
Cell-Surface MMP-9 is a Novel Functional Marker for Early Endothelial Progenitor Cells Derived from CD133+ Cells
*Corresponding Author(s):
Teruhide Yamaguchi1,Kanazawa Institute Of Technology, Ohgigaoka 7-1, Nonoichi, Ishikawa 921-8501, Japan
Tel:+81 762749307,
Fax:+81 762749378
Email:yamaguch@neptune.kanazawa-it.ac.jp
Abstract
Matrix Metalloproteinase-9 (MMP-9) plays an important role in the migration of and tissue invasion by vascular endothelial cells. Additionally, early Endothelial Progenitor Cells (EPCs) are known to play a key role in the recruitment of endothelial cells in angiogenesis. In angiogenesis, MMP-9, which binds on the plasma membranes of early EPCs, is important in neo-vascularization. Therefore, early EPCs with MMP-9 on their cell membranes are a novel candidate for vascular regeneration therapy.
Keywords
Angiogenesis; CD133; Endothelial progenitor cells; MMP-9
INTRODUCTION
The development of cell-based therapeutic products using endothelial progenitor cells (EPCs) for vascular disorders caused by ischemic symptoms such as myocardial infarction, cerebral infarction, and lower-limb Arteriosclerosis Obliterans (ASO) has been progressing. EPCs are divided into two categories: late EPCs/Endothelial Colony Forming Cells (ECFC), which differentiate into vascular endothelial cells and contribute to nascent blood vessels; and early EPCs, which do not differentiate into vascular endothelial cells, but induce neovascularization through the production of cytokines [1-4]. Former progenitor cells are induced relatively late in the in vitro culture system and have the ability to proliferate rapidly as cobblestone colonies. In contrast, early EPCs have a fibroblast-like morphology and do not exhibit high proliferative potential, but they can migrate and invade ischemic regions, and produce various cytokines. The production of cytokines such as Vascular Endothelial Growth Factor-A (VEGF-A), Platelet-Derived Growth Factor-AA (PDGF-AA) and Interleukin-8 (IL-8) will recruit and proliferate vascular endothelial cells into ischemic regions.
It is important to understand the potential of early EPCs to induce vascular endothelial cells, not only for basic research but also for cell therapy for ischemic diseases. A number of clinical trials have been conducted and their practical application is expected [5-7]. Two origins of early EPCs have been reported: EPCs are derived either from a fraction of CD34 or from CD133-positive Hematopoietic Stem Cells (HSCs), or from mononuclear cells/CD14-positive cells. EPCs in the latter category have been reported to have characteristics similar to those of M2 macrophages in terms of their differentiation antigens and cytokine production profiles [8].
On the other hand, early EPCs derived from CD34/CD133-positive cells have the ability to rapidly invade the ischemic area and enter the vascular system. They are known to have a high capacity for the production of cytokines, which are required in order for endothelial cells to proliferate [9-12]. Regarding these CD34/CD133-positive cell-derived early EPCs, we have found that Matrix Metalloproteinase-9 (MMP-9), which has been reported to be involved in the migration and invasion of vascular endothelial cells, binds to the cell membrane of early EPCs and regulates their ability to recruit endothelial cells. It has also been found that membrane-bound MMP-9 is a key factor in the development of the tube formation process [13]. While it has long been known that secreted MMP-9 enzymatically digests matrix gelatin protein and contributes to migration, invasion and the remodeling of vascular endothelial cells, a new role for MMP-9 has emerged. Furthermore, early EPCs that have MMP-9 bound on their plasma membranes are considered to be key players in the development of vascular cell therapy. In this article, we discuss the function of MMP-9 membrane-expressed EPCs and their potential for cell therapy.
