Chemopreventive and Antioxidative Effects of Averrhoa carambola (Star fruit) Extract against Diethylnitrosamine Induced Hepatocarcinogenesis
*Corresponding Author(s):
Goyal PKDepartment Of Zoology, Radiation & Cancer Biology Laboratory, University Of Rajasthan, Jaipur, India
Tel:+918387841051,
Email:pkgoyal2002@gmail.com
Abstract
Recent investigations have shown that the antioxidant properties of medicinal plants could be correlated to ameliorate the oxidative stress induced during different human diseases including cancer. In the present study, the ability of Averrhoa carambola fruit extract to reduce Diethylnitrosamine/Carbon tetrachloride (DENA/CCl4) induced oxidative stress and hepato-carcinogenesis in Swiss albino mice was investigated. Single intraperitoneal injection of DENA (15 mg/kg b.wt.) and oral administration of CCl4 (1.6g/kg b.wt. in corn oil thrice a week for 24 weeks) produced a marked increase in the lipid peroxidation and decrease in the antioxidant status of glutathione, catalase and superoxide dismutase. However, administration of fruit extract of Averrhoa carambola along with DENA/CCl4 treatment significantly reduced the Lipid Peroxidation (LPO) and increased the activities of Reduced Glutathione (GSH), Catalase (CAT) and Superoxide Dismutase (SOD) in mice. Therefore, the results from the present study demonstrate that the fruit extract of Averrhoa carambola has the potential to reduce the liver cancer by alleviating the oxidative stress during chemical induced carcinogenesis in mammals.
Keywords
Anti-oxidants; CCl4; DENA; Hepato-protective; Liver cancer; Oxidative stress
INTRODUCTION
Hepatocellular Carcinoma (HCC) represents a major health problem today in the world. HCC is a highly prevalent, treatment-resistant malignancy with a multifaceted molecular pathogenesis [1]. The process of HCC involves different genetic alterations that ultimately lead to malignant transformation of the hepatocytes [2]. Surgical resection and liver transplantation are the current curative options to treat liver cancer. However, recurrence or metastasis is quite common in the patients who have had a resection and survival rate 30% to 40% at 5 years post operatively [3]. Worldwide, liver cancer ranks fifth among the solid tumors and it is the third cause of cancer-related mortality with approx. 5 lakh deaths each year [4]. HCC is most prevalent in Asia and Africa as compared to Europe and USA, however, China being reporting the highest liver cancer rate [5].
The liver is a vital organ which regulates many important metabolic functions and is responsible for maintaining homeostasis of the body [6]. Hepatotoxicity caused by drugs or chemicals can occur due to occupational, environmental or dietary exposure. These chemical agents impose excessive stress on the liver filtering function. If accumulation of toxins is faster than the liver metabolizing activity, hepatic damage may occur [7]. It is hypothesized that the continuous exposure to these toxins leads to process of recurrent liver cell necrosis and regeneration. Both genetic and epigenetic changes may occur, eventually leading to the subsequent formation of dysplastic foci, nodules and finally hepatocellular carcinoma [8,9].
All forms of life maintain a reducing environment within their cells. The cellular redox environment is preserved by enzymes that can maintain this reduced state through a constant input of metabolic energy. An imbalance in the redox homeostasis results in an increased flux of reactive oxygen species accumulation of which leads to oxidative stress. During the metabolism, excessive free radicals are generated and may cause liver damage. These ROS can damage DNA, RNA, proteins and lipid components, and this may lead to mutations or apoptosis. Normally, the free radical level in the body is low because healthy organisms can neutralize, metabolize, or subtract the toxic effects by free radical scavengers. Antioxidant defenses, both enzymatic and non-enzymatic, protect the body against oxidative damage. In case of liver disease, ROS seem to play a crucial role in the pathogenesis and the progression of HCC irrespective of the etiology.
It has been realized that the focus and emphasis should be shifted to the control of carcinogenesis, rather than attempting to cure end-stage disease. Chemoprevention, a pharmacological approach to intervention in order to arrest or reverse the process of carcinogenesis, attempts to address this issue.
