
Bone Marrow Stem Cells in Appositional Bone Construction: A Pilot Study in Rabbit Calvaria
*Corresponding Author(s):
Daiane Cristina PeruzzoSao Leopoldo Mandic Dental Institute And Research Center, Rua José Rocha Junqueira, 13. CEP 13045-755, Campinas, SP, Brazil
Tel:+55 1932113600,
Email:daiane.peruzzo@slmandic.edu.br
Abstract
This study aimed to evaluate the expression of the following osteogenesis related markers: Alkaline Phosphatase (ALP), Bone Sialoprotein (BSP), Osteopontin (OPN), Bone Morphogenetic Protein 2 (BMP-2) and 7 (BMP-7), in xenogeneic bone graft associated to a culture of mesenchymal stem cells from bone marrow, induced osteogenic differentiation (MSCIOD), in appositional bone construction on rabbits’ calvaria. For this purpose hollow titanium cylinders were installed on 11 rabbit calvaria, which were divided into 3 groups, according to the material used to fill the cylinders: G1 (n=3): control - filled with clot, G2 (n=5): filled with isolated Deproteinized Bovine Bone Mineral (DBBM); and G3 (n=5): DBBM associated to MSCIOD. After 60 days, the animals were euthanized and the entire contents of the cylinder were collected for analysis by real-time PCR for ALP, BSP, OPN, BMP-2 and 7. Statistical difference between the groups were observed only for BMP-7 expression (p = 0.029), with significant difference only between G2 and 3 (p = 0.0304). It was concluded that the combination of the DBBM and MSCIOD resulted in increased expression of BMP-7, while for other genes (ALP, BMP-2, OPN and BSP), although greater numerical expression was found, no differences were observed.
Keywords
Bone reconstruction, Gene expression, Mesenchymal stem cells.
INTRODUCTION
With tooth loss, the lack of stimulus given by chewing leads to reabsorption of remnant bone tissue, which many times disables implant installation [1]. Thus, the remnant bone amount has huge importance in rehabilitation treatment prognosis. In an attempt of solving this issue, some alveolar reconstruction techniques were developed [2,3]. In this scope, it may be considered that the ideal material for grafting should present, besides bone scaffold, a considerable quantity of mesenchymal cells and osteoblasts to be transferred to the recipient bed [4].
Initially, autogenous graft use was recommended to the majority of reconstructive techniques [3,5]. This graft is still considered the biological gold standard, as it presents osteogenic, osteoconduction and osteoinduction potentials [5]. Autogenous graft contains some viable cells and, also, proteins that stimulate the mesenchymal cell differentiation in osteoprogenitor cells [2].
Nevertheless, despite this consensus concerning the standard presented by autogenous bone graft, new biomaterials have been developed to replace it, since autogenous graft harvest repercutes in morbidity at the donor site [6]. However, the majority of bone substitutes present just the osteoconductive property, as it does not contain bone cells and osteoinductive proteins. Therefore, one of the researchers' main focus in bone regeneration field has been to test the use of an alternative to enhance those substitutes with viable cells and growth factors, giving to those materials similar characteristics to the autogenous graft ones [5]. In this sense, adult bone marrow has been tested as a source tissue to cell therapy because it presents hematopoietic and mesenchymal stem cells [7]. Mesenchymal stem cells have the potential of differentiation in some lineages, such as bone tissue [8-11].
The association of bone marrow cells with biomaterials can activate bone formation, as this association offers new properties to the graft [12]. If the used scaffold biomaterial is a resorbable matrix, after the association the composite graft starts having osteoinductive, osteoconductive and osteogenic properties, since mesenchymal bone marrow cells have capacity of differentiation in osteoblasts [4].
Having that in mind, the present study has the aim of evaluating gene expression in proteins enrolled in the initial processes of osteogenesis, concerning the association of xenograft to bone marrow mesenchymal stem cells induced to the osteogenic differentiation, in appositional bone reconstructions in rabbits’ calvaria.