CATEGORIES OF EPCS AND THEIR BIOLOGICAL ACTIVITIES
The main function of early EPCs in angiogenesis is to recruit vascular endothelial cells into ischemic regions, and there are several possible candidates for their origins. As reported by Asahara and others, EPCs may arise from CD34-positive or CD133-positive cells derived from or similar to HSCs [14,15]. Early EPCs may be induced by the in vitro culture conditions for vascular induction from CD34-positive or CD133-positive cells from peripheral blood and cord blood. With incubation under these conditions, endothelial-like cells which express vascular endothelial markers such as Tie2, endothelial Nitric Oxide Synthase (eNOS), von Willebrand Factor (vWF) and Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1)/CD31 emerge within one week [16]. In addition to the expression of differentiation markers such as vascular endothelial cells, CD133-derived early EPCs also express c-Kit ligand/SCF, insulin-like growth factor-1 and VEGF-A, and are involved in basic fibroblast growth factor vascular induction as a paracrine factor, which is associated with other types of vascular induction such as growth [5,12], using a system that transplants early EPCs into nude mice, report that cytokines produced by early EPCs have the ability to induce angiogenesis. It has also been shown that the factors that produce early EPCs play a role in the promotion of endothelial tube formation. Additionally, different factors have been reported to be involved in vascular induction and lumen formation.
Another possible origin of early EPCs is differentiation from either peripheral blood mononuclear cell fractions or CD14-positive cells. There are cells that carry markers of differentiation of vascular endothelial progenitor cells that come from myeloid cell lineages, the so-called Myeloid Angiogenic Cells (MACs). Similar to AC133/CD34-derived early EPCs, these MACs release several cytokines involved in the amplification and chemotaxis of vascular endothelial cells and contribute to vascular induction. Recent studies suggest that these MACs have properties that are extremely similar to those of M2 macrophages, and some reports have questioned their function.
On the other hand, when peripheral blood mononuclear cells and bone marrow cells are cultured in vitro for vascular cell induction, highly proliferative EPCs are produced delay to early EPC. These are known as late EPCs, in contrast to early EPCs [2,17]. Late EPCs proliferate by forming cobblestone-like colonies, demonstrating their tube formation capacity and high proliferation. The difference between early and late EPCs is minor with regard to surface markers such as vascular endothelial cells, but early EPCs are unable to form tubes as endothelial cells. Early EPCs have a fibroblast-like morphology and their proliferative potential is not high; they show no luminal growth, unlike late EPCs.
FUNCTION OF AC133-DERIVED EARLY EPCS AND MEMBRANE-BOUND MMP-9
Many studies have reported that early EPCs induce neovascularization by recruiting vascular endothelial cells, and research into potential regenerative medicines with vascular induction ability is also in progress. Regarding cell therapy for neovascularization, it is essential to determine what factors influence how EPCs accumulate in ischemic regions. It is important that early EPCs can be made to accumulate at the site of ischemia, and cytokines produced by those EPCs will then be more effective for neovascularization. This allows vascular endothelial cells to migrate to the ischemic area, resulting in the formation of new blood vessels. The functions of these early EPCs include migration to ischemic sites, invasion, and recruitment of vascular endothelial cells through released cytokines. This is accomplished through several functions such as mobilization, migration, and vasculogenesis. Although various factors are assumed to be involved in these processes, extracellular matrix-digesting enzymes such as MMPs, including MMP-9, have attracted a lot of attention [18,19].
MMPs are enzymes that play an important role in cell migration and tissue invasion. They support cell motility by degrading extracellular matrices, such as collagen, gelatin or laminin. Furthermore, they have been reported to contribute to tissue remodeling [20]. MMPs that function in angiogenesis, and both MMP-2 and MMP-9, are reported to up- and down-regulate in ischemic sites, and to be regulated. Furthermore, a study using MMP-9-knocked-out mice reports that the migration of bone marrow-derived EPCs to the ischemic site is inhibited, as is neovascular induction [21]. Huang’s report suggests that MMP-9 plays a central role in angiogenesis, which is not inhibited when only MMP-2 is inhibited.
It was thought that the role of MMP-9 was simply to degrade the extracellular matrix and thereby facilitate cell migration and other processes, however, MMP-9 is now known to contribute not only to digesting the extracellular matrix in order to migrate but also to releasing either membrane-bound or matrix-bound growth factors that will then promote the growth of endothelial cells [21]. That is, MMP-9 increases in a soluble Kit-Ligand (sKitL) from hematopoietic and stromal cells, which is used in EPC angiogenesis.
