
Complex Cesarian Section
*Corresponding Author(s):
Salvatore FelisObstetric-Gynecology Department, IRCCS-San Martino Hospital, Genoa, Italy
Email:salvatorefelis8@gmail.com
Abstract
The caesarean section, in principle, is not a complex surgical procedure when compared to many others performed in our specialty. However, there is a complex set of physiological and anatomical elements and circumstances that must interact perfectly to obtain an optimal result. Surgical technique is a factor but it isn’t often the primary determinant of a positive outcome; concomitant circumstances interact in Caesarean section, such as: obstructed labor, abruptio placenta, morbid invasion of the placenta, previous pelvic infection, chorioamnionitis / endometritis, chronic and acute anemia, inadequate blood or insufficient transfusion capacity, oxytocics, anesthetics, lack of (or lack of appropriate administration) antibiotics and trained or motivated personnel. In all these cases, and in many other contexts, less than optimal results may occur, even in the face of a perfect surgical technique.
Keywords
Adeguate Resources; Complex Cesarian Section; Hemorrhage; Hysterectomy; Inadeguate Resources
Acronyms and Abbreviations
C-Section: Cesarean Section
CTG: Cardiotocography
OR: Odds Ratio
TOLAC: Trial of Labor after Cesarean Section
PROM: Premature Rupture of Membranes
MAP: Morbid Attachment of the Placenta
ICU: Intensive Care Unit
Introduction
Cesarean section is ideally not a complex surgical procedure compared to many others performed in our specialty. However, a complex set of elements, physiological and anatomical circumstances should/must interact perfectly to obtain a good result. Surgical technique is often not the primary determinant of a positive outcome, the outcome comprehends the interaction of many factors such as: obstructed labor, abruptio placenta, placenta previa, previous pelvic infection, chorioamnionitis / endometritis, chronic or acute anemia, insufficient transfusion capacity, oxytocic, anesthetics, absence of antibiotic therapy or wrong choice of antibiotic and trained or motivated staff. In all these situations and in many other contexts, far from optimal results may occur compared to a perfect surgical technique. As the number of caesarean sections in the world is growing, challenging interventions will become the standard rather than the exception. It is mandatory that obstetricians should receive initial and ongoing training focused on recognizing conditions of increased risk and those who are entrusted with the surgery have adequate experience in managing multiple complications frequently associated with caesarean section. This may involve a revision of the procedures and the development of guidelines appropriate for the referral of cases with the highest risk of complications at these centers of excellence.
Cesarean Section In Environments With Adequate Resources
Risks in full dilation caesarean section with absolute dystocia
The risks of a full dilation caesarean section appear to be less important when there is the availability of an operating room and the necessary support services to perform the intervention. While the risks are somehow lowered in this scenario, all the associated complications should be anticipated. A large retrospective cohort study conducted in Nova Scotia [1] assessed 55,273 births, comprising 549 full dilation caesarean sections. Authors proved that women who underwent a full dilation c-section were more likely to experience intraoperative trauma and perinatal asphyxiation, compared to the ones who underwent a caesarean section during the first stage of labor (OR, 2.57; 95% CI, 1.71-3.88 and OR, 1.5; 95% CI, 1.06-2.14). These results confirm that, even in high level hospital settings, maternal and neonatal outcomes can be adversely affected by prolonged labor leading to a full dilation caesarean section. Another study involved 627 singleton pregnancies in nulliparous women that underwent an emergency cesarean in the UK; of these, 199 (18.9%) had a full dilatation caesarean section [2]. Intraoperative complications and blood transfusions were more likely to occur in women undergoing a full dilation caesarean section (OR, 4.6; 95% CI, 2.7-7.9 and OR, 2.9; 95% CI,1.5-5.6). Apart from that, there were no differences in terms of the incidence of new hospitalization, hospitalization longer than 5 days or perinatal morbidity between the two groups. Another small retrospective study conducted at Singapore on 110 emergency full dilation caesarean sections showed no statistically significant adverse maternal or fetal outcomes [3]. It is not uncommon for the bladder to be injured or lacerated during a cesarean section for protracted or obstructed labor. Once the lesion is recognized, the bladder can be fixed with two-layer closure; if the ureters or the bladder trine are damaged, urological consultation has to be performed and if it is not available the patient should be immediately transferred to a center where it can be done. After the repair of the bladder injury, an urinary catheter should be left in for at least 7-10 days.
Rupture of the uterus
Rupture of the uterus during labor has dramatic consequences for both the mother and the fetus; following the uterine rupture, fetus passes into the abdominal cavity with low chances of survival unless rapid intervention is performed. This situation is often accompanied by some degree of placental abruption. If the patient is admitted to a high-level hospital, early identification of the rupture - one of the first signs is abnormal fetal heart rate on CTG [4] - and delivery within 18 minutes [5] by laparotomy can prevent fetal death and the onset of neurological complications. Risks of hypoxic ischemic encephalopathy and perinatal death with uterus rupture stand at 6.8% (1.8% -10.6%) and 1.8% (0.0% -4, respectively. 2%) respectively [6,7]. Women who decide to undergo a TOLAC (Trial of labor after cesarean) should be informed about the risk of uterine rupture during labor; for women with a previous Pfannenstiel incision this risk is about 1% and increases between 4% and 9% for those with a classic scar [8]. Uterine rupture rarely occurs in women who have never undergone full-thickness uterine surgery, especially in a low-resource environment, after prolonged obstructed labor and increased uterine contractions [9].
Pathological obesity
Obesity is a risk factor for caesarean section. Considering the obesity epidemic it is not surprising that doctors are increasingly called upon to perform caesarean sections for obese women (BMI ≥30) [10, 11]. The results of a secondary analysis of the FASTER study indicate that caesarean section is more common in obese and morbidly obese individuals than in normal weight subjects (20.7% vs 33.8% vs 47.4%, respectively) [12]. Caesarean section in morbidly obese subjects involves an increased risk of complications such as for example, increased technical difficulty of the surgical procedure, poor wound healing and increased potential risk of venous thromboembolism [13]. The need for cesarean section is due to both maternal and fetal factors: the greater difficulty in monitoring fetal heart rate and a more likely dystocia of labor lead to the execution of an earlier and more complex cesarean section. During surgery, the surgeon must assess the skin incision balancing the most efficient extraction of the fetus and the optimal wound healing (Figure 1).