MATERIALS AND METHODS
Ethical aspects
The present study was submitted to the Animal Experimentation Ethics Committee (CEUA) of the São Leopoldo Mandic Dental Institute and Research Center and was approved under protocol number 2010/0483.
Thirteen adult male rabbits were used, at 60 days of age, New Zealand breed, with weight between 2.5 and 3 kg. The rabbits were kept in a bioterium, with controlled temperature, separated in individual cages and fed with rabbit pelleted feed and water ad libitum.
Animal samples
The animals were randomly divided into 3 groups, them being 2 groups with 5 animals and the third group with 3 animals. In this regard, a randomization program available at www.randomization.com was used.
For each animal, appositional bone reconstruction situations were created, with the aid of hollow cylinders made of commercially pure titanium with a diameter of 6 mm and height of 5 mm with a screw-on lid, which insinuates 1 mm into the cylinder. The cylinders were fixed by two 3mm screws and filled with grafting material, bilaterally, on the parietal bones. A third cylinder was installed frontally to the other two, at the sagittal suture. Similar methods were used and published elsewhere [13].
The groups were divided according to the material filling the cylinders, as follows: Group 01 (n=3): Negative control - filling with blood clot; Group 02 (n=5): filling with Deproteinized Bovine Bone Mineral (DBBM) fine isolated granules (0.25-1mm, 2g~4cc - Bio-Oss™, Geistlich, Wolhusen, Suíça); and Group 03 (n=5): DBBM associated to mesenchymal stem cells culture from bone marrow induced to osteogenic differentiation (MSCIOD). After filling the cylinders with the materials, they were closed with a titanium screw cap (Figure 1).
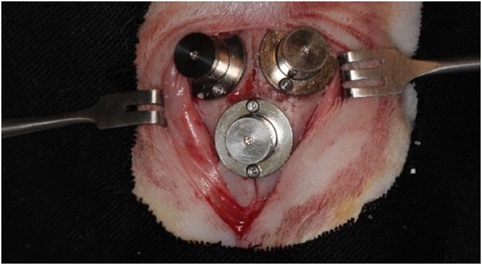 Figure 1: Titanium cylinders fixed on rabbits’ calvaria after being filled with the materials and closed with the titanium caps.
Figure 1: Titanium cylinders fixed on rabbits’ calvaria after being filled with the materials and closed with the titanium caps.
Bone Marrow Harvest
Autologous bone marrow was obtained by aspiration following the same protocol of general anesthesia described above. Two mL bone marrow aspirates were obtained from each tibia of the five rabbits using disposable 40 x 10 needles (1.10 mm x 38 mm) and 20 mL disposable syringes previously heparinized to prevent blood clotting.
Culture of bone marrow-derived adult mesenchymal stem cells
The following procedure was performed to obtain the BMMSCs. The bone marrow aspirate was transferred onto polypropylene tubes containing preservative-free heparin (1000 units/mL). The bone marrow and heparin were mixed thoroughly. The Bone Marrow Mononuclear Fraction (BMMF) was obtained from bone marrow tissue by density-gradient centrifugation using Ficoll Histopaque-1077 (Sigma-Aldrich, Saint Louis, MO, USA) according to the manufacturer’s instructions. Briefly, 3.5 mL bone marrow tissue was diluted in a 1:1 ratio with PBS, which was placed in a conical tube (15 mL) containing the same volume of Ficoll Histopaque-1077. The final solution was centrifuged at 400 g for 30 minutes at 22°C. The mononuclear cell layer was then pipetted and washed twice with PBS. A volume of 4 mL was obtained and another centrifugation was performed at 200 g for 10 minutes at room temperature. The supernatant was discarded and the pellet resuspended in 5 mL of DMEM (Sigma, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS, Sigma, St. Louis, MO, USA), 1% penicillin/streptomycin.
