in level to that in a 20-mm-long autologous nerve graft. However, DABL transplantation opens the possibility of organism contamination (Figure 3).
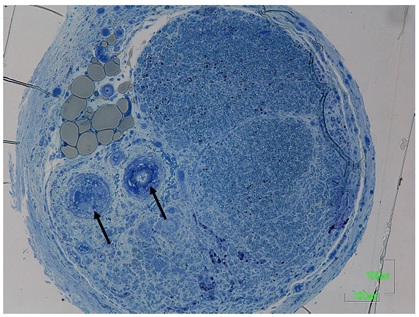 Figure 3: A transverse section taken from the most distal portion of the nerve regenerated through a silicone tube containing DABLs and BMSCs, which was transplanted to bridge a 20mm gap of rat sciatic nerve 24 weeks ago.
Figure 3: A transverse section taken from the most distal portion of the nerve regenerated through a silicone tube containing DABLs and BMSCs, which was transplanted to bridge a 20mm gap of rat sciatic nerve 24 weeks ago.
Arrows indicated the sural vessel pedicle.
Creation Of A Biodegradable And Flexible Tube Having Capillary Infiltrating Capability
Next, our attention was focused on creation of a biodegradable and flexible nerve conduit having capillary infiltration capability. Peripheral nerve injuries often occur in hands and fingers where there is thin subcutaneous tissue. The conduit should thus be biodegradable and flexible. If the tube wall has capillary infiltration capability, insertion of a vascular pedicle through the tubular lumen is not needed. If the site of transplantation is well vascularized, the tube may obtain vascularity from the surrounding tissue.
We were interested in a commercially available nerve induction tube, Nerbridge® (Toyobo CO. LTD, Osaka, Japan) [39-41]. This conduit is composed of an outer cylinder made of woven polyglycolic acid (PGA) fibers, giving flexibility to the tube, and an inner core made of collagen sponge. Nerbridge® is biodegradable and dissolves after about 3 months in vivo. The outer cylinder is semipermeable and passes particles less than 600 kDa, allowing capillaries to infiltrate the cylinder and reach the tubular lumen (Brochure Nerbridge® Version 1; http://www.toyobo-global.com/news/pdf,2018/08/press20180820atch.pdf). However, capillary invasion of the tubular lumen is also associated with water permeability (Figure 4).
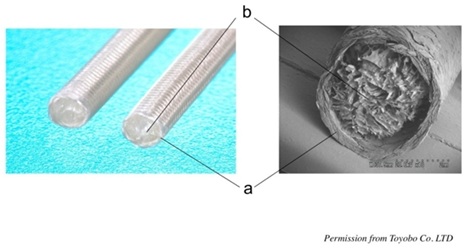 Figure 4: Right; a nerve induction tube “Nerbridge®” Toyobo Co. LTD, Osaka, Japan. Left: a scanning electron microscope picture of “Nerbridge®”. a) The outer cylinder made of meshed PGA fibers. b) The inner core made of collagen sponge. We used only the outer cylinder as a nerve conduit in our study.
Figure 4: Right; a nerve induction tube “Nerbridge®” Toyobo Co. LTD, Osaka, Japan. Left: a scanning electron microscope picture of “Nerbridge®”. a) The outer cylinder made of meshed PGA fibers. b) The inner core made of collagen sponge. We used only the outer cylinder as a nerve conduit in our study.
The role of a nerve conduit is to create an environment that provides a space for fibrin matrix formation, accumulation of neurochemical factors secreted from the nerve stumps, and axon regeneration. Ideally, the intrachamber space should be completely separated from the surrounding tissue. It is difficult to create a fibrin matrix structure in the tubular lumen in a water-permeable tube, especially with a long interstump gap. Neurochemical factors secreted from the nerve stumps may also leak from the tubular lumen through the tubular wall, resulting in poor nerve regeneration. In order to keep the fibrin matrix within a water-permeable nerve conduit, something that retains the fibrin matrix within the tubular lumen is necessary. We hypothesized that the intratubular DABLs may also play a role in retention of fibrin matrix in the space between the basal lamellae to which the extracellular matrix is attached. We expect the DABLs to act not only as a scaffold of nerve fiber regeneration but also to retain the transplanted BMSCs and fibrin matrix inside the tubular lumen.