Recently, considerable attention has been focused on identifying naturally occurring chemopreventive substances capable of inhibiting, retarding or reversing the preneoplastic lesion of carcinogenesis. This increasing interest with the antioxidants of natural origin is developed because they could suppress the oxidative damage of a tissue by stimulating the body own natural defense system [10]. The reasons for the use of herbal medicine over conventional drugs include the latter’s expensive cost, adverse drug reactions and their inefficacy [11]. Moreover, herbal drugs have been regarded as relatively safe in use; numerous types of herbal extracts have been tested in various in vivo or in vitro systems [12].
The 21st century has seen a paradigm shift towards therapeutic evaluation of herbal products in the management of various liver disorders in addition to promotion of natural healing process [13].Various herbs have been found to be efficacious against liver problems including milk thistle, dandelion root, licorice root, chicory root. Averrhoa carambola L. (Oxalidaceae) is also known as the star fruit tree. Studies have shown that the fruit of A. carambola has several medicinal properties and it is rich in antioxidants which act against reactive oxygen species. The ripe star fruit has digestive and biliousness properties. It is also good source of vitamin C and used to treat headache, vomiting, coughing, hangovers and eczemas [14,15]. Furthermore, it is used as an appetite stimulant, diuretic, anti-diarrheal and febrifugal agent. In addition, the extract obtained through the leaves of such plant has been used in the treatment of diabetes [16].
Looking into the pharmacological and medicinal properties of this plant, the present study has been targeted to investigate the possible anti-cancer potential of A. carambola fruit Extract (ACE) against chemical induced hepatocellular carcinoma in mammals.
MATERIALS AND METHODS
Chemicals
Diethylnitrosamine (DENA) and Carbon Tetrachloride (CCl4) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). DENA at a dose 15 mg/kg b.wt (single i.p. injection in normal saline) was injected to initiate hepatic carcinogenesis, while CCl4 (1.6 g/kg b.wt.) in 1:1 dilution with corn oil was given to animals by gavage to stimulate liver cell proliferation and regeneration.
Preparation of plant extract
Carambola fruits were cleaned, air dried and grinded into the form of fine powder. The powder was extracted with 90% ethyl alcohol using soxhlet apparatus and concentrated by evaporating its liquid contents. The required dose for further treatments was prepared by dissolving the extract in Double Distill Water (DDW).
Animals
Swiss albino mice (3 week old) were taken for the experiment from an inbred colony provided feed and water ad libitum. All studies were carried out in accordance with the guidelines of the Institutional Animal Ethics Committee and Indian National Science Academy, New Delhi, India.
Dose selection of ACE
Pilot experiments were done earlier to evaluate the desired dose of ACE for the treatment after evaluation of various biochemical parameters in the liver of mice. The optimum dose found out to be 25 mg/kg/b.wt./animal for this experiment.
Determination of anti-oxidative and anti-cancer activity of ACE
Animals for this experiment were divided into the following groups with 10 animals in each
Group I: Negative control (Vehicle treated normal mice) - In this group, animals were given single i.p. injection of normal saline and later administered with corn oil by oral gavage, three times in a week, for the entire experimental period i.e. 24 weeks.
Group II: Positive control (Carcinogen treated) - The animals in this group were given DENA in normal saline. After 2 weeks of DENA administration, CCl<sub>4</sub> was given 3 times in a week until the end of the experiment.
Group III: Drug treated control - In this group, the animals were administered Averrhoa carambola Extract (ACE) at a dose of 25 mg/kg/b.wt./animal/day for the entire experimental period.
Group IV: Pre-treated experimental - The animals of this group were provided ACE at a dose of 25mg/kg b.wt./day for 5 consecutive days. ACE source was withdrawn 48 hrs before the first administration of DENA. CCl4 was given after 2 weeks as 3 times a week for 24 weeks.
Group V: Peri-post treated experimental- Animals of this group were given ACE (25 mg/kg/b.wt/animal/day), as in Group IV, and continued daily even after the administration of CCl4 till the end of the experimental period i.e. 24 weeks.
Animals from all the above groups were necropsied after 24 weeks of respective treatments. The following parameters were taken into account for the study:
Morphological
- Body weight: The weights of the animals from each group were recorded at the beginning and at the termination of the experiments.
- Liver weight: The liver was taken out from the animals of each group and weighed at the termination of the experiments.
- Tumor incidence: Number of mice carrying at least one tumor expressed as percentage incidence.