The pellet was transferred to a 75-cm2 culture flask (Corning, Tewksbury, MA, USA) previously filled with 5 mL of the osteogenic culture medium (DMEM (Sigma, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS, Sigma, St. Louis, MO, USA), 1% penicillin/streptomycin, 50 µg/ml ascorbic acid (Sigma, St. Louis, MO, USA), 10 mmol beta-glycerophosphate (Sigma, St. Louis, MO, USA), and 10 nmol of dexamethasone (Sigma, St. Louis, MO, USA). The cells were incubated at 37°C in an atmosphere composed of 95% O2 and 5% CO2. The culture medium was changed every 48 hours and the culture was monitored daily using an inverted optical microscope until 80% of confluence was reached.
Osteogenic culture medium
The stem cells derived from bone marrow were stimulated by the addition of osteogenic factors to the culture medium. The osteogenic culture medium was constituted of the basic medium (DMEM) with bovine fetal serum at 20% (supplemented with iron), and Penicillin/Streptomycin (100 UI/ml - 100µg/ml) and buffered with sodium bicarbonate (1N) improved with 0,5µg/ml of ascorbic acid, 10 mmol/L β glycerophosphate and 10nmol/L of dexamethasone [14].
Surgical protocol
General anesthesia protocol was induced and maintained by a combination of 40mg/kg of ketamine, 2mg/kg of midazolam and 0.8 µg/kg of fentanyl citrate, all administered intramuscularly. The maintenance was conducted by the administration of 100% oxygen 2L/min and isoflurane in a universal vaporizer with facial mask.
After inducing general anesthesia, trichotomy on the animal's head and asepsis with iodopovidone was performed. Posteriorly, complementary infiltrative anesthesia was administered at the surgery location, using lidocaine with epinephrine 1:100.000, with the objective of promoting local ischemia.
Sagittal incision was performed, the skin and periosteum were folded and each one of the animals received three hollow cylinders made of pure machined titanium, which were fixed to the calvaria by two 3mm auto-screwing screws.
Along with the installation of the cylinders, bone decortication for graft nutrition was performed by means of 5 micro perforations on the bone cortical contained inside the cylinders. These perforations were conducted with the aid of 0.5 mm diameter spherical milling cutters. At this moment, in a randomized manner, Bio-Oss™, associated or not to cell culture of mesenchymal stem cells induced to osteogenic differentiation, was deposited inside group 1 and 2 cylinders. Group 3 devices were maintained only with the clot.
After insertion of the materials, the cylinders were hermetically closed with an internal screw-on lid for complete isolation of the material from the adjacent tissues. Continuous sutures with 4.0 nylon were performed to close the surgical field, which was made in planes. During the postoperative period, the animals were medicated with antibiotics (sodium cefazolin 30 mg/kg IM every 12 hours for 3 days) and anti-inflammatory (fluxene meglumine 1.0mg/kg IM every 24 hours for 3 days). In order to obtain analgesia, tramadol hydrochloride was used (2mg/kg/SC every 8 hours for 3 days).
The surgical wounds were cleaned with 0.9% physiological solution and posterior application of iodopovidone and bandages. This procedure was performed three times a day for a period of fifteen days. The sutures were removed at the end of 2 weeks of healing. After 8 weeks, the animals were euthanized with the application of ketamine (40mg/kg IM) and overdose of sodium thiopental via marginal auricular vein catheter. After euthanasia all the animals had the calvaria of the head removed with help from an oscillatory saw in a careful manner maintaining galea, periosteum and dura-mater preserved.
Analysis of gene expression
For the analysis of gene expression the material contained inside the frontal cylinder was collected with aid from a dentine curette. The material was stored in RNAlater (Ambion Inc., Austin, TX, USA) and frozen at -80°C. To extract RNA from the samples Trizol reactant was used according to the manufacturer’s recommendation. The RNA pellet was resuspended in diethylpyrocarbonate-treated water and stored at -70°C. RNA concentration was determined by optical density using a biophotometer (Eppendorff, Hamburg, Germany). Samples were assessed quantitatively for the expression of the following genes: BMP-2, BMP-7, Ssteopontin (OPN), Alkaline Phosphatase (ALP) and Bone Sialoprotein (BSP)
Real-Time Polymerase Chain Reaction (PCR) Reverse transcription. Total RNA was treated with DNase (Turbo DNA-free, Ambion.) and 1 mg was used for cDNA synthesis. The reaction was carried out using the first-strand cDNA synthesis kit (Transcriptor cDNA synthesis, Roche Diagnostics, Indianapolis, IN) following the manufacturer’s recommendations.