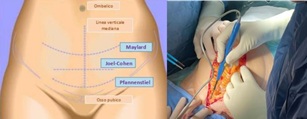 Figure 1: laparotomic abdominal incisions. a. Representation of variants. b. xifopubic incision.
Figure 1: laparotomic abdominal incisions. a. Representation of variants. b. xifopubic incision.
Rather than a vertical midline incision, the Pfannenstiel or Joel-Cohen incision are associated with less abdominal tension and less complications in situations of increased intra-abdominal pressure, such as a chronic cough. In a morbidly obese woman with a large abdominal panniculus wound healing may be impaired due to the continued presence of moisture in the area. If the adipose panniculus is not mobile or the anatomy of the abdomen is altered, it should be better to make a supra-umbilical incision, over the anterior and fundic portion of the uterus, in order to facilitate fetal extraction. This approach has to be preferred in women who opt for tubal ligation and who do not plan further pregnancies as high uterine incisions are associated with a greater risk of subsequent uterus ruptures. Regardless of the type of incision, the current recommendations provide for the execution of a subcutaneous suture when the subcutaneous fat has a thickness greater than 2 cm, to prevent the formation of a seroma and the dehiscence of the wound. If the incision is subjected to a large amount of moisture due to the presence of skin folds, it is wise to leave a dressing covering the healing skin until the risk of wound dehiscence has passed. formation of a seroma. In contrast, there is no evidence that subcutaneous drainage is effective in preventing postoperative morbidity. Additional special considerations must be taken into account when performing caesarean section on morbidly obese individuals. A pragmatic important indication is to make sure that the operating table could support the patient's weight, especially when tilted or placed in the Trendelenberg position. While lying in a supine position a patient may experience shortness of breath. This sensation may not be relieved by releasing the pressure of the uterus on the inferior vena cava with the insertion of a wedge to maintain the left lateral tilt position. In these situations, raising the head of the bed by 15°-20 ° is sufficient to relieve the feeling of shortness of breath. Obesity affects anesthesia choices; it may not be possible to apply epidural analgesia due to inadequate length of the spinal needle, inability to reach anatomical landmarks and irregular distribution of drugs in the blood. If regional anesthesia is ineffective or impossible, the patient must undergo general anesthesia, which involves its own risks, including aspiration of gastric material and transplacental transfer of drugs. Since there is an increased risk of venous thromboembolism, both mechanical and pharmacological prophylaxis must be performed in morbidly obese women. Finally, due attention should be paid to the administration of correct antibiotic dosage, taking into account the potential increased volume of distribution of these drugs [14].
Uterine leiomyomas
The incidence of uterine leiomyomas in pregnancy ranges from 0.1% to 3.9% [15,16]. Uterine leiomyomas can cause various complications during pregnancy, including recurrent miscarriage, preterm birth, premature birth, Premature Rupture of Membranes (PROM), and malpresentation. Furthermore, large uterine myomas are at risk of degeneration during pregnancy leading to inflammation and pain accompanying the degenerative process. Furthermore, women with uterine leiomyomas were more likely to experience complications of labor and delivery, such as dysfunctional or prolonged labor, breech presentation, and cesarean delivery (OR, 1.90; 95% CI, 1.65-2.18)., women with leiomyomas were more likely to require a caesarean section than women whose uterus was of regular morphology (OR, 7.59; 95% CI, 5.47-10.53). Even with medical indications for cesarean section, women with uterine leiomyomas were much more likely to require cesarean section (OR, 5.26; 95% CI, 3.98-6.95). A systematic review examining the influence of uterine leiomyomas on the whole course of pregnancy showed that the difficulties of a caesarean section depend on the number and location of the fibroids [16]. In a study based on hospital records among women who gave birth between 1987 and 1993 in Washington (USA), the prevalence of diagnosed uterine leiomyoma was 0.37% [15]. The study showed that women with uterine leiomyomas were more likely to have pregnancy complications, including first trimester bleeding, placental abruption, and PROM (OR, 1.87; 95% CI, 1.59-2.20). Several factors must be taken into consideration when determining the best way to deliver: women with single pregnancy and a single large fibroid should undergo primary cesarean section if the fetal head is unable to pass the myoma and engage in the pelvis. If the uterine myoma involves a significant portion of the lower uterine segment, thus making a low transverse incision difficult or impossible to access through the uterus, the only viable and safe option may be a classic incision. The safety of myomectomy at the time of caesarean section has long been debated due to the possibility of uncontrollable bleeding following the removal of uterine myomas in the presence of increased vascularity during pregnancy [17]. A retrospective case-control study of 120 women with uterine leiomyomas present during delivery, included 40 women who underwent myomectomy at the time of caesarean section and 80 controls without myomectomy [16]. The authors defined an excessive intraoperative blood loss as both a drop in hematocrit of 10 points and more from the preoperative value and the need for a transfusion. The surgical technique was described as: "A linear incision was made on the myoma and electrocautery was used to remove the myomas with minimal blood loss. The closure of the myometrium was made in one or two layers using absorbable broken sutures (1/0 vicryl caliber). The serosa is sutured using a continuous absorbable suture (2/0 or 3/0 vicryl caliber)." Women who had undergone myomectomy at the time of cesarean section had statistically larger uterine leiomyomas compared to those who did not have a myomectomy (8.1 ± 4.7 cm vs 5.7 ± 2.7 cm, P 0.05). There was no statistically significant difference in the rate of bleeding (12.5 % vs 11.3%) or postoperative fever (defined as temperature ≥38.0 ° C; 7.5% vs 10%) while myomectomy involved longer operations (53.3 ± 18.6 minutes vs 44, 4 ± 6.7 minutes, P P <0.05) and a longer length of hospitalization (3.3 ± 0.8 gi hours vs 2.7 ± 0.6 days, P <0.05). A large university hospital in China has adopted the policy of routine myomectomy at the time