- Tumor yield: It refers to the total number of tumors per group (No of tumors/total no of mice)
- Tumor burden: The average number of tumors per tumor bearing mouse (total no. of tumors in all mice/total no of tumor bearing mice).
Biochemical: All the animals were necropsied after the end of experiment i.e. 24 weeks, and the whole liver was taken out from each mice.
Biochemical analysis for the following parameters was performed in the liver:
- Lipid Peroxidation (LPO) - The level of LPO in liver was measured in terms of thiobarbituric acid reactive substances by the method of Okhawa et al. [17]. Briefly, thiobarbituric acid (0.8%), sodium dodecyl sulfate (0.1%), and acetic acid (20%) were added to 100 ml. of the tissue homogenate for 60 min. It was cooled and extracted with N-butanol-pyridine, and the optical density was recorded at 532 nm. The content of TBAS was expressed in nmol/mg.
- Glutathione (GSH) - The level of reduced GSH was estimated by the method of Moron et al. [18]. The GSH content in the liver was measured spectrophotometrically, using Ellman’s reagent with 5,5’-Dithiobis 2-Nitrobenzoic acid (DTNB) as a coloring agent, according to the method of Beutlar et al. [19]. The absorbance was recorded at 412 nm with levels expressed as nmol/mg of protein.
- Catalase (CAT) - The enzyme activity was assayed in the liver by the method of Aebi et al. [20].The content was estimated at 240 nm by monitoring the disappearance of H2O2.
- Superoxide Dismutase (SOD) - The activity of this enzyme was measured by utilizing the method of Marklund et al. [21].
- Total Proteins - The protein contents in liver were measured by the method of Lowry et al. [22]. The absorbance was recorded at 680 nm.
Histopathological analysis: The paraffin block was cut into uniform sections of 5-6 μm thickness using a microtome and tissue sections were stained with hematoxylin and eosin. Light microscopic examinations were made at 100, 200 and 400 magnifications.
Statistical analysis: All the data is normally distributed and the statistical significance of differences for various parameters among different groups was analyzed by Student’s t-test. Data are presented as means ± SEM.
RESULTS AND DISCUSSION
Morphological Study
The animals treated with DENA depicted morphological changes such as increase in liver weights and decrease in body weights at the end of experimentation i.e. 24 weeks. The mice receiving DENA and CCl4 (carcinogen control) exhibited a slight decrease in mean body weights from that of the untreated (Group I) and ACE treated mice (Group III). There was no considerable change in the average body weight of ACE (Group III) and DENA+ACE (Group IV and V) treated animals when compared to vehicle treated control (Group I). The ACE treated mice (Group III) exhibited a spontaneous gain in body weight as similar to the negative control mice. The liver weights were significantly increased (p≤0.01) in DENA/CCl4 treated animals higher as compared to the normal ones. However, the gain in the liver weights was not noticeable among the other groups (Table 1).
| Group | Body Weight (gm) |
Liver Weight (gm) |
Tumor | |||
| Initial | Final | Incidence (%) |
Burden (%) |
Yield (%) |
||
| I | 13.10 ± 1.48 | 32.60 ± 0.37 | 2.54 ± 0.92 | 0 | 0 | 0 |
| II | 12.60 ± 0.34 | 30.06 ± 0.44 | 3.90 ± 0.54* | 100 | 24 | 24 ± 1.31 |
| III | 15.40 ± 1.83 | 33.83 ± 0.97 | 2.60 ± 0.28 | 0 | 0 | 0 |
| IV | 12.50 ± 1.19 | 30.93 ± 0.22 | 3.05 ± 0.33 | 80 | 12.62 | 10.10 ± 1.73* |
| V | 10.3 ± 1.76 | 37.65 ± 2.90 | 3.63 ± 0.68 | 80 | 5.87 | 8.62 ± 1.11* |
Significance level – Normal vs. Carcinogen treated control * = p<0.01; Carcinogen treated control vs. ACE treated experimental ** = p<0.001.