Primer design
Primers were designed using probe design software (LightCycler Probe design 2.0, Roche Diagnostics) as shown in table 1.
|
PRIMER NAME |
|
SEQUENCE (5' to 3') |
|
TIME (s) |
|
TEMPERATURE (°C) |
|
|
|
|
|
|
|
|
|
BMP2-F |
|
TGT AAG AGA CAC CCT TTG TAC G |
|
7 |
|
56 |
|
BMP2-R |
|
CTT AGA GTT CAC GGA GTT GAC |
|
|||
|
|
|
|
|
|
|
|
|
OPN-F |
|
CGG TTA AAC ACG CTG ATT CG |
|
7 |
|
56 |
|
OPN-R |
|
GGA GGG TCT CTT GTT TAA GGT |
|
|
||
|
|
|
|
|
|
|
|
|
BMP7-F |
|
ACA AGG ACT ACA TCC GGG |
|
6 |
|
56 |
|
BPM7-R |
|
GTG ATG TCG AAG ACC AGC C |
|
|
||
|
|
|
|
|
|
|
|
|
GAPDH-F |
|
CTG CGA CTT CAA CAG TGC |
|
7 |
|
56 |
|
GAPDH-R |
|
GGT GGT TTG AGG GCT CTT AC |
|
|
||
|
|
|
|
|
|
|
|
|
BSP-F |
|
CCA CTC ACT CTA GTG AGC TTG |
|
6 |
|
56 |
|
BSP-R |
|
TGG CCT GAA CTT AAA GAC CC |
|
|
||
|
|
|
|
|
|
|
|
|
ALP-F |
|
GGA CAT CTG GAG GAG CTT C |
|
6 |
|
57 |
|
ALP-R |
|
TGT TGT TCC TGT TCA GCT CG |
|
|
Table 1: Characteristics of the primers with regards to sequence, time and temperature.
Quantitative PCR (qPCR)
All of the primers were verified according to their specificity with use of the Melting curve verification (obtained after running in the LightCycler) and running the gel to verify the products.
Real time PCR
RT-PCR reactions were performed with the LightCycler system (Roche Diagnostics GmbH, Mannheim, Germany), using FastStart DNA Master SYBR Green I™ kit (Roche Diagnostic Co.). For each of the runs, DPEC water was used as a negative control, and the product of the reactions was quantified by the manufacturer’s own program (LightCycler Relative Quantification Software™ - Roche Diagnostics GmbH). GAPDH was used as the reference gene (housekeeping) in order to normalize the values. The calculation of the gene expression was based on the threshold cycle values (TC), using the formula 2 ?Δ Δ TC.
Statistical Analysis
Previous to statistical analysis properly said, the Kolmogorov-Smirnov test was conducted to evaluate the normality of the data. Since the data did not present normal distribution, the nonparametric Kruskal Wallis test for intragroup comparisons was used. The program used for data analysis was BioEstat 5.0 (Instituto Sustentável Mamirauá, Belém, PA, Brasil). A 5% significance level was considered for all the analysis.
RESULTS
Of the 13 animals used for the study, two did not resist the surgical procedure, them being one from Group 02 and the other from Group 03. The 11 remaining animals were divided among the respective groups: Samples 01 to 03 in group 01; Samples 04 to 07 in group 02 and samples 08 to 11 in Group 03.
After analyzing the samples with real time PCR, it was possible to observe the expression of the ALP, BSP, OPN, BMP-2 and BMP-7 genes by the relation of the concentration of mRNA of these genes with the concentration of GAPDH (mRNA/GAPDH). A grand variability on the distribution of the data can be observed, identified by the large standard deviation.