Nerve Regeneration In The Water-Permeable Tube And Our Future Research
We created a nerve conduit composed of the PGA meshed filaments (outer cylindrical part of Nerbridge®) containing thermally created DABLs seeded with BMSCs and transplanted it to a 20-mm gap in a rat sciatic nerve. This conduit demonstrated successful nerve regeneration, which was almost equal to that of a 20-mm-long autologous nerve graft. From the perspective of clinical application of this conduit, chemically created DABLs would reduce the chance of organism contamination compared with the thermally created ones during the transplantation process [35-38]. We have recently created DABLs using surfactants combined with irradiation and started transplantation surgery. In the near future, we expect to report the outcome of nerve regeneration through a PGA-meshed tube containing chemically created DABLs seeded with BMSCs. We believe that this might be an optimal substitute for autologous nerve graft in peripheral nerve injury with an interstump gap (Figures 5 and 6).
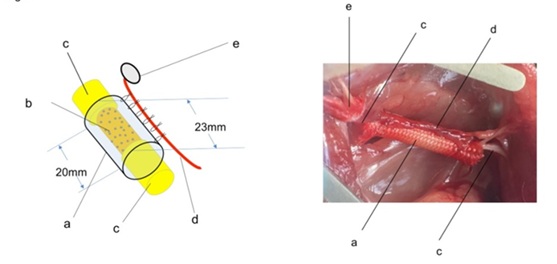 Figure 5 Left; a schematic diagram of a PGA-meshed tube containing DABLs and BMSCs. Right; an intraoperative photo of the PGA tube containing DABLs and BMSCs, which was transplanted to a 20mm gap of the sciatic nerve. a) PGA-meshed tube, b) DABLs implanted with BMSCs, c) transected sciatic nerve stumps, d) sural vessels, e) a monitor myocutaneous flap.
Figure 5 Left; a schematic diagram of a PGA-meshed tube containing DABLs and BMSCs. Right; an intraoperative photo of the PGA tube containing DABLs and BMSCs, which was transplanted to a 20mm gap of the sciatic nerve. a) PGA-meshed tube, b) DABLs implanted with BMSCs, c) transected sciatic nerve stumps, d) sural vessels, e) a monitor myocutaneous flap.
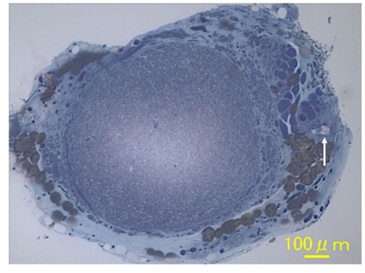 Figure 6: A transverse section taken from the most distal portion of the nerve regenerated through a PGA-meshed tube containing DABLs and BMSCs, which was transplanted to bridge a 20mm gap of rat sciatic nerve 24 weeks ago. Arrows indicated a sural vessel pedicle.
Figure 6: A transverse section taken from the most distal portion of the nerve regenerated through a PGA-meshed tube containing DABLs and BMSCs, which was transplanted to bridge a 20mm gap of rat sciatic nerve 24 weeks ago. Arrows indicated a sural vessel pedicle.
Conclusion
We created an artificial nerve conduit model using a tube containing a blood vessel pedicle and DABLs seeded with BMSCs. To bridge a long gap using artificial nerve conduits, intrachamber vascularity is essential. DABLs act as a retainer of fibrin matrix and transplanted BMSCs within the tube, and a scaffold for axon extension. However, the use of DABLs poses a risk of organism contamination. The optimal amount of transplanted BMSCs is not known. We demonstrated that some transplanted BMSCs differentiated into cells with glial cell markers in our nerve conduits. However, it is not known if the cells demonstrating glial cell markers actually function to facilitate nerve regeneration like normal Schwann cells.
Funding
We received no funding or grants with regard to this article.