Several small white- grayish foci were detected on the liver of DENA/CCl4 treated mice at the end of 24 weeks. However, animals of vehicle treated (Group I) as well as ACE treated (Group III) did not show any such foci on the liver while DENA treated mice (Group II) had the appearance of the tumor incidence as 100%. On the other hand, concomitant treatment of ACE with DENA (Group III and IV), the number of visible foci was found to be radically decreased and the liver surface was much smoother when compared with Group II animals. The tumor incidence was reduced to 80% when DENA treated mice were orally administered ACE in both the groups (IV and V) but the considerable difference was observed in tumor burden and tumor yield that was reduced to 52.58 and 42.5% respectively in the animals pre-treated with ACE. When such extract was administered during the peri-post treatment, the levels of tumor burden and tumor yield were found to be further reduced by 24.45 and 19.5%, respectively (Table 1).
Biochemical Study
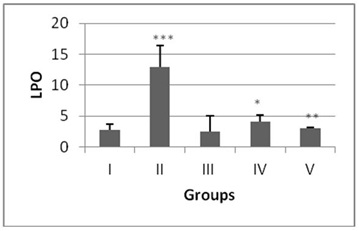
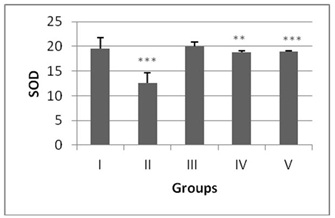
The antioxidant enzyme levels in negative control group were observed to be significantly higher than the DENA treated (positive control). The LPO content in the liver homogenate was significantly increased (p<0.001) by 4.6 fold in DENA treated animals when compared to the normal mice (Figure 1). The antioxidant activities of SOD, CAT, GSH and level of total proteins were significantly decreased (p<0.001) in DENA treated group as compared to normal. Nearly 0.64, 0.16, 0.07 and 0.20 fold decrease was noted from normal in SOD, CAT, GSH and total proteins respectively (Figures 2-5).
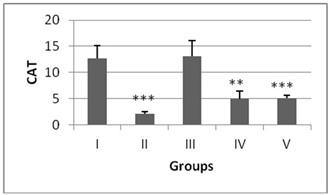
Figure 3: CAT (U/mg tissue) levels levels in liver of mice after DENA/CCl4 treatment with or without ACE administration. Significance level.
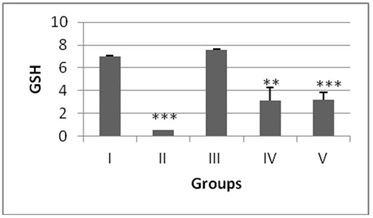
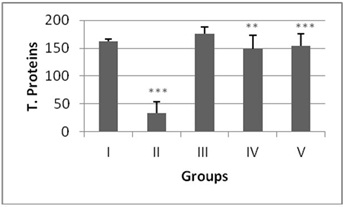
Administration of hydro-ethanolic extract A. carambola fruit at pre-initiational stage led to a significant (p<0.05) drop of 0.31 fold from normal in LPO while the peri-post treatment contributed highly significant (p>0.01) decrease of 0.23 fold (Figure 1). Concurrently, intake of ACE at pre-initiational stage with DENA significantly (p>0.01) increased the levels of antioxidant enzymes viz. SOD by 1.49 fold, CAT by 2.38 fold and GSH by 6.09 fold whereas, the similar extract during the peri-post initiation stage significantly (p<0.001) regained the activities of such enzymes in the liver of mice i.e. 1.50 fold in SOD, 2.4 fold in CAT, 6.27 fold in GSH. Further, the ACE treatment significantly elevated total protein levels by 4.46 (p>0.01) and 4.61 (p>0.001) folds during peri-initiation and peri-post initiation stage, respectively (Figures 2-5).
Histopathology Study
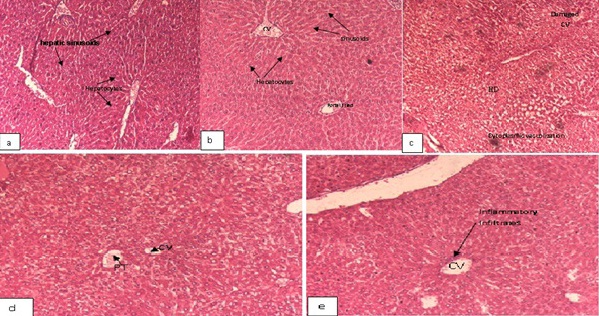
Light microscopic investigations revealed that vehicle treated control (Figure 6a) and ACE treated control (Figure 6b) mice demonstrated normal architecture of liver with hepatocytes arranged in cords located between the sinusoidal capillaries and oriented radially to the terminal venula. The section of the liver showed large number of hepatic lobules, central vein and portal vein. Hepatocytes have more or less centrally situated one large round nucleus and homogenous cytoplasm. No evidence of pathological lesions like necrosis or degenerative changes was observed.