Levels of expression of the ALP, BSP, OPN and BMP-2 presented a tendency of increase in values from G3 with regards to G2 and thesewith regards to G1 (Table 2), however without statistical differences among the groups (p>0.05).
|
Group |
ALP |
BMP-2 |
OPN |
BMP-7 |
BSP |
|
G1 (Clot) |
0.64 (0.56) |
0.28 (0.35) |
0.011 (0.017) |
0.0002 (0.0001) |
2.1567 (2.5267) |
|
G2 (DBBM) |
0.89 (0.60) |
0.38 (0.27) |
0.012 (0.013) |
0.0009 (0.0015) |
7.3325 (6.5598) |
|
G3 (DBM+MSCBM) |
1.09 (1.09) |
2.91 (3.32) |
0.017 (0.028) |
0.219* (0.343) |
10.915 (20.720) |
Table 2: Average and standard of the triplicate relative to the genes ALP, BMP-2, BMP-7, OPN and BSP, for G1, G2 and G3.
*Represents the statistical difference between groups (Kruskal-Wallis test; p = 0.029).
Regarding the expression of BMP-7, differences were not observed (p>0.05) between the averages of G1 (0.0002 ± 0.0001) and G2 (0.00090002 ± 0.0015). When G3 was evaluated (0.219 ± 0.343) it was statistically different from G1 and G2 (p = 0.029), indicating an increase in the expression of BMP-7 (Table 2).
DISCUSSION
The challenge to achieve a bone reconstruction using bone substitutes has already motivated a larger number of researchers to present their ideas, specially using animal models. The gold standard in bone reconstruction keeps being the autologous bone graft, and its advantages are many e.g.: osteoconductive properties, but there are some disadvantages e.g.: donor site morbidity [15]. In order to overcome these disadvantages, numerous tissue-engineering approaches have been developed to take advantages of using physiological osteoinductive signals [16]. Osteoinductive factors released from resident cells or after osteoclast bone resorption regulate the recruitment and differentiation of osteoblastic cells. Some markers of bone metabolism have correlative and prognostic value, especially products of osteogenesis or osteoblast-secreted factors and they can provide insight into the ongoing levels of bone formation [17].
The expression of Alkaline Phosphatase (ALP), Osteopontin (OPN), Bone Sialoprotein (BSP) and BMP-2 and BMP-7 have been used in several studies to evaluate bone formation because it is known that in physiological conditions they are released into extracellular matrix. The analysis of these osteogenic factors altogether, can provide information about early and late osteogenic marker expressions.
This present study was undertaken to assess the effects of bone marrow mesenchymal stem cells induced to osteogenic differentiation (MSCIOD) associated to a bone xenograft, in an in vivo appositional reconstructive model after 8 weeks of healing. The models were designed as titanium cylinders with a removal caps, fixed with screws on rabbits’ calvaria, as described by Pelegrine et al. [18]. Our hypothesis was that by using MSCIOD, it would be possible to have the distribution throughout the biomaterial of the differentiated cells in a more predictable way, since it would not depend on the proximity between the BMSC within biomaterial to native bone for induction toward osteo differentiation.
In this study the gene expression of ALP, OPN, BSP and BMP-2 were not affected by the presence of biomaterial associated with MSCIOD (p >0.05), with the ALP level in all experimental groups close to the negative control group (G1) and OPN, BSP and BMP-2 levels with a higher level in the test group (G3) in comparison with G1 and G2, but without statistical significance.
These results were expected in an experimental model with 8 weeks of healing time, since ALP, OPN and BSP are considered early markers of osteogenic expression [19]. ALP is the marker most commonly used to evaluate bone formation and plays an important role in osteoid formation and bone mineralization. It is detectable in the early stages of osteoblast formation [20]. Moreover, it is one of the major stimulants of OPN expression, which can also be detected in early stages of differentiation of bone precursors, although their levels are increased in osteoblasts [21]. The trend toward greater expression of ALP and OPN in the group where stem cells were used may indicate higher levels of osteoblast formation, suggesting a greater osteogenic potential presented by this group. OPN is very important in osteogenesis by the ease of adhering osteoblasts to this extracellular matrix. In addition, it plays a crucial role in the mineralization of this matrix [22]. BSP is only to mineralized tissues and is a specific marker of osteoblast differentiation, being highly specific for tissue mineralization [23].