of caesarean section for women with uterine leiomyomas; a retrospective study was subsequently performed to evaluate the safety and efficacy of the procedure [18]. A total of 1967 pregnant women also had uterine fibroids, 1438 of whom underwent caesarean section (73.1%): 1242 (86.4%) were simultaneously subjected to myomectomy, 51 (5.3%) to hysterectomy and 145 (10.1%) underwent only a caesarean section. The surgical technique has been described extensively and electrocautery has not been used. Excessive bleeding was defined as a 10% decrease in hematocrit or the need for intraoperative transfusion. Women who had caesarean section associated with hysterectomy had leiomyomas of a larger mean diameter (8.1 ± 3.9 cm) than women who underwent myomectomy at the time of cesarean section (7.3 ± 4.6 cm) and to women undergoing caesarean section without myomectomy (3.6 ± 2.1 cm). There were no significant differences between the three groups in terms of length of hospitalization. The mean length of hospitalization, even routine, among patients with cesarean section without myomas was quite long (4.9 ± 1.9 days). There were also no significant differences between the groups in the frequency of bleeding or the incidence of post-operative fever (defined as temperature ≥38.0 °C). The duration of surgery was longer in women undergoing myomectomy at the time of cesarean section (83.6 ± 10.8 minutes) compared to cesarean section in women without uterine leiomyomas (40.2 ± 8.9 minutes) and cesarean section in women with uterine leiomyomas (41.9 ± 9.1 minutes). Cumulative data suggest that, in appropriately selected pregnant women, myomectomy can be performed safely at the time of caesarean section without concern for uncontrolled bleeding or post-operative fever. However, the optimal technique to use in the context of a caesarean section remains unclear, as several techniques are described, including the use of tourniquets and electrocautery. Patients should be informed of the longer operating time and length of hospital stay when a myomectomy is performed.
Pelvic adhesive disease
The formation of an adhesion is a complex process that results from the imbalance between the deposition of fibrin and its disintegration during the healing process. Most of the adhesions rise during the first postoperative week. While opening the abdomen the obstetrician could sometimes be faced with severe pelvic adhesions, that can be the result of previous caesarean sections, pelvic inflammatory disease or bowel, bladder and gynecological surgeries. Unless there is an immediate danger to the fetal condition, it would be better to restore the anatomy before performing the cesarean section even if there could be a greater risk of sectioning the underlying bowel or bladder. When faced with impenetrable adhesions, it is advisable to make a higher incision in order to expose nonscarred tissue; once the abdomen has been entered and the distinct structures recognized, an orderly approach can be undertaken. The option of an inverted T incision, a J incision or even a separate supraumbilical incision should be taken under consideration. If adhesions make the lower uterine segment completely inaccessible, a fundus or even a posterior uterine incision may be required to deliver the newborn. The presence of adhesions during the caesarean section increases with each subsequent operation affects both the time of delivery of the newborn and the overall surgical operating time. A recent study showed that, in the presence of adhesions, delivery time was increased by 18.2, 20.3 and 27.5 minutes, respectively from second to fourth delivery [19]. When the abdomen is full of severe, extensive and dense adhesions, delivery times will increase by 8.4, 12.6 and 21.5 minutes, from the second to the fourth cesarean section compared to the first cesarean section. The proportional decrease in the pH values of the umbilical cord and in Apgar scores was reported in newborns delivered after a woman already underwent multiple cesarean sections. This study was limited by its retrospective nature and reliance on the interpretation of surgical reports, but the presence and severity of adhesion suggest a potential impact on fetal well-being as a consequence of caesarean sections. These results are consistent with another retrospective review showing that delivery times are significantly increased by 3-5 minutes as the number of caesarean sections increases [20]. The surgical technique focused on the prevention of postoperative adhesions has been studied with variable results, including the closure or non-closure of the rectus muscles and the peritoneum. The presence of intraperitoneal and bladder adhesions have been related to the creation of a bladder flap at the time of hysterotomy: in conclusion it would seem that the creation of a bladder flap causes inflammation and fibrotic reaction, sometimes responsible for inflammation, with subsequent processes reactive and regenerative mesothelial agents that lead to subomesothelial hyperplasia and fibrosis [21]. The same authors, as well as others, have suggested that the closure of the visceral peritoneum during cesarean section can produce an inflammatory reaction and adhesions [22,23]. Therefore, scientific evidence seems to support the non-creation of a bladder flap and the non-closure of the visceral peritoneum at the time of hysterectomy and uterine closure. A recent study has suggested that the presence of severe striae gravidarum may indicate the presence of underlying intraperitoneal adhesions in women who have had a previous caesarean section [24]. The authors used the Davey striae gravidarum score to quantify the severity of the striae and found that 50% of women with severe striae and a previous cesarean section had intraperitoneal adhesions, versus 9% of women without striae. Similarly, the relationship between keloid presence and adhesion formation among women of different races was examined [20]: while adhesion formation appears to be comparable, women with caesarean scar keloids have more adhesions between the uterus and the anterior abdominal wall.
Abnormal fetal station/fetus not engaged
Understanding the fetal station, presentation and position of the head prior to entry into the abdomen or uterus saves valuable minutes at delivery by enabling a strategic approach to surgery. Advantages include proper planning of the incision and correct positioning of the hand, which allows for the best grasp and flexion of the fetal head in the case of cephalic birth (or of the feet in the case of breech birth) and to ensure that any additional maneuvers, such as forceps or suction cups, are available in the room for quick use.