Figure 6: Photomicrograph of transverse section showing liver architecture (H and E, ×200) of mice.
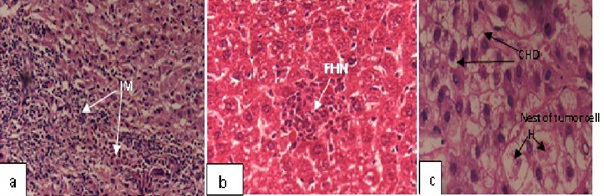
However, the liver of mice treated with DENA/CCl4 showed significant loss of liver architecture. The histologic appearance of HCC is extremely variegated. Damaged hepatocytes were seen with manifestations of extensive cytoplasmic vacuolization, hydropic degeneration and hyaline globules that represent protein produced by the tumor cells and hyperchromatic nuclei (Figure 6c) and seen in nest. Several focal hepatocellular necrosis and necroinflammation (inflammation due to necrotic hepatocytes) was also evident (Figures 7a and 7b).
Figure 7: Photomicrograph of transverse section showing liver architecture (H and E, ×400) of mice treated with DENA/CCl4 showed, (a) Severe Infilteration of Monocytes (IM), (b) Several Focal Hepatocellular Necrosis (FHN), (c) Nests of tumor cells with Cytoplasmic Hydropic Degeneration (CHD), and hyalinization (H).
Experimental group i.e., ACE-DENA/CCl4 treated mice (Figures 7a-7c) demonstrated dilatation in blood sinusoids, slight vacuolization in the cytoplasm of the hepatocytes, less monocytic infilteration in the liver. The histological study confirms the protective effects on DENA/CCl4 induced mice by ACE. The pre treated group with ACE showed well-defined structures and hepatocytes maintaining near normal architecture (Figure 6d), whereas in the peri-post treated ACE group showed normal hepatocyte architecture with well-defined aggregation of hepatic and portal veins (Figure 6e). The potential beneficial effects of ACE on the liver tissue were also visible evident from histological findings. Histological examination also showed substantial improvement in the overall tissue architecture.
Studies on the Carambola have demonstrated that the fruit is rich in several important antioxidants especially polyphenols. These offered protection against the various disorders like cancer, diabetes, osteoporosis, neurodegenerative and cardiovascular diseases [23,24]. Polyphenols act as chemopreventive agents by mediating anti-proliferation, induction of cell cycle arrest or apoptosis, prevention of oxidation, induction of detoxification enzymes, regulation of the host immune system, anti-inflammatory activity and changes in cellular signaling mechanisms. They also influence the metabolism of pro-carcinogens and facilitate their excretion [25].
The major polyphenols reported in star fruit are quercetin-3-O-b-D-glucoside, rutin, lupeol etc. Quercetin mediate the anti-carcinogenic properties by increasing the apoptosis of mutated cells, inhibition of DNA synthesis, inhibition of cancerous cell growth, decrease and modification of cellular signal transduction pathways. Rutin has been found to display chemopreventive properties by acting as an agent that blocks carcinogenesis induced by heterocyclic amines [26]. Quercetin 3-O-glucoside and rutin contribute to the relaxation of smooth muscles in mammals and they also aid in absorption of vitamin-C. Lupeol reported to provide hepatoprotection against aflatoxin B1 [27,28] that causes acute hepatotoxicity and liver carcinoma. It was observed that lupeol can substantially normalized degenerative alterations in the hepatocytes [29], and its treatment induces growth inhibition and apoptosis in HCC cells by down-regulating the death receptors expressed on these cells [30].
In the present study, the rise in LPO and the decline in GSH, CAT, SOD and total proteins in the DENA/CCl4treated liver suggest failure of antioxidant defense mechanism in animals to prevent formation of excessive free radicals.