BMP-2 and BMP-7 have a strong osteoinductive potential, playing a key role in bone repair, and participating in the differentiation of mesenchymal cells into osteoblastic cells [24]. Only a limited number of studies have focused on BMP-7 and mesenchymal stem cells (MSC). In particular, Burastero et al., [25] used the association of human MSCs and BMP7 and Schiavi et al., [26] tested a novel 3D collagen nanofiber functionalized with BMP-7, and both have demonstrated the correlation between BMP-7 and bone formation. In this present study only the expression of BMP-7 was statistically higher in the test group.
Even though in this study we did not achieve statistically significant results for most of the osteogenic markers, it was evident that there was a tendency for an increase in its expression in the group where stem cells derived from bone marrow with osteogenic stimulation were used. Other studies involving the same model but with cells derived from bone marrow without osteogenic stimulation showed a greater presence of vital mineralized tissue in the same healing period than in the control group where only the biomaterial was used [27]. Thus, it could be observed that the increase of BMP-7 gene expression and the increase of the amount of vital mineralized tissue could be related in this particular experimental model, but a greater number of studies involving this theme will be necessary to corroborate these inferences. Additionally, expression analysis of genes involved in bone formation by real-time quantitative PCR should be supported by histochemical staining of tissue sections obtained from each experimental,as well as the analysis of other important proteins that participate in the osteogenesis process, such as collagen type I and osteocalcin. Considering the results, it could be concluded that the combination of the DBBM and MSCBM resulted in increased expression of BMP-7, while for other genes (ALP, BMP-2, OPN and BSP), although it has been found greater numerical expression, no differences were observed.
ACKNOWLEDGEMENT
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
REFERENCES
- Barber HD, Betts NJ (1993) Rehabilitation of maxillofacial trauma patients with dental implants. Implant Dent 2: 191-193.
- Gordh M, Alberius P, Johnell O, Lindberg L, Linde A (1999) Effects of rhBMP-2 and osteopromotive membranes on experimental bone grafting. Plast Reconstr Surg 103: 1909-1918.
- Chiapasco M, Casentini P, Zaniboni M (2009) Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants 24: 237-259.
- Smiler D, Soltan M, Lee JW (2007) A histomorphogenic analysis of bone grafts augmented with adult stem cells. Implant Dent 16: 42-53.
- Dym H, Pierse J (2011) Advanced techniques in bone grafting procedures. Dent Clin North Am 55: 453-460.
- Jensen SS, Terheyden H (2009) Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int J Oral Maxillofac Implants 24: 218-236.
- Kotobuki N, Hirose M, Takakura Y, Ohgushi H (2004) Cultured autologous human cells for hard tissue regeneration: preparation and characterization of mesenchymal stem cells from bone marrow. Arti?cial Organs 28: 33-39.
- Pelegrine AA, Aloise AC, Zimmermann A, de Mello E Oliveira R, Ferreira LM (2014) Repair of critical-size bone defects using bone marrow stromal cells: a histomorphometric study in rabbit calvaria. Part I: use of fresh bone marrow or bone marrow mononuclear fraction. Clin Oral Implants Res 25: 567-572.
- Aloise AC, Pelegrine AA, Zimmermann A, de Mello E Oliveira R, Ferreira LM (2015) Repair of critical-size bone defects using bone marrow stem cells or autogenous bone with or without collagen membrane: a histomorphometric study in rabbit calvaria. Int J Oral Maxillofac Implants 30: 208-215.
- Brown C, McKee C, Bakshi S, Walker K, Hakman E, et al. (2019) Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med 13: 1738-1755.
- Fu X, Liu G, Halim A, Ju Y, Luo Q, et al. (2019) Mesenchymal Stem Cell Migration and Tissue Repair. Cells 8: 784.