Fetal head not engaged
When an uncommitted or "floating" fetal head is encountered, it can be difficult to guide the head to a transverse uterine incision, in particular when the fetal head deflects. In this case, the use of a suction cup or forceps can be particularly useful in extracting the fetus. However, it is not always possible to know when the forceps or the suction cup will be needed and, if these instruments are not part of the normal caesarean section set, it is advisable to have them available in the operating room to avoid a delayed extraction caused by the recovery of these instruments. To apply a suction cup at the time of cesarean section, the same general principles should be followed as for operative vaginal delivery. Almost all types of forceps can be used at the time of caesarean section, however, long handles and standard handles can create problems in its use and it is therefore advisable to use forceps with short handles and handles during cesarean delivery. The technique for applying these tools is different from the one used in the vagina. First, the fetal head must be palpated to determine presentation; once presentation is confirmed, the posterior blade should be guided under the fetal head and held in place by the assistant. Only at this time can the anterior blade be positioned: depending on the maternal anatomy and the type of forceps available, the anterior blade can be placed directly on the fetal head or it may be necessary to position it posteriorly and guided into position before locking the handles. It is important to lock the handles before exerting any traction on the blades. Finally, the surgical assistant should provide pressure on the fundus of the uterus while gentle upward traction of the forceps is exerted, guiding the fetus out of the hysterotomy; conversely, pulling down and towards the mother's feet can cause an extension of the hysterotomy.
Transverse posterior spine situation
Delivery of a fetus in a transverse posterior position is technically challenging and often requires a classic incision. When transverse uterine incisions are attempted for this presentation it is often necessary to use a J incision or a T incision on the uterus to extract the infant. Extension of the uterine incision is associated with increased blood loss, broad ligament hematoma and uterine artery tear [25]; thus, the preoperative execution of an ultrasound for the evaluation of the fetus, when the presentation is uncertain, can be the key to a safe birth.
Perimortem caesarean section
The incidence of cardiac arrest in pregnancy in the United States from 1998 to 2011 was estimated to be 1 in 12,000 pregnant women [26] and although this number is quite low, it is higher than the most recent estimated incidence in the United Kingdom of 1: 20,000. [27]. This increase is potentially attributable to a number of causes, including an increase in pregnant women with acquired or congenital heart disease, who survive to reproductive age, and events such as acute bleeding, amniotic fluid embolism and sepsis. An International Consensus on cardiopulmonary resuscitation and cardiovascular emergency care, in 2010, established therapeutic recommendations trying to determine if any specific interventions would improve the outcome of pregnant women in cardiac arrest [28]. During full-term pregnancy, occlusion of the inferior vena cava by the uterus and fetus is significant, resulting in a 30% decrease in blood volume for the pregnant woman compared to that of a non-pregnant woman [28]. Placing pregnant women on a left lateral inclined plane, to prevent aorto-caval compression by the pregnant uterus, has numerous maternal and fetal benefits in non-arrest situations:
- For the mother: increased blood pressure and cardiac output.
- For the fetus: better oxygenation and a non-stress situation.
The sum of these benefits suggests that positioning on a left lateral inclined plane should be desirable in case of cardiac arrest to improve maternal and fetal status during resuscitation; however, the method chosen to position the woman in a left lateral tilt during chest compressions may be important for maternal survival. There are few clinical studies on resuscitation techniques for pregnant women. In a systematic review [27] only two studies were identified that used manikins to test the effectiveness of chest compression. These studies suggest that, although chest compressions can be performed in the left lateral inclination position, they are not as effective as those performed in the supine position: with the increase in the inclination angle, the effectiveness of chest compressions decreases. Considering the importance of effective and uninterrupted chest compressions in maintaining the perfusion of critical organs, the authors recommend manual displacement of the uterus as a valid alternative to the left lateral tilt position, stressing that the former is equally effective in relieving aortic compression. Caval during cesarean delivery in patients not in arrest [27]. For the mother, the extraction of the fetus and the afterbirth of the placenta can lead to a rapid improvement in the haemodynamic state, including the return of the pulse and the improvement of blood pressure. Maternal resuscitation is in fact improved from delivery, allowing more blood to return to the heart through the inferior vena cava once the fetus has been extracted [28]. Immediate extraction is even more important when you have a viable fetus, as quick action can make a difference in its survival. Based on data collected between 1900 and 1985, delivery of a viable fetus within 5 minutes of the mother's cardiac arrest is associated with survival with intact neurological status [29] while newborns delivered more than 5 minutes after the onset of 'arrest are more likely to undergo neurological sequelae, the severity of which appears less with increasing gestational age. A perimortem caesarean section should be performed normally but with an emergency character [29]: time should not be wasted in determining fetal heart tones, since the extraction of the fetus from the uterus guarantees greater chances of fetal and maternal survival. The patient may or may not be moved to the operating room, depending on the logistics and time required for transit: since the procedure will be essentially bloodless, due to the absence of maternal cardiac output, the surgery can be performed in almost all places with relative ease. Only after the extraction of the fetus, with the return of cardiac output, and the consequent appearance of blood loss, the closure of the various abdominal layers must comply, as efficiently as possible, with the standard procedure. In general, a vertical skin incision is recommended because of the speed with which the fetus can be extracted but, ultimately, any type of incision that can be done quickly should be done. Where defibrillation is required, the absence of difference in transthoracic impedance during pregnancy suggests that the standard adult energy settings appear appropriate [26], although the study making such observations was performed on an undersized patient sample [27].
