
Associating Insulin Instillation with Negative Pressure Wound Therapy Reduces Insulin Resistance in Diabetic Patients with Infected Foot Wounds
*Corresponding Author(s):
Xin-long ChenBurn And Plastic Surgery Department, Sanya Central Hospital (Hainan Third People’s Hospital), Sanya, Hainan Province, China
Tel:+86-21-2507-0600,
Email:cxlcxlzxy@hotmail.com
Abstract
In this work we investigated whether a joint treatment with insulin instillation and negative pressure wound therapy (NPWT) would reduce insulin resistance (IR) in diabetic patients with infected diabetic foot ulcers (DFUs) and improve their healing. We also studied the effects of insulin+NPWT treatment on the inflammatory response coupled with wound healing. Seventy-five diabetic patients with infected DFUs were recruited and randomly divided into equal (n=25) groups treated respectively with NPWT alone, NPWT+insulin, and conventional dressings (controls). Thereafter, the ulcers’ healing progress was assessed. The serum levels of Tumor Necrosis Factor-α (TNF-α), Interleukin-2 (IL-2), and Interleukin-6 (IL-6) were measured at 1 day before and at 7, 14, and 21 days after each treatment using specific double-antibody sandwich enzyme-linked immunosorbent assays (ELISA). Serum glucose was measured via a glucose-oxidase method, and serum insulin via radioimmunoassay. In each patient, IR was appraised via the Homeostasis Model Assessment (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI). Our results showed that at 7, 14 and 21 days post-treatment, with respect to the control group the two NPWT-treated groups had (i) significantly (p < 0.05) lower serum levels of TNF-α, IL-2 and IL-6; (ii) a significantly (p < 0.01) greater reduction in IR; and (iii) significantly (p < 0.05) higher wound healing rates. Moreover, at 14- and 21-days post-treatment, the NPWT+insulin-treated group had a significantly (p < 0.05) higher wound healing rate than the NPWT alone-treated group.
In conclusion, the combined NPWT+insulin instillation treatment gave superior results by improving wound healing, decreasing inflammation at the wounds surface, and reducing IR in diabetic patients with infected DFUs.
Keywords
Diabetic foot wound; Insulin; Insulin resistance; Negative pressure wound therapy; Wound healing
ABBREVIATIONS
DFUs: Diabetic foot ulcers; ELISA: Enzyme-linked immunosorbent assay; FBG: Fasting blood glucose; HOMA: Homeostasis Model Assessment; IGF-1: Insulin-like growth factor-1; IL-6: Interleukin- 6; IR: Insulin resistance; IRI: Insulin resistance index; NPWT: Negative pressure wound therapy; QUICKI: Quantitative Insulin Sensitivity Check Index; T2DM: Type 2 diabetes mellitus; TNF-α: Tumor Necrosis Factor-α
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a worldwide public health problem that affects the quality of life and increases mortality rates. It is estimated that throughout the world the number of people suffering from T2DM will increase from 246 million as in 2012 [1] to 592 million by 2035 [2]. The T2DM prevalence has rapidly increased in China from 1% as in 1980 to 5% in 2001[3] and to 9.7% in 2008, thus affecting about 92.4 million adults [4]. Diabetic foot ulcers (DFUs) are a global health problem being the main cause of hospitalization for diabetic patients. In recent years, improvements in T2DM therapy and its reinforced guidelines have curtailed the rate of limb amputations [5]. Furthermore, the increased knowledge of the clinical approaches to DFUs has made available several medical options aimed at ensuring the best local conditions and wounds healing.
Over the past years, various studies have been focusing on the usefulness of the negative pressure wound therapy (NPWT) for the treatment of chronic wounds, DFUs included [6-10]. NPWT is a non-invasive therapeutic approach that promotes wound healing through the controlled use of a negative (sub-atmospheric) pressure produced by a vacuum device. NPWT removes fluids exuding from open wounds via a sealed dressing or a foam dressing connected to a collecting container [11].
NPWT’s application to wound healing became popular over a decade ago [12,13]. NPWT’s positive effects on wounds healing were demonstrated by both laboratory investigations [13,14] and a multicenter randomized controlled study carried out in diabetic patients that had undergone partial amputations [6].
Improvements on traditional NPWT devices now allow to combine it with fluid instillation therapy. The latter aims at reducing the bioburden within the wound thus advancing the healing process. Moreover, instillation therapy helps control pain in selected cases [15-17].