The effects of MMP-9 on late EPCs have been analyzed and it has been reported that, while MMP-9 has a significant effect on the migration and invasion abilities of late EPCs, it has no effect on their proliferation or other functions. Late EPCs are involved in angiogenesis through their incorporation into vascular endothelial cells in the neovascular system, and MMP-9 plays an important role in angiogenesis by promoting their invasion and migration to ischemic sites. On the other hand, the role of MMP-9 in early EPCs has not yet been fully elucidated.
FUNCTION OF EARLY EPCS AND ROLE OF MMP-9
Kanayasu-Toyoda T et al., report that culturing CD133-positive cells from cord blood and peripheral blood under vascular-inducing conditions induce early EPCs (CD133-EPCs) that express vascular endothelial cell differentiation antigens such as KDR, eNOS, vWF or PECAM-1/CD31 [16]. These early EPCs show a higher production than Human Umbilical Vein Endothelial Cells (HUVECs) of cytokines for vascular induction such as IL-8, VEGF-A and PDGF-AA. A marked correlation has been identified between a high production of relevant cytokines and high vascular induction capacity [16]. On the other hand, CD133-EPCs have also been found to exhibit an ability to produce high amounts of MMP-9, and it has been noted that that some MMP-9 can bind to the cell membrane of CD133-EPCs. Furthermore, cells that express MMP-9 on the cell membrane (MMP-9+ EPCs) can be prepared using MMP-9 antibody. An analysis of biological abilities has revealed that MMP-9+ EPCs promote tube formation by HUVECs in vitro, and this promoting effect is inhibited by the addition of anti-IL-8 antibody. On the other hand, early EPCs that do not express MMP-9 on the cell membrane (MMP-9- EPCs) did not show this promoting effect on HUVEC tube formation. Furthermore, when MMP-9+ EPCs were transplanted into nude mice and their vascular induction activity was analyzed, only MMP-9+ EPCs showed vascular inducing activity and, interestingly, anti-IL-8 antibodies were able to inhibit the acceleration ability of MMP-9+ EPCs. MMP-9- EPCs did not show in vivo vascular induction.
The invasion ability of MMP-9+ EPCs to VEGF-induced MatrigelTM was found to be much higher than that of MMP-9- EPCs. In addition, their IL-8 production capacity is 10 times higher than that of MMP-9- EPCs, though there was little difference between their VEGF-A and PDGF-AA production abilities. The high infiltration activity of MMP-9+ EPCs was also confirmed by in-situ zymography, but this invasive activity is inhibited by MMP-2/9 inhibitors. On the other hand, the results of a super-array analysis of vascular endothelial cell-associated protein expression demonstrate that both cell types contain little MMP2, suggesting that the effect of MMP2/9 inhibitor invasion activity on MatrigelTM may be due to an MMP-9-dependent mechanism.
MMP-9+ EPCs not only have high invasion ability but also induce vascular endothelial cells in vivo. In particular, while various cytokines are assumed to be involved in vascular induction ability, the present results using anti-IL-8 antibodies indicate that the most important processes are chemokine IL-8-mediated actions. In addition, MMP-9+ EPCs strongly promote tube formation of HUVECs in vitro, but this effect is also inhibited by anti-IL-8 antibodies. Therefore, MMP-9+ EPCs are able to recruit vascular endothelial cells into ischemic regions and to promote vascular architecture (tube formation) through high IL-8 production. These data suggest that IL-8 expressed by MMP-9+ EPCs plays a key role in vascular regeneration and tube formation (Figure 1). Furthermore, MMP-9+ EPCs are expected to be useful in vascular regenerative medicine focusing on this function.