Management of Morbidly Adherent Placenta (MAP)
Morbid Attachment of the Placenta (MAP) can encompass many forms ranging from small focal areas of attachment, which may not be recognized, to overt trophoblastic invasion of the bladder or other structures. Some complications can occur in both these situations. Difficult removal of a placenta at the time of caesarean section should always suggest a MAP; in such cases it is necessary to prepare what is necessary to stop a possible bleeding (which can develop before, during and after the closure of the hysterotomy), to perform a blood transfusion and to prevent the development of coagulopathy. Even small focal areas of placenta accreta can weaken uterine integrity and predispose to placental bed rupture, broad ligament hemorrhage, and development of intramyometrial hematoma. In the recognition of a focal MAP, observation of the placental bed and an in-depth evaluation of the uterine wall are fundamental; in most cases the use of haemostatic sutures will be sufficient to control bleeding but if these fail, O'Leary-type uterine artery ligation [30], uterine compression sutures (B -Lynch or some modification thereof) or gradual devascularization [31,32]. An ongoing bleeding, despite these efforts, should lead to a necessary reassessment of the strategy. In the presence of dangerous bleeding and a persistent risk to maternal life, a hysterectomy must be resorted to as a last resort and appropriate measures must be taken for this type of surgery. In such cases, arterial embolization is not an option, given the temporal latency of this procedure and the consequent risk of delays. Very important is the development of algorithms for the treatment of postpartum hemorrhage and the establishment of regular training and simulation courses to ensure staff readiness and familiarity with the disease. Once a decision has been made for an emergency hysterectomy, sustained pressure on the bleeding areas can allow the bleeding to be contained sufficiently to ensure resuscitation and preparation for the procedure. In cases where rapid and safe surgical access to the uterine and utero-ovarian arteries is possible, early clamping or ligation of these vessels (via O'Leary-type sutures or progressively higher lateral uterine sutures) can reduce the loss of blood. In any case, as long as preparations for definitive surgery are in place, blood loss should be attenuated in order to stabilize the patient, maintaining a good hemodynamic situation and normal coagulation and electrolyte parameters [33]. The intervention performed more often are: - direct uterine compression - B-Lynch suture [31] - direct aortic compression - endo-aortic balloon placement [32] - crossed aortic clamping. In those situations in which the bleeding continues persistently, the hemorrhage of the placental bed can be controlled with the implementation of haemostatic sutures and, after the hysterotomy has been closed, with the inflation of a Bakri-type balloon. Following inflation of the balloon with sterile saline, the hysterotomy suture can be visually inspected and its integrity checked. Particular attention should be paid to progressive distension of the lower uterine segment (including after contraction of the upper segment or balloon placement), as such distension suggests that there is ongoing bleeding and may signal the need for further procedures. In those hospital units where arterial embolization instruments are available in the operating room and in the presence of a hemodynamically stable patient, the use of selective arterial embolization is very effective in controlling bleeding. In those contexts where this procedure is not feasible, it is recommended to proceed with the hysterectomy rather than transporting the patient to a radiology department where rapid hysterectomy could not be performed. Sometimes focal MAP is recognized only once most of the placenta has been removed, with areas of deep invasion found; when accompanied by massive bleeding, this circumstance becomes one of the most dangerous situations in obstetrics. In these cases there is a rapid deterioration of vital parameters and haemodynamic instability therefore the workload of both the surgical and anesthetic teams increases exponentially, often with delays in communicating the mutual needs. Concerted efforts for effective interteam communication will ensure that both teams have minute-by-minute situational awareness and the ability to coordinate their actions. Rapid recognition of the need for hysterectomy is essential and all efforts should be directed to performing this procedure with minimal blood loss; in the immediate future, blood loss can be minimized by applying pressure to the infrared portion of the aorta and with bimanual uterine compression. If these efforts succeed in reducing bleeding, they could allow the patient to be resuscitated and stabilized so that definitive surgery can be performed. The start of a massive transfusion protocol should be practiced in all units where a c- section is performed; both before and after the infusion of red blood cells, it is necessary to measure and determine the basal electrolyte levels (in particular Potassium and Calcium), blood count and coagulation profile. If conservative management fails, the team has to proceed with the hysterectomy and a post-operative investigation should be performed for any surgical complications (section or ureter ligature, bladder or intestinal injury, nerve injury or development of bruising). The general principles regarding emergency surgery for placenta accreta include those described below. The following suggestions are in most cases anecdotal, gathered from about 50 years of operational experience and from the results of the international literature [30-35].
Surgical preparation for prenatal assessment of MAP
- The pregnant woman must be hospitalized between 33 and 34 weeks of gestation with planned csection between 34 and 35 weeks and subsequent hysterectomy.
- Preoperative consultation and planning for surgery and following care should be performed by a multidisciplinary team that includes a midwife, gynecologist surgeon, anesthetist, urologist, interventional radiologist, neonatologist, ICU specialist and a hematologist. It is important to have a designated team and standard operating procedures to handle patients even in emergency settings.
- It is essential to establish the degree of postpartum hemorrhage, have adequate massive transfusion protocols and have frequent simulations to keep the team well-trained.
- To place an arterial line, which will be used to monitor blood pressure, to collect specimen and to place a central venous catheter.
- If possible, ureteral stents should be placed bilaterally to help intraoperative identification of the ureters, which are frequently displaced or deviated by the protruding lower uterine segment.
- Patients should be placed in lithotomic position with low stirrups to allow visualization of vaginal bleeding and, if necessary, to allow a third co-surgeon to access the surgical field.
- The abdominal access should consist in a peri-umbilical abdominal incision in the midline, with delicate exteriorization of the pregnant uterus to allow classical posterior or fundic hysterotomy.
- No attempt to remove the placenta should be made when there is a suspicion of MAP.
- In those situations where a high-level facility is not available nearby, the C-section should be performed through an incision as far from the placenta as possible. No attempt to remove the placenta should be made and the uterus should be closed with the placenta in place.
- In those cases where the placental site is not bleeding and the patient is stable, this approach is safer than proceeding with the hysterectomy in an unprepared setting. If the placenta remains in place, the patient must remain in the hospital for at least the first few weeks of observation to avoid postpartum hemorrhage and sepsis.
- There are reports of resection of the placenta and part of the myometrium and reconstitution of the uterus in patients in whom the placenta is not praevia and it is possible to isolate the MAP region [34].
- If the patient starts bleeding during c-section and hysterectomy (or resection) appears to be necessary, as life-saving intervention, every effort should be focused on reducing bleeding as much as possible. Methods such as intermittent aorta compression, crossed blockage of aorta, the placement of an aortic balloon catheter and uterine and pelvic compression should be utilized, until blood transfusion or further help are not available.
MAP surgical technique
- One of the most important aspects of c-section in case of suspected or known placenta accreta is refraining from trying to remove it. Since a greater risk of MAP is increasingly recognized in women undergoing uterine surgery, performing a second trimester ultrasound can allow early detection of this condition and adequate preparation for childbirth. In conclusion, discoverying a MAP at the time of delivery should become a less common occurrence.