The solution currently used for the instillation therapy combined with NPWT has been hitherto restricted to the normal saline. Solutions of insulin, phenytoin (Dilantin), sodium hypochlorite (Dakin’s solution), and polyhexanide (Prontosan, Braun Medical) have been suggested as being therapeutically useful [7, 18, 19]. Insulin has long been recognized as an important hormone contributing to wound repair [20]. Various studies have shown the beneficial effects of insulin to wound healing [21-24]. In their study involving the systemic administration of insulin to nondiabetic burnt patients, Pierre and associates [22] found that chronically administering insulin at high doses together with glucose significantly decreased the donor sites’ healing time by two to three days and improved wounds’ structural matrix formation and protein kinetics. Lopez and Mena [25] reported the use of local insulin irrigation (30-60 IU daily of isophane insulin) in two cases of diabetic infectious gangrene which were resistant to all the other current therapies. They found that the insulin irrigation treatment accelerated wound healing with no systemic side effects worth paying attention to. Greenway et al. [24] published results from a randomized double-blind placebo-controlled trial that investigated the relative roles of insulin (Iletin-II) and zinc in accelerating the healing of the forearm wounds of diabetic and non-diabetic human subjects. The investigators concluded that the topical application of insulin hastened wounds healing in both groups of patients. Wilson et al. [26] reported the case of an 80-year-old woman in which a post-laparotomy chronic nonhealing wound was topically irrigated with 20 ml normal saline plus 2 IU of human soluble insulin (Actrapid) after all conventional dressing attempts and a three-week NPWT alone course had failed. The authors observed a visible improvement in wound healing after seven days of irrigation with normal saline plus insulin with no concomitant systemic side effects, e.g. hypoglycemia. Although a certain degree of IR persisted, the skin tissue of the diabetic patient showed a prompt insulin responsiveness, which could be ascribed to the cellular and molecular mechanisms driven by insulin to speed the diabetic patient’s wound healing up. In a double-blind randomized placebo-controlled trial, Rezvani and colleagues [27] evaluated the effects of topical insulin on wound healing and concluded that the healing rates in the insulin treated group were higher than in the controls, regardless of the initial wound dimensions, while no major systemic side effects occurred. Similarly, the topical application of a low dose of insulin stimulated wound healing in rats affected by type II diabetes [28]. Also, the systemic administration of insulin remarkably increased the phosphorylated AKT levels in the skin, but not in the liver, of diabetic rats [28]. Moreover, the results of a study by Tianyi Yu and colleagues [29] using insulin-resistant diabetic rats revealed in the diabetic skin tissue the presence of a subtle responsiveness to insulin, regardless of any coexisting insulin sensitivity impairment, which could result from the insulin-driven cellular and molecular mechanisms accelerating wound healing.
IR is a complex metabolic defect that has several causes depending on the ongoing pathophysiologic conditions. Patients with T2DM have a genetic component promoting the IR (or decreased insulin sensitivity). It has been suggested that among the contributory factors are inherited defects in mitochondrial function entailing a decreased capacity to oxidize fatty acids [30]. In addition, obesity is an IR major cause in T2DM and up-to-date information proves that a chronic, low-grade inflammation that associates with obesity is an important mechanism decreasing the signaling of insulin. In this regard, it is known that inflammation causes a cell-autonomous IR in muscle, hepatic, and adipose tissues, and that activated tissue macrophages can drive tissue-autonomous inflammatory processes [31]. Although so far IR’s causative factors have not been fully identified, it is widely accepted that oxidative stress, inflammation, and genetic, behavioral, environmental, and other epigenetic factors play a significant role in IR [32]. Therefore, IR is one of the most typical and common pathological occurrences in T2DM patients with infected DFUs.
In the present study, we evaluated the effects of NPWT alone and combined with insulin instillation on IR and wound healing rates in T2DM patients with infected DFUs.
MATERIALS AND METHODS
This randomized control study was performed in the Department of Burn and Plastic Surgery at the Third People's Hospital of Hainan Province in P. R. China. The study population included 75 diabetic patients aged between 20 and 70 years, with stage 2 or 3 DFUs, as defined according to Agner’s classification [33]. The patients were randomly divided (n=25/group) into
- • the NPWT+insulin treatment group;
- • the NPWT alone-treated group; and
- • the control (standard dressings) group. The Medical Ethics Committee of the Third People's Hospital of Hainan Province reviewed and approved the present study. Patients or their next-of-kin signed the corresponding informed consent forms. The study did not include patients aged less than 20 years or more than 70 years; pregnant women; nursing mothers; and subjects suffering from peripheral vascular diseases or from comorbidities involving the respiratory or cardiovascular or other bodily systems. Moreover, the study excluded people on medications such as corticosteroids or immunosuppressive or chemotherapeutic agents. Data related to the detailed history, clinical examination, and relevant laboratory investigations were collected for all the patients.
Initially, the ulcers of all the patients involved in the study underwent a sharp surgical debridement that was repeated during each subsequent dressing change to remove necrotic and sloughing off tissues. After carrying the debridement out under strict aseptic conditions in an operating room, the wounds of all the study patients were covered with a foam-based dressing. In their turn, the foam-based dressings were covered with an adhesive tape to create airtight seals. An evacuation tube embedded in the foam-based dressings was connected to a negative pressure drainage device (Shandong Weigao New Life Medical Devices Co., Ltd., Weihai, China). Next, a sub-atmospheric (negative) pressure whose values ranged from 80 to 125 mmHg was applied on a continuous basis for three weeks. The vacuum device was changed once weekly. The NPWT and compressive dressings were applied exclusively during the treatment phase. Wounds irrigation with insulin solution used the doses defined by Wilson and associates [26]. We ran the infusion drip into the NPWT foam at 40 ml/h for three weeks. The conventional dressings group (control) received saline-soaked gauze dressings once daily. Standard antibiotic regimens were administered to all patients. They initially consisted of broad-spectrum antibiotics and later of antibiotics identified by culture sensitivity reports. Blood glucose levels were strictly monitored during the treatments and kept under control by systemic administrations of proper doses of insulin.