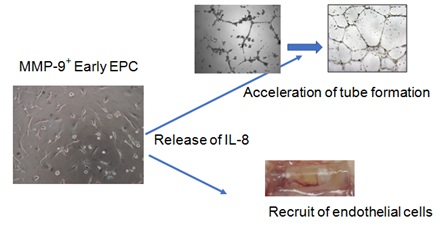 Figure 1: MMP-9+ EPC have an ability to produce high amount of IL-8, which enhances both tube formation of HUVEC and recruit of endothelial cells into ischemia regions.
Figure 1: MMP-9+ EPC have an ability to produce high amount of IL-8, which enhances both tube formation of HUVEC and recruit of endothelial cells into ischemia regions.
To clarify the function of membrane-bound MMP-9 in MMP-9+ EPCs, the effect of MMP-9 release from plasma membranes on the biological function of MMP-9+ EPCs was explored. Since MMP-9 is reported to attach to CD44 through hyaluronic acid [22,23], we analyzed the effect of hyaluronidase treatment on the induction ability of vascular endothelial cells by MMP-9+ EPCs. The membrane-bound MMP-9 of MMP-9+ EPCs was markedly reduced by hyaluronidase treatment, and the invasive activity of MMP-9+ EPCs was greatly inhibited. On the other hand, the enzymatic activity of MMP-9 in the condition medium of MMP-9+ EPCs was not affected by hyaluronidase treatment, suggesting that this treatment does not affect the enzymatic activity of MMP-9. These results indicate that MMP-9+ EPCs bind MMP-9 on the cell membrane via hyaluronic acid, and that membrane-bound MMP-9 plays an important role in invasive activity (Figure 2). Additionally, it should be noted that MMP-9-bound-to-plasma-membrane of EPCs has a physiological function and plays an important role in angiogenesis, suggesting that EPC of which plasma-membrane carry the MMP-9 are expected to play a key role for angiogenesis.
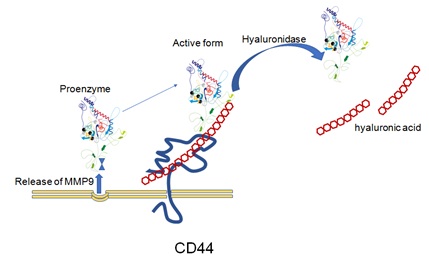 Figure 2: MMP-9+ EPC produce and release the MMP-9, a part of which binds to CD44 through the hyaluronic acid. The treatment with hyaluronidase causes the release of MMP-9 from the plasma membrane of MMP-9+ EPC, resulting the loss of function to invade.
Figure 2: MMP-9+ EPC produce and release the MMP-9, a part of which binds to CD44 through the hyaluronic acid. The treatment with hyaluronidase causes the release of MMP-9 from the plasma membrane of MMP-9+ EPC, resulting the loss of function to invade.
However, hyaluronidase treatment did not affect the expression of CD44 itself, and MMP-9 activity in the supernatant was hardly affected by this treatment, suggesting that MMP-9which is anchored to plasma membrane through the hyaluronic acid-on CD44 may have strong enzymatic activity. Although the physiological function of membrane-bound MMP-9 has not yet been fully elucidated, it is possible that it may dissolve the matrix during invasion more efficiently than soluble form.
Morancho A et al., [24] used MMP-9-knockout mice (MMP-9-/-) to analyze the role of MMP-9 in induced infarction, and report that, although early EPCs increased in mice with induced infarction, there was no induction of early EPCs in MMP-9-/-mice, and tube formation ability was found to be reduced. Interestingly, biological activity was not restored by the addition of MMP-9 to MMP-9-/-mice. However, the results reported by Morancho A et al., seem to be explained if the role of MMP-9 in angiogenesis is due to its binding to the cell membrane.
CONCLUSION
Early EPCs secrete cytokines and other related factors to induce vascular endothelial cells into ischemic regions, playing a key role in neovascularization. On the other hand, it has been reported that gelatinase MMP-9 plays an important role in vascular induction. Kanayasu-Toyoda K et al., [13] report that early EPCs, which have a membrane-associated MMP-9 (MMP-9+ EPCs), have marked IL-8 expression and induce angiogenesis through this important cytokine. We also found that IL-8 plays a central role in vascular induction induced by MMP-9+ EPCs. These data indicate that MMP-9+ EPCs are a novel candidate for cell therapy for stroke, myocardial infarction, and lower extremity arterial infarction.