- Common practices such as delayed umbilical cord clamping and spontaneous placental abruption at the time of c-section can actually have an unintended positive effect. Waiting longer before attempting manual placental removal allows the clinician to monitor spontaneous placental separation and closely observe the uterus. In case of a placenta that does not separate at the time of c-section, after an appropriate waiting time, the clinician should hypothesize a MAP. This approach can be taught and will become the norm over time; a number of evidence supports this strategy. A partial separation of the placenta with a partial removal of the placenta in a fragmented way and the creation of a forced separation plane in the myometrium switches a stable and reasonable situation to an emerging and life-threatening scenario for the woman with fewer options available.
- Similarly, if areas of thinned myometrium or dilated venous sinuses are observed on the surface of the uterus, even in a patient who does not have a history of multiple uterine surgeries, the best approach is not to apply excessive traction to the cord or attempting to remove a placenta that is not separating spontaneously.
- Reproductive technologies have recently been found to significantly increase the risk of MAP; this observation may explain the increase in the incidence of MAP in women at their first birth.
- Due to the abnormal anatomy observed in MAP, the technique of c-section and hysterectomy is very different from that for an atonic uterus. Due to the very thin lining of the myometrium covering a highly vascularized placental tissue, placing clamps close to the sides of the uterus may result in tearing of the vascular peduncle and underlying uterine tissue, with the exposure of the placental mass and a consequent massive hemorrhage. For this reason, in case of severa placenta accreta, it is essential to perform a wider lateral pelvic dissection of the broad ligament: the round ligaments and the utero-ovarian are ligated, if possible, after closing the uterus. Whether other myometrial regions are invaded, it is required to separate them from the surrounding tissue. After this step, that reduces blood flow to the uterus, most of the blood from the uterus will go through the uterine arteries, upper bladder arteries, cervicovaginal arteries, and vessels which developed through the collateral circulation due to the placenta.
- Before performing hysterectomy in a patient with percrete placenta and clear lateral invasion, or in cases with significant myometrial swelling (indicating a myometrium almost completely replaced by placental tissue), it is recommended to open the retroperitoneal spaces, lateral and parallel to the round ligaments. This step allows an adequate border of avascular parametria to be left between the thinned uterine wall and the vascular peduncle and allows for the safe identification of ureters and retroperitoneal structures. Before ligating the uterine arteries, ureterolysis is performed and an attempt is made to ligate the artery lateral to the ureter; it may be helpful to use a bipolar cauterizer.
- It is advisable to administer blood products early at the first sign of severe bleeding according to a massive transfusion protocol (transfusion of red blood cells and fresh frozen plasma in a 1: 2 ratio). When transfusion is performed, it is important that electrolyte levels are measured frequently, especially calcium and potassium; in the case of placenta percreta every 20-30 minutes must be performed: arterial blood gas analysis, Potassium, Calcium, Magnesium, complete blood count, prothrombin time and partial thromboplastin time, fibrinogen, D-dimer and glucose. The platelet count should ideally be above 100,000 / mL.
- The placement of intra-abdominal drainages to reveal any further bleeding is not usually necessary but can be useful in case of doubts.
Specific considerations in placenta percreta
- It is important to be aware that the blood supply to the lower uterine segment is extremely abnormal in the case of a placenta percreta. Frequently, the same bladder tissue supplies arterial blood to the placenta and drains venous blood through a network of tiny tiny vessels that individually appear insignificant. However, the totality of the surface of these vessels exceeds that of the uterine artery itself. The interface between the bladder tissue and the uterus involved in a placenta percreta must be treated with the same caution as a pulsating artery or a distended vein. Usually, these new vessels have a thin muscular layer and trying to cauterize them with a diathermocoagulator can cause problems. It is also contraindicated to perform a blunt dissection of the invasion areas because this maneuver can cause massive bleeding (Figures 1-5).
- Intraoperative arterial embolization of the placental bed (after c-section but before hysterectomy) may be necessary when the placenta percreta involves the lateral pelvic walls and dissection seems too risky.
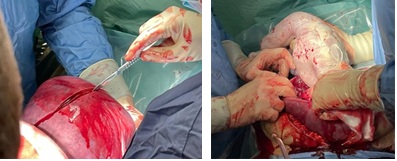 Figure 2-3: Fundic incision in the face of a low placental insertion (a) and subsequent breech extraction of the fetus.
Figure 2-3: Fundic incision in the face of a low placental insertion (a) and subsequent breech extraction of the fetus.
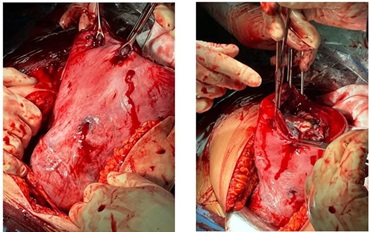 Figure 4: Placenta firmly adhering to the anterior aspect of the lower uterine segment (left) left in situ (right).
Figure 4: Placenta firmly adhering to the anterior aspect of the lower uterine segment (left) left in situ (right).
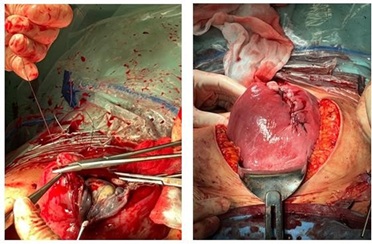 Figure 5: Fundus closure with placenta left in situ (left). Completion of the fundus suture (right).
Figure 5: Fundus closure with placenta left in situ (left). Completion of the fundus suture (right).
- Whenever possible, a careful and thorough dissection of the bladder tissue from the myometrium with a bipolar cautery device (LigaSure) is preferable in order to retain as much bladder wall as possible. The device allows to clamp small pieces of vascular tissue, to dry the tissue mass and to separate the two interface zones. Using this technique, it is often necessary to support and suture the bladder wall after completing the hysterectomy due to significant bladder thinning and damage; sometimes a cystotomy and excision of the attached piece of bladder is done. This approach is preferable to persistent bladder dissection attempts in cases of deep placental invasion.