During the drainage period, in both the NPWT alone- and the NPWT+insulin-treated groups the wounds were rinsed daily with copious amounts of saline. The NPWT procedure was carried out for three weeks in both groups. The wound healing rate was determined in terms of percentage of ulcer’s area reduction in each of the three groups of patients at the end of each week according to the formula:
Ulcer area at week 0 - ulcer area at week “X”/ulcer area at week 0×100
The mean ulcer size reductions pertaining to each group are reported in Tables.
Assay of IL-6, IL-2, and TNF-α serum levels
To keep track of any changes in the levels of proinflammatory factors, venous blood (5 ml) samples were drawn in the morning from the upper limbs of fasting patients at 1 day before and at 7, 14, and 21 days after the onset of treatments. The serum levels of IL-6, IL-2, and TNF-α were measured using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA). The kits used were bought from Shanghai Beyotime Biotechnology Inc. (Shanghai, China).
Measurement of biochemical parameters
While the hyperinsulinemic euglycemic clamp technique is the gold standard to evaluate insulin sensitivity, the Homeostasis Model Assessment (HOMA) for IR (HOMA-IR) and the QUICKI are widely used as noninvasive surrogate markers of IR and insulin sensitivity, respectively [34,35]. At baseline, in the morning following an overnight fast, venous blood was sampled to assess the serum concentration of glucose via a glucose-oxidase method. Serum insulin was measured via radioimmunoassay. In each patient, the IR’s degree was estimated via the HOMA-IR and QUICKI according to the method described by Matthews et al. [34-37]. Low HOMA-IR values reveal a high insulin sensitivity, whereas high HOMA-IR values are signs of a low insulin sensitivity (IR). The formula we used to this purpose was:
QUICKI = 1/[log fasting insulin (mU/L) + log fasting glucose (mg/dL)] [36].
Statistical analysis
Data were processed and analyzed using the SPSS 20.0 statistical software package (IBM SPSS, Armonk, NY, USA). Numerical data were expressed as mean values ± SDs. Intergroup comparisons before and after treatments were performed by t-tests, while paired t-tests were used for intra-group comparisons.
RESULTS
Patients’ general data
Patients’ age, sex ratio, and wound areas were comparable among the three groups since the corresponding differences were not statistically significant (p > 0.05) (Table 1). No patient of the three groups withdrew from the study during the entire treatment procedure, and no patient was lost to follow-up because of adverse events.
|
Clinical characteristics |
Control group |
NPWT group |
NPWT+insulin group |
P |
|
Men/women |
12/13 |
12/13 |
13+12 |
>0.05 |
|
Age (years) |
58.6±5.3 |
57.9±6.3 |
58.1±7.4 |
>0.05 |
|
Diabetes duration (years) |
15.0 ± 5.4 |
14.7 ± 4.8 |
14.9±2.6 |
>0.05 |
|
Days with ulcer |
54.1±6.9 |
52.16±5.3 |
53.6±4.8 |
>0.05 |
|
Ulcer area (cm2) |
) 9.38±1.2 |
8.90±1.2 |
9.15±1.1 |
>0.05 |
|
Wagner stage III (n) |
13 |
13 |
12 |
>0.05 |
|
Wagner stage IV (n) |
12 |
12 |
13 |
>0.05 |
|
Local infection (%) |
100 |
100 |
100 |
>0.05 |
Data are mean values ± SD. None of the parameters are significantly different among the three groups investigated. Wagner stage III: Deep ulcer with infection. Wagner stage IV: Ulcer with gangrene of 1-2 toes.
Table 1: Clinical characteristics of the study subjects (n=25 for each group).
Changes in the levels of proinflammatory factors
No statistically significant (p > 0.05) differences in the serum levels of the proinflammatory mediators IL-6, IL-2, and TNF-α occurred at 1 day before the treatments in the three groups. At 7, 14, and 21 days after the treatment’s onset,
- the levels of the three proinflammatory mediators were all significantly (p < 0.05) lower than they were at 1 day before the treatments in the three groups of patients; and
- in both the combined NPWT+insulin and the NPWT-alone treatment group the levels of three pro inflammatory mediators were significantly (p < 0.05) lower than in the control group (Tables 2, 3 and 4).
|
Controls |
NPWT alone |
NPWT+insulin |
P |
|
|
1 day before treatment |
94.37±12.56 |
95.64±13.56 |
96.34±11.64 |
>0.05 |
|
7 days after treatment |
68.64±8.91 |
58.34±6.98 |
59.39±10.26 |
<0.05 |
|
14 days after treatment |
60.52±7.62 |
50.23±8.95 |
49.36±9.13 |
<0.05 |
|
21 days after treatment |
55.69±6.89 |
43.69±7.28 |
39.62±8.16 |
<0.05 |
Table 2: Serum levels of IL-6 before and after treatment in the three groups (pg/ml; n=25/group).
|
Controls |
NPWT alone |
NPWT+insulin |
P |
|
|
1 day before treatment |
28.54±2.16 |
27.69±3.20 |
30.21±4.21 |
>0.05 |
|
7 days after treatment |
20.08±1.52 |
15.68±2.56 |
16.16±2.66 |
<0.05 |
|
14 days after treatment |
16.39±3.12 |
12.78±1.95 |
11.25±1.69 |
<0.05 |
|
21 days after treatment |
15.26±2.94 |
11.67±1.19 |
10.39±2.10 |
<0.05 |
Table 3: Serum levels of IL-2 before and after treatment in the three groups (pg/ml; n=25/group).