LPO is regarded as one of the basic mechanisms of cellular damage caused by free radicals. It is an autocatalytic process which causes oxidative tissue damage due to toxicity of xenobiotics [31]. DENA impart hepato-carcinogenicity by its metabolic activation that leads to the release of ethylcarbonium ions which bind to DNA, making adducts and generate superoxide radicals through lipid peroxidation of phospholipids membrane fatty acids [32]. Malondialdehyde (MDA) is an important marker for oxidative stress that is formed during lipid peroxidation. The present data show that a single injection of DENA followed by CCl4 administration elicited a dramatic induction of hepatic lipid peroxidation in mice, as evidenced from an increase in Malondialdehyde levels, indicating severe oxidative stress due to excessive generation of free radicals. The finding also reveals that ACE significantly reduces the DENA-CCl4 induced lipid peroxidation and raises the ability for scavenging free radicals produced by such carcinogens.
There is no single parameter that can be representative of “liver function.” However, the enzymes and the substances released by damaged hepatic cells can serve as the most useful biomarkers of possible liver injury [33]. The detoxification ability of the liver resides in its rich metabolizing antioxidant enzyme profile that includes superoxide dismutase, catalase, intracellular non-enzymatic reserve (glutathione and vitamin C) which serve as important biochemical parameters in estimation of hepatic carcinogenesis.
SOD and CAT provide the first defense against oxygen toxicity by catalyzing the dismutation of superoxide anion to hydrogen peroxide and decomposition of hydrogen peroxide to water and molecular oxygen. Studies have showed the decreased activities of SOD and CAT in DENA-treated mice could be due to overutilization of these non-enzymatic and enzymatic antioxidants to scavenge the products of lipid peroxidation [34]. The current study exhibited a significant decrease in SOD and CAT activities in liver of mice treated with DENA/CCl4 while ACE treatment depicted an extremely significant rise in their levels, which signifies well-defined antioxidant activity in liver of the treated animals. As ACE has rutin which quenches the free radicals especially O2?• produced by DENA, and hence protects the liver cells from further oxidative stress [35].
Glutathione is the most abundant tri-peptide antioxidant present in liver. It is non-protein sulfhydryl-containing compound in the cell and constitutes the major thiol buffer which protects thiol groups of protein from oxidation induced by free radicals [36]. It neutralizes the electrophilic site by providing –SH group and renders the metabolite more water soluble. The GSH levels in biological tissues were found to be decreased after DENA administration [37,38] In the present study, DENA administration led to a significant decrease in the liver GSH levels in comparison with the positive control. Pre-treatment with ACE resulted in the partial restoration of GSH levels, whereas during the peri-post treatment, a significant increase in the levels of GSH was observed when compared with the positive control. GSH plays a crucial role in the detoxification process of majority of alkylating agents including DENA and plays major role in combating oxidative stress [39,40].
It has been suggested that star fruit is rich in polyphenols and flavonoids which mainly include quercetin-3-O-b-D-glucoside, rutin, lupeol, catechin, epicatechin, proanthocyanidins and saponins that mediate hepato-protective role [41,42-44] which is responsible for the significant decrease in tumor incidences.
Histological examinations in this study, revealed noticeable changes that were observed in the architecture of liver in HCC bearing animals like distorted and disorganized liver histology, highly damaged portal tracts and hydropic degeneration. These indicate the presence of neoplastic conditions following DENA and CCl4 administration as observed in other studies [45,46]. In animals treated with ACE (in both peri and peri-post treatments), the liver architecture was well preserved and DENA/CCl4 induced damage was recovered. Hence, the regression of the tumors in liver may be due to the protective effect of A. carambola.
CONCLUSION
The results of the present study indicate that dietary intake of A. carambola, rich in antioxidants, may be an important component of a healthy living strategy to increase the resistance to liver cancer. ACE exhibited significant protection given in peri-post treatment than pre treatment against DENA/CCl4-induced hepatotoxicity. It might be due to its active constituents present in ACE which mediated the free radical scavenging activity, thereby combating the oxidative stress and suppressing the inflammatory cascade against chemical induced hepatic carcinogenesis in the mammals.
SUPPLEMENTAL DATA
The supplemental data include seven figures and one table.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest in the publication of this manuscript.
ACKNOWLEDGEMENTS
The authors are thankful to Council of Scientific and Industrial Research, New Delhi (India) for providing the financial assistance in the form of Senior Research Fellowship to Ms. Ritu Singh under the supervision of Prof. P. K. Goyal.