Post-surgical assistance
- In those facilities that have an intensive care unit, it is preferable to admit these patients after surgery. Frequently, a massive transfusion is performed and electrolyte imbalances are present that require correction and minimal monitoring.
- Secondary bleeding is always a possibility in the first hours after surgery, therefore close monitoring of vital parameters (blood pressure, heart rate and diuresis) is essential.
- In case of long lasting surgery with massive fluid loss and fluid redistribution, prolonged postoperative intubation and assisted ventilation are advisable to reduce the risk of pulmonary edema and respiratory difficulties.
- If minimal residual bleeding is suspected, causing a small collection of intra-abdominal blood, it is recommended to use blood and blood products to resuscitate the patient and correct any coagulopathy. Infusion of large volumes of crystalloids in such patients will produce an expansion of the intravascular volume, a temporary increase in blood pressure and a decrease in heart rate. This situation is usually temporary and it will be necessary to bring the patient back to the operating room, because crystalloids infusion causes hemodilution and the reduction of the intravascular concentration of coagulation factors with consequent collection of blood-clots in the abdomen, leading to abdominal distension and worsening of the hemoperitoneum. Patients often need to be reoperated only to drain the hemoperitoneum. The source of bleeding is usually not detected, and the patient can be closed without further problems once the hemoperitoneum is drained.
Retroperitoneal hemorrhage during Cesarean Section
The most common cause of retroperitoneal hemorrhage at the time of c-section is the extension of a hysterotomy in the broad ligament with injury to the uterine artery. This lesion may be unrecognized for some time and often it is discovered once the blood loss is significant and may have caused the formation of hematomas. In most cases, unless there is an immediate need to drain the blood collection into the retroperitoneum, the safest and most effective way to control bleeding may be the embolization of the uterine arteries or other supply arteries. Attempting to locate the bleeding vessel within an established retroperitoneal hematoma is difficult; if possible and only if the patient is hemodynamically stable, selective embolization may be an option. If embolization is ineffective or impractical and in the presence of continuous life-threatening bleeding, it may become impossible to avoid surgery. In this case, regardless of the initial incision, it is preferable to make a midline abdominal incision, to allow access to the upper retroperitoneum; it is necessary to include in the team a vascular surgeon who can resort to methods such as aortic compression, aortic cross-locking and the positioning of intraortic balloon devices [32,33]. It is preferable to enter the retroperitoneum at the top and where there is no hematoma in order to correctly identify the vascular structures and the possible source of the bleeding. In an unstable patient with coagulopathy, compression of the pelvic structures to temporarily reduce blood loss may provide time for life-saving resuscitation. The placement of a pelvic pressure gauze pack [35] or a balloon device to compress the pelvic lateral walls [36] may allow additional time for the aforementioned resuscitation efforts. The combination of pelvic pressure and embolization can allow definitive control of the bleeding.
C-Section In Low Resource Countries
Most of the complications seen in well-resourced obstetric settings also occur in limited-resource settings, often with a higher incidence. Fetal heart rate monitoring during labor is relatively rare in contexts with limited resources, as it is infrequent to perform a c-section due to impaired fetal conditions; most urgent or emergent caesarean sections are performed for: - obstructed labour, - antepartum hemorrhage, - uterine rupture Prolonged labor is followed by dehydration, anemia, infection and sepsis. The uterus is often edematous and ischemic, with areas of bleeding in the myometrium and, in many of these cases, fetal death has already occurred. Stabilization of the maternal condition prior to surgery is crucial in patients with precarious balance. An urgent C-section performed to attempt to rescue a compromised fetus must be carefully weighed against the risk of losing the mother during or after surgery due to inadequate resuscitation capacity in the face of sepsis, hypovolemic or hemorrhagic shock. In many cases the mother dies from hypovolemic shock after a technically successful cesarean section with average blood loss simply because she cannot tolerate such loss. Such a situation may exist in women with severe chronic anemia due to malaria or other causes (e.g. malnutrition, hookworm, or other helminth infestation) where there has been time for the mother to compensate for a chronically low hemoglobin level (such as 4 or 5 mg / dl) but are unable to sustain an average blood loss of 500cc as heart rate and output are already at the limits of their capacity. In these cases, blood pressure drops with slight changes in stroke volume and there is no compensatory response. Performing spinal or general anesthesia on a woman in septic shock will often represent insurmountable stress. The clinician should understand that maternal outcome is primary and that some decisions that are absolutely not contemplated in high resource settings, must necessarily be implemented in such scenarios. The obstetric surgery algorithm includes acts that are different from those used in a well-resourced environment, such as assessing whether the mother can reasonably survive the anesthesia, stress and blood loss of a normal c-section compared to her state of health. It may be reasonable to proceed with the surgery if the setting has all the characteristics to safely perform the surgery such as skilled anesthetist and medical team, required equipment and blood products. If, on the other hand, she is in a facility without access to emergency resuscitation and blood transfusion, the wisest action could be to delay any surgery until the maternal condition improves or to transport the patient to a more equipped facility; such a decision could inevitably involve the loss of the fetus. In case of fetal death and obstructed labor, performing a destructive fetal procedure to allow vaginal delivery and avoid major abdominal surgery may be an acceptable option in expert hands [37,38]. Although the application of these procedures is a rare event and training in such practices is almost nonexistent in well-resourced environments, it would be advisable to carry out specific training considering multiple circumstances. Considering the low-resources, abdominal surgery can be avoided in women with fetal death and placental abruption and vaginal delivery can be performed safely without serious complications or loss of maternal life [39,40]. In many resource-limited settings, the rate of uterine rupture is estimated to be much higher than high-resource settings, possibly due to traditional practices that encourage the use of herbs and drugs to accelerate labor. Many of the decision-making problems mentioned above are not the ones that most physicians trained in high-resource settings usually face, so it would be necessary to be trained in high-level institutions before being employed in low-resource settings. This preparation may include participation in conferences, counseling by a mental health professional, simulated scenarios and interviews with people with experience in this area. The staff should be closely monitored for signs of stress, demoralization, feelings of hopelessness, anger and frustration with the local system and PTSD (post-traumatic stress disorder), which can occur at any time during their stay.