|
Controls |
NPWT alone |
NPWT+insulin |
P |
|
|
1 day before treatment |
224.43±24.58 |
230.56±22.69 |
229.67±20.69 |
>0.05 |
|
7 days after treatment |
181.32±22.86 |
131.36±16.39 |
128.34±19.67 |
<0.05 |
|
14 days after treatment |
169.32±15.89 |
105.26±9.89 |
99.36±18.62 |
<0.05 |
|
21 days after treatment |
159.98±16.45 |
98.64±10.23 |
88.31±15.68 |
<0.05 |
Table 4: Serum levels of TNF-α before and after treatment in the three groups (pg/ml; n=25/group).
Wound areas and healing rates in the three groups
The residual wound areas and wound healing rates were compared among the patients of the three groups. The observed differences among the three groups were all statistically significant (p < 0.05) at 7, 14, and 21 days after the treatments’ onset (Tables 5, 6, and 7) (Figures 1, 2, and 3).
|
Controls |
NPWT alone |
NPWT+insulin |
P |
|
|
Residual wound area (cm2) |
15.32±1.46 |
12.69±1.96 |
12.06±2.19 |
<0.05 |
|
Wound healing rate (%) |
22.50±4.75 |
33.65±3.46 |
35.36±2.06 |
>0.05 |
Table 5: Wound healing at 7 days post-treatment in the three groups (n=25/group).
|
Controls |
NPWT alone |
NPWT+insulin |
P |
|
|
Residual wound area (cm2) |
12.32±1.46 |
8.69±1.66 |
5.36±0.98 |
>0.05 |
|
Wound healing rate (%) |
30.50±4.75 |
45.39±3.61 |
55.36±3.98 |
>0.05 |
Table 6: Wound healing at 14 days post-treatment in the three groups (n=25/group).
|
Controls |
NPWT alone |
NPWT+insulin |
P |
|
|
Residual wound area (cm2) |
10.15±2.09 |
5.69±1.69 |
3.29±1.03 |
>0.05 |
|
Wound healing rate (%) |
41.63±3.98 |
68.3±3.46 |
81.69±3.61 |
>0.05 |
Table 7: Wound healing at 21 days post-treatment in the three groups (n=25/group).
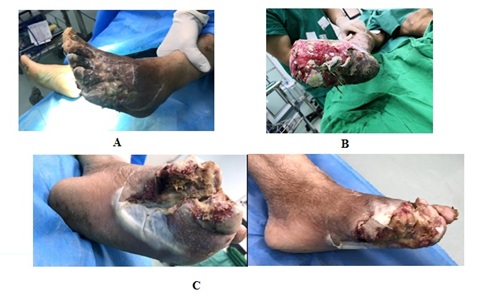 Figure 1: Wound aspects before treatments’ onset in the three groups of patients. A: Wound’s appearance prior to treatment in a patient of the control group. B: Wound’s aspect in advance of treatment in a patient of the NPWT alone group. C: Wound’s semblance ahead of treatment in a patient of the NPWT+insulin group.
Figure 1: Wound aspects before treatments’ onset in the three groups of patients. A: Wound’s appearance prior to treatment in a patient of the control group. B: Wound’s aspect in advance of treatment in a patient of the NPWT alone group. C: Wound’s semblance ahead of treatment in a patient of the NPWT+insulin group.
 Figure 2: Wound healing at 7 days post-treatment in the three groups of patients.
Figure 2: Wound healing at 7 days post-treatment in the three groups of patients.
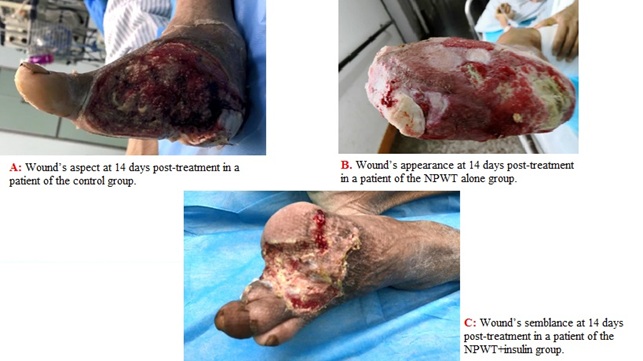 Figure 3: Wound healing at 14 days post-treatment in the three groups of patients.
Figure 3: Wound healing at 14 days post-treatment in the three groups of patients.