REFERENCES
- Whittaker S, Marais R, Zhu AX (2010) The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 29: 4989-5005.
- Eriksson L, Ahluwalia M, Spiewak J, Lee G, Sarma DSR, et al. (1983) Distinctive biochemical pattern associated with resistance hepatocytes in hepatocyte nodules during liver carcinogenesis. Environ Health Perspect 49: 171-174.
- Blum HE (2005) Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol 11: 7391-7400.
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74-108.
- Zeinab F, Pourhoseingholi MA, Vahedi M, Zali MR (2011) Burden of Hepatocellular Carcinoma in Asia. Asian Pacific J Cancer Prev 13: 5955-5958.
- Mayuren C, Reddy VV, Priya SV, Devi VA (2010) Protective effect of Livactine against CCl(4) and paracetamol induced hepatotoxicity in adult Wistar rats. N Am J Med Sci 2: 491-495.
- Bigoniya P, Singh CS, Shukla A (2009) A Comprehensive Review of Different Liver Toxicants used in Experimental Pharmocology. Int J Pharmc Sci and Drug Res 1: 124-135.
- Kojiro M, Roskams T (2005) Early hepatocellular carcinoma and dysplastic nodules. Semin Liver Dis 25: 133-142.
- Roskams TA, Libbrecht L, Desmet VJ (2003) Progenitor cells in diseased human liver. Semin Liver Dis 23: 385-396.
- Jodynis-Liebert J, Mat?awska I, Bylka W, Murias M (2005) Protective effect of Aquilegia vulgaris (L.) on APAP-induced oxidative stress in rats. J Ethnopharmacol 97: 351-358.
- Aghel N, Rashidi I, Mombeini A (2007) Hepatoprotective activity of Capparis spinosa root bark against CCl4 induced hepatic damage in mice. Iranian J Pharmaceut Res 6: 285-290.
- Lee HS, Ahn HC, Ku SK (2006) Hypolipidemic effect of water extracts of picorrhiza kurroa in PX-407 induced hyperlipemic ICR mouse model with hepatoprotective effects: A prevention study. J Ethanopahrmacol 105: 380-386.
- Del Prete A, Scalera A, Iadevaia MD, Miranda A, Zulli C, et al. (2012) Herbal Products: Benefits, Limits, and Applications in Chronic Liver Disease. Evid Based Complement Alternat Med 2012: 837939.
- Corrˆea AD (1998) Plantas Medicinais: Do Cultivo `a Terapˆeutica, Editora Vozes, Petr´opolis, Brazil.
- Carolino RO, Beleboni RO, Pizzo AB, Vecchio FD, Garcia-Cairasco N, et al. (2005) Convulsant activity and neurochemical alterations induced by a fraction obtained from fruit Averrhoa carambola (Oxalidaceae: Geraniales). Neurochem Int 46: 523 - 531.
- Provasi M, Oliveira CE, Martino MC, Pessini LG, Bazotte RB, et al. (2001) Avaliação da toxicidade e do potencial antihiperglicemiante da Averrhoa carambola L. Oxalidaceae. Acta Scientiarum 23: 665–669.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582: 67-78.
- BEUTLER E, DURON O, KELLY BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882-888.
- Aebi H (1984) Catalase in vitro. Methods Enzymol 105: 121-126.
- Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47: 469-474.
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- Arts IC, Hollman PC (2005) Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 81: 317S-325S.
- Graf BA, Milbury PE, Blumberg JB (2005) Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food 8: 281-290.
- García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA (2009) Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res 58: 537-552.
- Subrahmanyam Naidu PV, Prakash MMSK, Kalyani P, Muralidhar P (2012) Characterization and biological activities of quercetin thiosemicarbazone derivatives: potential anti cancer drugs. Int J Pharm Biomed Sci 3: 24-27.
- Luyendyk JP, Copple BL, Barton CC, Ganey PE, Roth RA (2003) Augmentation of aflatoxin B1 hepatotoxicity by endotoxin: involvement of endothelium and the coagulation system. Toxicol Sci 72: 171-181.
- Preetha SP, Kanniappan M, Selvakumar E, Nagaraj M, Varalakshmi P (2006) Lupeol ameliorates aflatoxin B1-induced peroxidative hepatic damage in rats. Comp Biochem Physiol C Toxicol Pharmacol 143: 333-339.