References
- Lundborg G, Gelberman RH, Longo FM, Powell HC, Varon S (1982) In vivo regeneration of cut nerves encased in silicone tubes: Growth across a six-millimeter gap. J Neuropathol Exp Neurol 41: 412-422.
- Dahlin LB, Lundborg G (2001) Use of tubes in peripheral nerve repair. Neurosurg Clin North Am 12: 341-352.
- Williams LR, Longo FM, Powell HC, Lundborg G, Varon S (1983) Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay. J Comp Neurol 218: 460-470.
- Meek MF, Coert JH (2008) US Food and Drug Administration/Conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann Plast Surg 60: 466-472.
- Ray WZ, Mackinnon SE (2010) Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol 223: 77-85.
- Lundborg G, Dahlin LB, Danielsen N, Gelberman RH, Longo FM, et al. (1982) Nerve regeneration in silicone chambers: Influence of gap and of distal stump components. Exp Neurol 76: 361-375.
- Weddell G (1942) Axonal regeneration in cutaneous nerve plexuses. J Anat 77: 49-62.
- Nukada H (1988) Post-traumatic endoneurial neovascularization and nerve regeneration: A morphometric study. Brain Res 449: 89-96.
- Weerasuriya A (1988) Pattern of change in endoneurial capillary permeability and vascular space during Wallerian degeneration. Brain Res 445: 181-187.
- Weerasuriya A (1990) Pattern of change in endoneurial capillary permeability and vascular space during nerve regeneration. Brain Res 510: 135-139.
- Hobson MI, Brown R, Green CJ, Terenghi G (1997) Inter-relationships between angiogenesis and nerve regeneration: A histochemical study. Br J Plast Surg 50: 125-131.
- Hobson MI, Green CJ, Terenghi G (2000) VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat 197: 591-605.
- Zhao B, Zhao Z, Ma J, Ma X (2019) Modulation of angiogenic potential of tissue-engineered peripheral nerve by covalent incorporation of heparin and loading with vascular endothelial growth factor. Neurosci Lett 705: 259-264.
- Aebischer P, Guénard V, Brace S (1989) Peripheral nerve regeneration through blind-ended semipermeable guidance channels: Effect of the molecular weight cutoff. J Neurosci 9: 3590-3595.
- Kakinoki R, Nishijima N, Ueba Y, Oka M, Yamamuro T (1995) Relationship between axonal regeneration and vascularity in tabulation-an experimental study in rats. Neurosci Res 23: 35-45.
- Kakinoki R, Nishijima N, Ueba Y, Oka M, Yamamuro T, et al. (1998) Nerve regeneration over a 20-mm gap through a nerve conduit containing blood vessels in rats: The influence of interstump distance on nerve regeneration. J Neurosurg Sci 42: 11-21.
- Kakinoki R, Nishijima N, Ueba Y, Oka M, Yamamuro T, et al. (1997) Nerve regeneration over a 25 mm gap in rat sciatic nerves using tubes containing blood vessels: The possibility of clinical application. Int Orthop 21: 332-336.
- Yamakawa T, Kakinoki R, Ikeguchi R, Nakayama K, Morimoto Y, et al. (2007) Nerve regeneration promoted in a tube with vascularity containing bone marrow-derived cells. Cell Transplant 16: 811-822.
- Kaizawa Y, Kakinoki R, Ikeguchi R, Ohta S, Noguchi T, et al. (2016) Bridging a 30 mm defect in the canine ulnar nerve using vessel-containing conduits with implantation of bone marrow stromal cells. Microsurgery 36: 316-324.
- Mackenzie TC, Flake AW (2001) Human mesenchymal stem cells persist, demonstrate site-specific multipotential differentiation, and are present in sites of wound healing and tissue regeneration after transplantation into fetal sheep. Blood Cells Mol Dis 27: 601-604.
- Bittira B, Shum-Tim D, Al-Khaldi A, Chiu RCJ (2003) Mobilization and homing of bone marrow stromal cells in myocardial infarction. Eur J Cardiothorac Surg 24: 393-398.