Fasting blood glucose, insulin, HOMA-IR, and QUICKI in the three groups
Fasting serum glucose, insulin levels, HOMA-IR, and QUICKI did not significantly differ among three groups at 1 day before the treatments (Table 8).
|
Controls |
NPWT alone |
NPWT+insulin |
P |
|
|
FBG (mmol/l) |
10.7 ± 0.6 |
11.3 ± 0.8 |
11.1 ± 0.9 |
>0.05 |
|
Insulin (pmol/l) |
123.6 ± 3.6 |
118.9 ± 2.6 |
111.7 ± 3.4 |
>0.05 |
|
HOMA-IR |
58.9 ± 6.7 |
59.7 ± 8.6 |
55.1 ± 6.2 |
>0.05 |
|
QUICKI |
0.32 (0.28, 0.39) |
0.32 (0.27, 0.38) |
0.32 (0.29, 0.36) |
>0.05 |
Except where otherwise indicated, values are means (95% confidence interval) ±SD. FBG: fasting blood glucose. QUICKI: Quantitative Insulin Sensitivity Check Index (IRI) = 1/[log (Insulin) + log (FBG)]. Homeostasis Model Assessment for insulin resistance (HOMA-IR) value = IRI×glucose/22.5.
Table 8: Fasting blood glucose, insulin, HOMA-IR, and QUICKI at 1 day before treatment in the three groups (n=25/group).
In these patients, significant decreases in serum insulin (p < 0.01), and improvements in HOMA-IR and QUICKI (p < 0.01) values were seen with the treatments. At 7, 14, and 21 days after the treatment’s onset, the patients of the NPWT+insulin and NPWT alone treatment groups had a lower fasting serum insulin level (p < 0.01) than did the patients of the control group (Figure 4) (Tables 9, 10, and 11).
|
Controls |
NPWT alone |
NPWT+insulin |
P |
|
|
FBG (mmol/l) |
9.9±0.7 |
9.1 ±0.6 |
9.3±0.8 |
>0.05 |
|
Insulin (pmol/l) |
108.6±14.5 |
58.7±2.9 |
60.6±2.4 |
>0.05 |
|
HOMA-IR |
47.8±5.8 |
30.8±3.2 |
30.7±2.98 |
>0.05 |
|
QUICKI |
0.33 (0.30, 0.36) |
0.37(0.32, 0.53) |
0.36 (0.32, 0.51) |
>0.05 |
Except where otherwise indicated, values are means (95% confidence interval) ±SD. FBG: fasting blood glucose. QUICKI: Quantitative Insulin Sensitivity Check Index (IRI) = 1/[log (Insulin) + log(FBG)]. HOMA for IR (HOMA-IR) value = IRI×glucose/22.5.
Table 9: Fasting blood glucose, insulin, HOMA-IR, and QUICKI at 7 days post-treatment in the three groups (n=25).
|
Controls |
NPWT alone |
NPWT+insulin |
P |
|
|
FBG (mmol/l) |
9.8±0.5 |
8.6±0.6 |
8.4 ±0.7 |
>0.05 |
|
Insulin (pmol/l) |
101.8 ±6.6 |
38.6±2.9 |
22.2±3.1 |
>0.05 |
|
HOMA-IR |
44.3 ±4.5 |
14.8±3.3 |
8.3 ±1.1 |
>0.05 |
|
QUICKI |
0.33 (0.28, 0.46) |
0.40(0.36, 0.60) |
0.44 (0.28, 0.65) |
>0.05 |
Except where otherwise indicated, values are means (95% confidence interval) ±SD. FBG: fasting blood glucose. QUICKI: Quantitative Insulin Sensitivity Check Index (IRI) = 1/[log (Insulin) + log (FBG)]. HOMA-IR value = IRI × glucose/22.5.
Table 10: Fasting blood glucose, insulin, HOMA-IR, and QUICKI at 14 days post-treatment in the three groups (n=25/group).
|
Controls |
NPWT alone |
NPWT+insulin |
P |
|
|
FBG (mmol/l) |
9.5±0.4 |
8.2±0.5 |
8.3 ±0.3 |
>0.05 |
|
Insulin (pmol/l) |
96.3 ±9.4 |
18.6±5.8 |
9.4±2.1 |
>0.05 |
|
HOMA-IR |
40.7 ±6.3 |
6.8±1.3 |
3.5 ±0.62 |
>0.05 |
|
QUICKI |
0.34 (0.27, 0.45) |
0.46(0.56, 0.64) |
0.53 (0.60, 0.69) |
>0.05 |
Except where otherwise indicated, values are means (95% confidence interval) ±SD. FBG: fasting blood glucose. QUICKI: Quantitative Insulin Sensitivity Check Index (IRI) = 1/[log (Insulin) + log (FBG)]. HOMA-IR = IRI × glucose/22.5.
Table 11: Fasting blood glucose, insulin, HOMA-IR, and QUICKI at 21 days post-treatment in the three groups (n=25/group).
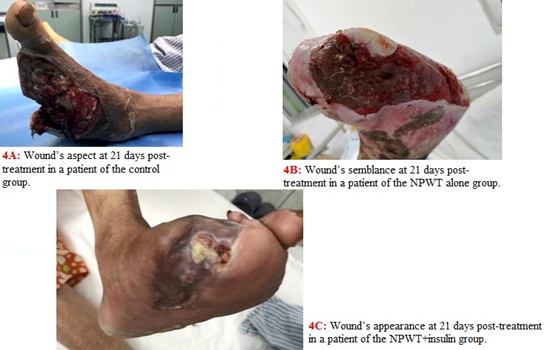 Figure 4: Wound healing at 21 days post-treatment in the three groups of patients.
Figure 4: Wound healing at 21 days post-treatment in the three groups of patients.