- Akçam M, Artan R, Yilmaz A, Ozdem S, Gelen T, et al. (2013) Caffeic acid phenethyl ester modulates aflatoxin B1-induced hepatotoxicity in rats. Cell Biochem Funct 31: 692-697.
- Zhang L, Zhang Y, Zhang L, Yang X, Lv Z (2009) Lupeol, a dietary triterpene, inhibited growth, and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Invest 27: 163-170.
- Gupta M, Mazumder UK, Kumar TS, Gomathi P, Kumar RS (2004). Antioxidant and hepatoprotective effects of Bauhinia racemosa against paracetamol and carbon tetrachloride induced liver damage in rats. Iranian J Pharmacol Therapeutics 3: 12-20.
- Bishayee A, Bhatia D, Thoppil RJ, Darvesh AS, Nevo E et al. (2011) Pomegranate-mediated chemoprevention of experimental hepatocarcinogenesis involves Nrf2-regulated antioxidant mechanisms. Carcinogenesis 32: 888–896.
- Plaa GL (2010) Evaluation of hepatotoxicity: Physiology and biochemical measures of hepatic function in animals. Comprehensive Toxicol 9: 129-140.
- Afzal M, Kazmi I, Anwar F (2013) Antineoplastic potential of Bryophyllum pinnatum lam. on chemically induced hepatocarcinogenesis in rats. Pharmacognosy Res 5: 247-253.
- Guardia T, Rotelli AE, Juarez AO, Pelzer LE (2001) Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 56: 683-687.
- Biswas K, Kumar A, Babaria BA, Setty RS, Prabhu K (2010) Hepatoprotective effect of leaves of Peltophorum pterocarpum against paracetamol induced acute liver damage in rats. J Basic Clin Pharm 1: 10-15.
- Sehrawat A, Sultana S (2006) Evaluation of possible mechanisms of protective role of Tamarix gallica against DEN initiated and 2-AAF promoted hepatocarcinogenesis in male Wistar rats. Life Sci 79: 1456-1465.
- Pradeep K, Mohan CV, Gobianand K, Karthikeyan S (2007) Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol 560: 110-116.
- Chan JY, Stout DL, Becker FF (1986) Protective role of thiols in carcinogen-induced DNA damage in rat liver. Carcinogenesis 7: 1621-1624.
- Conway JG, Neptun DA, Garvey LK, Popp JA (1987) Carcinogen treatment increases glutathione hydrolysis by gamma-glutamyl transpeptidase. Carcinogenesis 8: 999-1004.
- Shui G, Leong LP (2004) Analysis of polyphenolic antioxidants in star fruit using liquid chromatography and mass spectrometry. J Chromatogr A 1022: 67-75.
- Ghosh J, Das J, Manna P, Sil PC (2008) Cytoprotective effect of arjunolic acid in response to sodium fluoride mediated oxidative stress and cell death via necrotic pathway. Toxicol In Vitro 22: 1918-1926.
- Yao LH, Jiang YM, Shi J, Tomás-Barberán FA, Datta N, et al. (2004) Flavonoids in food and their health benefits. Plant Foods Hum Nutr 59: 113-122.
- Kang DH (2002) Oxidative stress, DNA damage, and breast cancer. AACN Clin Issues 13: 540-549.
- Kartik R, Rao CV, Trivedi SP, Pushpangadan P, Reddy GD (2010) Amelioration effects against N-nitrosodiethylamine and CCl4- induced hepatocarcinogenesis in Swiss albino rats by whole plant extract of Achyranthes aspera. Indian J Pharmacol 42: 370-375.
- Moustafa D, Gamal-Eldeen AM, Saleh S, El-Daly SM (2014) The Pharmacological Effect of Gum Arabic on Liver Hyperplasia in the Presence or Absence of Laser Beam. International Journal of Innovative Research and Development 3: 269-273.
Citation: Singh R, Sharma J, Goyal PK (2014) Chemopreventive and Antioxidative Effects of Averrhoa carambola (Star fruit) Extract against Diethylnitrosamine Induced Hepatocarcinogenesis. J Cancer Biol Treat 1: 003.
Copyright: © 2014 Jyoti Sharma, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.