- Victorelli G, Aloise AC, Passador-Santos F, de Mello e Oliveira R, Pelegrine AA (2016) Ectopic Implantation of Hydroxyapatite Xenograft Scaffold Loaded with Bone Marrow Aspirate Concentrate or Osteodifferentiated Bone Marrow Mesenchymal Stem Cells: A Pilot Study in Mice. Int J Oral Maxillofac Implants 31: 18-23.
- Pelegrine AA, Sorgi da Costa CE, Sendyk WR, Gromatzky A (2011) The comparative analysis of homologous fresh frozen bone and autogenous bone graft, associated or not with autogenous bone marrow, in rabbit calvaria: a clinical and histomorphometric study. Cell Tissue Bank 12: 171-184.
- Duplomb L, Dagouassat M, Jourdon P, Heymann D (2007) Concise review: embryonic stem cells: a new tool to study osteoblast and osteoclast differentiation. Stem Cells 25: 544-552.
- Rawashdeh MA, Telfah H (2008) Secondary alveolar bone grafting: the dilemma of donor site selection and morbidity. Br J Oral Maxillofac Surg 46: 665-670.
- Henkel J, Woodruff MA, Epari DR, Steck R, Glatt V, et al. (2013) Bone Regeneration Based on Tissue Engineering Conceptions - A 21st Century Perspective. Bone Res 1: 216-248.
- Nguyen TT, Bae TS, Yang DH, Park MS, Yoon SJ (2017) Effects of Titanium Mesh Surfaces-Coated with Hydroxyapatite/β-Tricalcium Phosphate Nanotubes on Acetabular Bone Defects in Rabbits. Int J Mol Sci 18: 1462-1475.
- Pelegrine AA, Costa CE, Correa ME, Marques Junior JF (2010) Clinical and histomorphometric evaluation of extraction sockets treated with an autologous bone marrow graft. Clin Oral Implants Res 21: 535-542.
- Aquino-Martínez R, Artigas N, Gámez B, Rosa JL, Ventura F (2017) Extracellular calcium promotes bone formation from bone marrow mesenchymal stem cells by amplifying the effects of BMP-2 on SMAD signalling. PLoS One 12: 0178158.
- Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T (2012) Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J Biochem 151: 247-254.
- Ellis E, Sinn DP (1993) Use of homologous bone in maxillofacial surgery. J Oral Maxillofac Surg 51: 1181-1193.
- Rust PA, Kolsi P, Briggs TW, Cannon SR, Blunn GW (2007) Will mesenchymal stem cells differentiate into osteoblasts on allograft? Clin Orthop Relat Res 457: 220-226.
- Ganss B, Kim RH, Sodek J (1999) Bone Sialoprotein. Crit Rev Oral Biol Med 10: 79-98.
- Giannoudis PV, Al-Lami MK, Tzioupis C, Zavras D, Grotz MR (2005) Tricortical bone graft for primary reconstruction of comminuted distal humerus fractures. J Orthop Trauma 19: 741-743.
- Burastero G, Scarfì S, Ferraris C, Fresia C, Sessarego N, et al. (2010) The association of human mesenchymal stem cells with BMP-7 improves bone regeneration of critical-size segmental boné defects in athymic rats. Bone 47: 117-126.
- Schiavi J, Keller L, Morand DN, De Isla N, Huck O, et al. (2015) Active implant combining human stem cell microtissues and growth factors for bone-regenerative nanomedicine. Nanomedicine (Lond) 10: 753-763.
- Coelho de Faria AB, Chiantia FB, Teixeira ML, Aloise AC, Pelegrine AA (2016) Comparative Study Between Mesenchymal Stem Cells Derived from Bone Marrow and from Adipose Tissue, Associated with Xenograft, in Appositional Reconstructions: Histomorphometric Study in Rabbit Calvaria. Int J Oral Maxillofac Implants 31: 155-161.
Citation: Peruzzo DC, Teixeira ML, Aguiar FM, Duboc L, Joly JC, et al. (2019) Bone Marrow Stem Cells in Appositional Bone Construction: A Pilot Study in Rabbit Calvaria. J Stem Cell Res Dev Ther 5: 018.
Copyright: © 2019 Daiane Cristina Peruzzo, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