At 7, 14, and 21 days after the treatments onset, NPWT resulted in a significant reduction in serum insulin (p < 0.01) and in improvements in HOMA-IR (p < 0.01) and QUICKI (p < 0.01) in the diabetic patients with infected DFUs. Even applying NPWT for only 1 week significantly improved insulin sensitivity as proved by the reduced HOMA-IR values and the increased QUICKI values as compared with the control patients.
These findings showed that NPWT can improve insulin sensitivity in diabetic patients with infected DFUs. They also suggest that NPWT’s metabolic effects may be more pronounced in this category of patients.
DISCUSSION
IR has long been held as an essential feature and a major hallmark of the etiology and pathogenesis of T2DM. IR consists in the inability of classic insulin targets, like adipose tissue, liver, skin, [38] and skeletal muscle to respond to insulin [38]. IR was detectable at 1 day before and at 7, 14, and 21days after the onset of treatments in diabetic patients with infected DFUs. Most often, IR development associates with low-grade tissue-specific inflammatory responses induced by various proinflammatory and/or oxidative stress mediators. Of note, these include proinflammatory cytokines such as IL-1β, IL-6, TNF-α, and a set of chemokines and adipokines [39-41]. Indeed, our results show that the levels of proinflammatory cytokines such as TNF-α, IL-6, and IL-2 were high at 1 day before treatments. Chronic exposure to proinflammatory mediators stimulates the activation of cytokine signaling pathways which eventually block the activation of insulin receptors signaling in the β-cells of pancreatic islets [39]. In the clinical settings, the altered function of insulin target cells combines with the effects of circulating antagonists of insulin, and with the accumulation of macrophages that secrete proinflammatory mediators advancing IR [42]. At the molecular level, both a reduction in insulin receptors expression by the target cells and an impairment of the insulin dependent PI3K activation and of its downstream signaling play crucial roles in the pathological progression of IR [43]. Remarkably, over one hundred known pathologic factors could contribute to a defective wound healing [44]. The disruption of insulin’s signaling pathways, one of the most distinctive pathological changes of T2DM [45], might also negatively affect wound healing. In addition to the effect on glucose disposal, insulin plays a role in stimulating DNA synthesis and cell proliferation. Insulin dysfunction is frequently associated with proliferative tissue abnormalities at the level of the skin (acanthosis nigricans), ovary, and heart [46].
To our knowledge, this is the first study performed to evaluate NPWT’s effectiveness on IR in the treatment of infected DFUs in patients with T2DM. Importantly, we observed that a one-week treatment with NPWT (i) exerted a positive effect on wound healing; and (ii) signi?cantly (p < 0.01) decreased the serum levels of proinflammatory factors like TNF-α, IL-6, and IL-2 when administered either alone or in combination with insulin instillation to diabetic patients bearing infected DFUs.
Almost all the adipokines regulate metabolism and in?ammation and thus play a vital role in the pathogenesis of metabolic disorders, T2DM included [47,48]. TNF-α, IL-2, and IL-6 are proin?ammatory cytokines that not only immune cells, but also adipocytes produce. Therefore, increases in the levels of TNF-α, IL-2, and IL-6 associate with obesity and T2DM [49]. The present results showed that at 7, 14, and 21-day post-treatment the TNF-α, IL-2, and IL-6 serum levels had decreased when compared to the controls’ ones in the diabetic patients with infected DFUs belonging to both the NPWT alone and NPWT+insulin instillation treatment groups.
As seen in experimental animal wounds [50], the diminished levels of insulin or IGF-1 in the diabetic skin partially result from the increased activity of insulin-degrading enzymes. Such an activity causes an “invisible damage” to the diabetic skin, while also hindering diabetic wounds healing. Our results show that already after 1 week, as compared with the control patients, the NPWT±insulin instillation treatments significantly reduced serum insulin levels and improved HOMA-IR and QUICKI values in diabetic patients with infected DFUs. These same decreases could also be seen after 2 and 3 weeks in the both groups of treated patients as compared with the control group. Notably, insulin, which can be applied topically with minimal systemic side effects, successfully decreased the wound healing time. In an experimental diabetic wound model, insulin administration significantly increased the healing rate through the modulation of the inflammatory response and of tissue-repairing cellular functions [28,29]. After an injury, a topical insulin application rapidly induced the upregulation of insulin-related signaling proteins in the wounded areas [23, 24, 27, 28]. Evidence is also available that the systemic insulin administration increased the prevalence of biofilms in the wounds area of diabetic mice [51]. Remarkably, topical insulin application might be a promising therapeutic intervention because by activating insulin signaling it accelerates wound healing without any of the noxious effects that associate with the hormones’ systemic administration.
As previously noted, the NPWT+insulin instillation treatment was already effective after the first week of treatment, causing the wound’s symptoms to subside quickly. In addition, the same one-week treatment improved the wounds’ healing rate and shortened the time required for wounds’ epithelialization and closure. These findings strongly suggest that the insulin’s effect on epidermal cell growth promotion was enhanced by NPWT’s continuous negative pressure that improved local blood circulation while mitigating local inflammation.