- Wu GD, Nolta JA, Jin YS, Barr ML, Yu H, et al. (2003) Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation 75: 679-685.
- Wang J, Ding F, Gu Y, Liu J, Gu X (2009) Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Res 1262: 7-15.
- Crigler L, Robey CR, Asawachaicharn A, Gaupp D, Phinney DG (2006) Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol 198: 54-64.
- Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY, et al. (2007) Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol 204: 443-453.
- Fansa H, Keilhoff G, Plogmeier K, Frerichs O, Wolf G, et al. (1999) Successful implantation of Schwann cells in acellular muscles. J Reconstr Microsurg 15: 61-65.
- Tohill M, Mantovani C, Wiberg M, Terenghi G (2004) Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett 362: 200-203.
- Nijhuis TH, Brzezicki G, Klimczak A, Siemionow M (2010) Isogenic venous graft supported with bone marrow stromal cells as a natural conduit for bridging a 20 mm nerve gap. Microsurgery 30: 639-645.
- Siemionow M, Duggan W, Brzezicki G, Klimczak A, Grykien C, et al. (2011) Peripheral nerve defect repair with epineural tubes supported with bone marrow stromal cells: a preliminary report. Ann Plast Surg 67: 73-84.
- Chen YS, Hsieh CL, Tsai CC, Chen TH, Cheng WC, et al. (2000) Peripheral nerve regeneration using silicone rubber chambers filled with collagen, laminin and fibronectin. Biomaterials 21: 1541-1547.
- Cuevas P, Carceller F, Dujovny M, Garcia-Gómez I, Cuevas B, et al. (2002) Peripheral nerve regeneration by bone marrow stromal cells. Neurol Res 24: 634-638.
- Jia H, Wang Y, Tong XJ, Liu GB, Li Q, et al. (2012) Sciatic nerve repair by acellular nerve xenografts implanted with BMSCs in rats xenograft combined with BMSCs. Synapse 66: 256-269.
- Ide C, Tohyama K, Yokota R, Nitatori T, Onodera S (1998) Schwann cell basal lamina and nerve regeneration. Brain Res 288: 61-75.
- Kaizawa Y, Kakinoki R, Ikeguchi R, Ohta S, Noguchi T, et al. (2017) A Nerve Conduit Containing a Vascular Bundle and Implanted With Bone Marrow Stromal Cells and Decellularized Allogenic Nerve Matrix. Cell Transplant 16: 215-228.
- Mackinnon SE, Hudson AR, Falk RE, Kline D, Hunter D (1984) Peripheral nerve allograft: an assessment of regeneration across pretreated nerve allografts. Neurosurgery 15: 690-693.
- Sondell M, Lundborg G, Kanje M (1998) Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res 795: 44-54.
- Haase SC, Rovak JM, Dennis RG, Kuzon WM Jr, Cederna PS (2003) Recovery of muscle contractile function following nerve gap repair with chemically acellularized peripheral nerve grafts. J Reconstr Microsurg 19: 241-248.
- Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, et al. (2004) Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng 10: 1641-1651.
- Inada Y, Hosoi H, Yamashita A, Morimoto S, Tatsumi H, et al. (2007) Regeneration of peripheral motor nerve gaps with a polyglycolic acid-collagen tube: technical case report. Neurosurgery 61: 1105-1107.
- Ichihara S, Inada Y, Nakamura T (2008) Artificial nerve tubes and their application for repair of peripheral nerve injury: an update of current concepts. Injury 39: 29-39.
- Yamanaka T, Hosoi H, Murai T, Kobayashi T, Inada Y, et al. (2014) Regeneration of the nerves in the aerial cavity with an artificial nerve conduit –reconstruction of chorda tympani nerve gaps–. PLoS One 9: 92258.
Citation: Kakinoki R, Kaizawa Y, Tanaka H, Akagi M (2021) Artificial Nerve Conduits with Stem Cell Transplantation used for Bridging a Long Nerve Gap: A Review of Experimental Studies in Rats. J Stem Cell Res Dev Ther 7: 068.
Copyright: © 2021 Ryosuke Kakinoki, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