ACKNOWLEDGEMENT
This work was supported by a grand form the Agency for Medical Research and Development.
REFERENCES
- Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, et al. (2001) HMGCoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest 108: 391-397.
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, et al. (2004) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 24: 288-293.
- Akita T, Murohara T, Ikeda H, Sasaki K, Shimada T, et al. (2003) Hypoxic preconditioning augments efficacy of human endothelial progenitor cells for therapeutic neovascularization. Lab Invest 83: 65-73.
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, et al. (2004) Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104: 2752-2760.
- Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, et al. (2000) Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 97: 3422-3427.
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, et al. (2004) Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 114: 330-338.
- Moubarik C, Guillet B, Youssef B, Codaccioni JL, Piercecchi MD, et al. (2011) Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev 7: 208-220.
- Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, et al. (2017) Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med 6: 1316-1320.
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964-967.
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, et al. (2000) Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95: 952-958.
- Rafii S, Lyden D (2003) Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 9: 702-712.
- Ratajczak J, Kucia M, Mierzejewska K, Marlicz W, Pietrzkowski Z, et al. (2013) Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+ cells-implications for stem cell therapies in regenerative medicine. Stem Cells Dev 22: 422-430.
- Kanayasu-Toyoda T, Tanaka T, Kikuchi Y, Uchida E, Matsuyama A, et al. (2016) Cell-surface MMP-9 protein is a novel functional marker to identify and separate pro-angiogenic cells from early endothelial progenitor cells derived from CD133+ cells. StemCells 34: 1251-1262.
- Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, et al. (2001) Numerous growth factors, cytokines, and chemokines are secreted by human CD34+ cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 97: 3075-3085.
- Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, et al. (2011) Exosomes from human CD34 + stem cells mediate their proangiopoietic paracrine activity. Circ Res 109: 724-728.
- Kanayasu-Toyoda T, Ishii-Watabe A, Suzuki T, Oshizawa T, Yamaguchi T (2007) A new role of thrombopoietin enhancing ex vivo expansion of endothelial precursor cells derived from AC133-positive cells. J Biol Chem 282: 33507-33514.
- Mukai N, Akahori T, Komaki M, Li Q, Kanayasu-Toyoda T, et al. (2008) A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res 314: 430-440.
- Pepper MS (2001) Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 21: 1104-1117.
- Johnson C, Sung HJ, Lessner SM, Fini ME, Galis ZS (2004) Matrix metalloprotinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching. Circ Res 94: 262-268.
- Roldán V, Marín F, Gimeno JR, Ruiz-Espejo F, González J, et al. (2008) Matrix metalloproteinases and tissue remodeling in hypertrophic cardiomyopathy. Am Heart J 156: 85-91.
- Huang PH, Chen YH, Wang CH, Chen JS, Tsai HY, et al. (2009) Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Anterioscler Thromb Vasc Biol 29: 1179-1184.
- Dufour A, Zucker S, Sampson NS, Kuscu C, Cao J (2010) Role of matrix metalloproteinase-9 dimers in cell migration: design of inhibitory peptides. J Biol Chem 285: 35944-35956.
- Yu Q, Stamenkovic I (2000) Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14: 163-176.
- Morancho A, Hernandez-Guillamon M, Boada A, Barceló V, Giralt D, et al. (2013) Cerebral ischaemia and matrix metalloproteinase-9 modulate the angiogenic function of early and late outgrowth endothelial progenitor cells. J Cell Mol Med 17: 1543-1553.
Citation: Kanayasu-Toyoda T, Uchida E, Yamaguchi T (2020) Cell-Surface MMP-9 is a Novel Functional Marker for Early Endothelial Progenitor Cells Derived from CD133+ Cells. J Stem Cell Res Dev Ther 6: 042.
Copyright: © 2020 Toshie Kanayasu-Toyoda, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