Patients affected by refractory wounds experience strong local inflammatory responses sustained by elevated levels of IL-6, IL-2, and TNF-α [52]. Nasole et al. [52] reported that IL-6 and TNF-α, two key mediators of general inflammation, both directly and indirectly induce the production of other cytokines and growth factors thereby impacting on the function of endothelial cells and fibroblasts. IL-6 is necessary for epithelialization because of its role in granulation tissue formation, as the delayed wound healing process occurring in IL-6 knockout mice showed [53]. The healing of local wounds associates with a rapid reduction of local inflammation and normalization of proinflammatory factors levels. The upshot is a decreased decomposition of wound tissue proteins and the promotion of wound healing [54]. The present results proved that the NPWT+insulin instillation treatment was more effective than was the standard control treatment in reducing the levels of inflammatory factors.
In our patients, we saw a statistically significant post-treatment decrease in proinflammatory cytokines such as TNF-α, IL-6, and IL-2 and, concurrently, a reduction of IR’s values. Therefore, we posit that combining NPWT with insulin irrigation can change the infected wound status from an elevated risk to a negligible risk, while reducing IR and controlling inflammation. We proved our belief from a molecular biology standpoint. The NPWT+insulin irrigation treatment resulted more effective than NPWT alone in reducing the levels of proinflammatory factors. Certainly, when used alone, NPWT may also increase angiogenesis. However, adding insulin irrigation to NPWT advances DFUs healing by regulating wound’s inflammatory cells and tissue repair cell functions. From our perspective, combining NPWT with insulin irrigation is more beneficial than NPWT alone in treating diabetic patients with infected DFUs. Unquestionably, further studies will ascertain an insulin dose-effect relationship in the infected DFUs.
In conclusion, the insulin instillation+NPWT treatment improved wound healing, decreased the inflammatory response at the wound surface, and reduced IR’s values in diabetic patients with infected DFUs.
REFERENCES
- Garduno-Diaz SD, Khokhar S (2012) Prevalence, risk factors and complications associated with type 2 diabetes in migrant South Asians. Diabetes Metab Res Rev 28: 6-24.
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, et al. (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103: 137-149.
- Gu D, Reynolds K, Duan X, Xin X, Chen J, et al. (2003) Prevalence of diabetes and impaired fasting glucose in the Chinese adult population: International Collaborative Study of Cardiovascular Disease in Asia (InterASIA). Diabetologia 46: 1190-1198.
- Yang W, Lu J, Weng J, Jia W, Ji L, et al. (2010) Prevalence of diabetes among men and women in China. N Engl J Med 362: 1090-1101.
- Anichini R, Zecchini F, Cerretini I, Meucci G, Fusilli D, et al. (2007) Improvement of diabetic foot care after the Implementation of the International Consensus on the Diabetic Foot (ICDF): results of a 5-year prospective study. Diabetes Res Clin Pract 75: 153-158.
- Armstrong DG, Lavery LA, Diabetic Foot Study Consortium (2005) Negative pressure wound therapy after partial diabetic foot amputation: a multicenter randomized controlled trial. Lancet 366: 1704-1710.
- Meloni M, Izzo V, Vainieri E, Giurato L, Ruotolo V, et al. (2015) Management of negative pressure wound therapy in the treatment of diabetic foot ulcers. World J Orthop 6: 387-393.
- Guffanti A (2014) Negative pressure wound therapy in the treatment of diabetic foot ulcers: a systematic review of the literature. J Wound Ostomy Continence Nurs 41: 233-237.
- Zhang J, Hu ZC, Chen D, Guo D, Zhu JY, et al. (2014) Effectiveness and safety of negative-pressure wound therapy of diabetic foot ulcers: a meta-analysis. Plast Reconstr Surg 134: 141-151.
- Hasan MY, Teo R, Nather A (2015) Negative-pressure wound therapy for management of diabetic foot wounds: a review of the mechanism of action, clinical applications, and recent developments. Diabetic Foot Ankle 6: 27618.
- Nain PS, Uppal SK, Garg R, Bajaj K, Garg S (2011) Role of negative pressure wound therapy in healing of diabetic foot ulcers. J Surg Tech Case Rep 3: 17-22.
- Argenta LC, Morykwas MJ (1997) Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 38: 563-576.
- Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W (1997) Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 38: 553-562.
- Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC (2001) Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 47: 547-551.
- Wolvos T (2004) Wound instillation--the next step in negative pressure wound therapy. Lessons learned from initial experiences. Stomy Wound Manage 50: 56-66.
- Baxter CR (1994) Immunologic reactions in chronic wounds. Am J Surg 167: 12S-14S.
- Falanga V (1992) Growth factors and chronic wounds: the need to understand the microenvironment. J Dermatol 19: 667-672.
- Giovinco NA, Bui TD, Fisher T, Mills JL, Armstrong DG (2010) Wound Chemotherapy by the use of Negative Pressure Wound Therapy and Infusion. Eplasty 10: e9.
- Scimeca CL, Bharara M, Fisher TK, Kimbriel H, Mills JL, et al. (2010) Novel Use of Insulin in Continuous-Instillation Negative Pressure Wound Therapy as “Wound Chemotherapy”. J Diabetes Sci Technol 4: 820-824.
- Grewal RS, Gupta SC, Singhal GM, Gupta SN (1972) Wound healing in relation to insulin. Int Surg 57: 229-232.
- Rosenthal SP (1968) Acceleration of primary wound healing by insulin. Arch Surg 96: 53-55.
- Pierre EJ, Barrow RE, Hawkins HK, Nguyen TT, Sakurai Y, et al. (1998) Effects of insulin on wound healing. J Trauma 44: 342-345.
- Hanam SR, Singleton CE, Rudek W (1983) The effect of topical insulin on infected cutaneous ulcerations in diabetic and nondiabetic mice. J Foot Surg 22: 298-301.
- Greenway SE, Filler LE, Greenway FL (1999) Topical insulin in wound healing: a randomised, double-blind, placebo-controlled trial. J Wound Care 8: 526-528.
- Lopez JE, Mena B (1968) Local insulin for diabetic gangrene. Lancet 1: 1199.
- Wilson JM, Baines R, Babu ED, Kelley CJ (2008) A role for topical insulin in the management problematic surgical wounds. Ann R Coll Surg Engl 90: 160.
- Rezvani O, Shabbak E, Aslani A, Bidar R, Jafari M, et al. (2009) A randomized, double-blind, placebo-controlled trial to determine the effects of topical insulin on wound healing. Ostomy Wound Manage 55: 22-28.
- Li C, Yu T, Liu Y, Chen X, Zhang X (2015) Topical application of insulin accelerates vessel maturation of wounds by regulating angiopoietin-1 in diabetic mice. Int J Low Extrem Wounds 14: 353-364.
- Yu T, Gao M, Yang P, Pei Q, Liu D, et al. (2017) Topical insulin accelerates cutaneous wound healing in insulin-resistant diabetic rats. Am J Transl Res 9: 4682-4693.
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI (2004) Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664-671.
- de Luca C, Olefsky JM (2008) Inflammation and insulin resistance. FEBS Letters 582: 97-105.
- Ndisang JF, Vannacci A, Rastogi S (2017) Insulin Resistance, Type 1 and Type 2 Diabetes, and Related Complications 2017. J Diabetes Res 15: 1478294.
- Wagner FW (1981) The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 2: 64-122.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting serum glucose and insulin concentrations in man. Diabetologia 28: 412-419.
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, et al. (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85: 2402-2410.
- McAuley KA, Mann JI, Chase JG, Lotz TF, Shaw GM (2007) Point: HOMA--satisfactory for the time being: HOMA: the best bet for the simple determination of insulin sensitivity, until something better comes along. Diabetes Care 30: 2411-2413.
- Borai A, Livingstone C, Kaddam IB, Ferns G (2011) Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol 11: 158.
- O’Doherty R, Stein D, Foley J (1997) Insulin resistance. Diabetologia 40: B10-B15.
- Feve B, Bastard JP (2009) The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 5: 305-311.
- Hotamisligil GS (2003) Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord 27: S53-S55.
- Moller DE (2000) Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab 11: 212-217.
- Yang P, Pei Q, Yu T, Chang Q, Wang D, et al. (2016) Compromised wound healing in ischemic type 2 diabetic rats. PLoS One 11: e0152068.
- Choi K, Kim YB (2010) Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J Intern Med 25: 119-129.
- Brem H, Tomic-Canic M (2007) Cellular and molecular basis of wound healing in diabetes. J Clin Invest 117: 1219-1222.
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813-820.
- Geffner ME, Golde DW (1988) Selective insulin action on skin, ovary, and heart in insulin-resistant states. Diabetes Care 11: 500-505.
- Knights AJ, Funnell AP, Pearson RC, Crossley M, Bell-Anderson KS (2014) Adipokines and insulin action: a sensitive issue. Adipocyte 3: 88-96.
- Kwon H, Pessin JE (2013) Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne) 4: 71.
- Tilg H, Moschen AR (2006) Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772-783.
- Duckworth WC, Fawcett J, Reddy S, Page JC (2004) Insulin-degrading activity in wound fluid. J Clin Endocrinol Metab 89: 847-851.
- Watters C, Everett JA, Haley C, Clinton A, Rumbaugh KP (2014) Insulin treatment modulates the host immune system to enhance pseudomonas aeruginosa wound biofilms. Infect Immun 82: 92-100.
- Nasole E, Nicoletti C, Yang ZJ, Girelli A, Rubini A, et al. (2014) Effects of alpha lipoic acid and its R+ enantiomer supplemented to hyperbaric oxygen therapy on interleukin-6, TNF-α and EGF production in chronic leg wound healing. J Enzyme Inhib Med Chem 29: 297-302.
- Rozenblum N, Zeira E, Bulvik B, Gourevitch S, Yotvat H, et al. (2015) Radiofrequency ablation: inflammatory changes in the periablative zone can induce global organ effects, including liver regeneration. Radiology 276: 416-425.
- Okada Y, Shirai K, Reinach PS, Kitano-Izutani A, Miyajima M, et al. (2014) TRPA1 is required for TGF-β signaling and its loss blocks inflammatory fibrosis in mouse corneal stroma. Lab Invest 94: 1030-1041.
Citation: Chen X, Li Y, Liang X, Chen F (2020) Associating Insulin Instillation with Negative Pressure Wound Therapy Reduces Insulin Resistance in Diabetic Patients with Infected Foot Wounds. J Emerg Med Trauma Surg Care 7: 056
Copyright: © 2020 Xin-long Chen, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