C-section after obstructed labour
In some cases, c-section after obstructed labor can cause high morbidity or mortality for the woman, so in case of fetal death and a vaginal destructive procedure is possible, this may be the most advisable course of action. If the fetus is in a cephalic presentation, the skull can be shattered to remove the brain material, allowing the skull to collapse and the stillbirth to be delivered. Although morally disturbing, in most cases a vaginal delivery is preferable to a c-section. These women are particularly at risk of uterine atony and post-partum haemorrhage so delivery should only be started in contexts where resuscitation is present; these women often have a history of recent or past infection and an increased risk of placental abruption: it is essential to start an antibiotic treatment and / or antithrombotic prophylaxis [41]. After childbirth and for several days of the puerperium it is mandatory to perform a careful evaluation of the vagina and cervix to promptly diagnose vaginal, cervical and bladder necrosis. All women who have had a long period of obstructed labor may need a bladder catheter for 5-7 days and any fistula formation should be promptly diagnosed [41]. In those cases where there is a live fetus (or, in special circumstances, stillbirth) and an informed decision has been made to perform a c-section, there are some technical issues that deserve to be considered:
- Frequently the baby will be deeply engaged in the pelvis due to prolonged labor;
- The lower uterine segment will be significantly thinned and can be retracted so that the bladder occupies its anatomical space;
- It is common for unsuspecting and inexperienced surgeons to perform an abdominal birth by dissecting the bladder during the creation of the uterine breach or the extraction of the baby.
The resulting damage can cause permanent damage to the urinary tract. The best way to avoid this situation is to initially disengage the fetal head vaginally, with a slight upward pressure, before starting the actual cesarean section. Disengagement may take a few minutes and is usually associated with a loud sucking sound and upward movement of the fetal head returning to the maternal pelvis. Overall, it is advisable to operate with the patient in a low lithotomy position to allow vaginal access during surgery, in addition, a midline incision should be made with extensive exposure of the lower uterine segment. The hysterotomy, after careful identification of the bladder, should be performed higher on the uterus than in an unobstructed labor; a narrow U-shaped incision, directed upward away from the uterine arteries, remains the preferable cutting mode. Before the extraction of the fetal head, an assistant can gently lift the head into the pelvis, to minimize trauma on the lower uterine segment and on the bladder. Uterine atony and postpartum haemorrhage may develop after the extraction, so it is necessary to administer oxytocin and start a uterine massage with manual compression of the myometrium. At the slightest sign of uterine atony and persistent haemorrhage, balloon tamponade (Bakri), compression sutures and concomitant use of other uterotonic drugs should be resorted to immediately. In most low-resource settings, early recognition of bleeding and the aggressive use of methods to reduce blood loss is mandatory. In this setting, a full dilated c-section can be performed even after a long labor and such delays can cause further complications, including a longer hospital stay, increased risk of haemorrhage, the extension of the surgical incision with laceration of the vagina or uterine arteries, the development of lesions to the genitourinary and / or gastrointestinal tract and the onset of postoperative fever [24]. In case of extremely prolonged and hindered labor, necrosis of the cervix may develop with separation of the uterus and vagina and it is almost always accompanied by the presence of a stillbirth, infection or sepsis and extensive destruction of the bladder, usually at the level of the trine. At the same time, the risk of other complications, such as acute respiratory distress syndrome and pulmonary compromise, increases; it is advisable to transfer the patient to a reference center to avoid such occurrences. Hysterectomy may be the best option due to the likely presence of micro-abscesses in the damaged myometrial tissue, with such significant lesions and the high risk of uterine necrosis. Particular attention should be paid to the ureters to ensure their patency and integrity; in the event that the ureters were detached and no urological expertise is available, catheterization with ureteral stents and urine drainage in a sterile bag are used while the patient is transported to a special department and undergoes definitive repair. It has recently been suggested that healthcare professionals should be trained to perform the symphysiotomy in all settings [42]. Symphysiotomy is an old operation in which the fibers of the pubic symphysis are partially divided to allow the separation of the symphysis and therefore the enlargement of the pelvic dimensions, in order to facilitate vaginal birth in the presence of cephalopelvic disproportion; this surgery can be performed under local anesthesia and does not require an operating room or advanced surgical skills [42,43]. A recent review concluded that symphysiotomy can be a life-saving procedure in certain circumstances and that appropriate guidelines should be drawn up for the indications of this procedure [43].
C-section in obese women
Overweight and obesity (BMI 30-45) are more common than underweight in young women residing in both urban and rural areas of many countries with adequate economic resources. In the teaching hospital of the University of Nigeria, Enugu, from May 2008 to December 2010, there was an incidence of 12.4% of patological maternal obesity [44]. Complication rates in obese women are similar in different parts of the world and include intra-partum and postoperative complications such as wound infection and endometritis, wound opening, hematoma or seroma.
Myomectomy during C-Section
Since access to blood products is insufficient in countries with limited resources and myomectomy is associated with an increase in blood loss, there is little experience on performing these two interventions in these settings. A study conducted in Accra, Ghana, comparing cesarean sections with and without myomectomy [45] enrolled 24 women, of whom 12 with leiomyomas and 12 without. In women undergoing myomectomy within the cesarean section, the surgery duration was 11.25 minutes longer than that performed on women with a regular uterus; however, this difference was not statistically significant. There was comparable blood loss in the two groups, with an estimated mean of 392 mL in patients undergoing myomectomy versus 388 mL in patients in whom the procedure was not performed. In a systematic review of nine studies in women who underwent myomectomy during cesarean section there was a greater than 0.30 g / dL drop in hemoglobin in the myomectomy and cesarean group compared to the control group, but the difference was not it was found to be significant [46]. These data suggest that cesarean section and myomectomy may be a reasonable option for those women (e.g. with anterior leiomyoma) who are potentially at risk for postoperative bleeding.
Conclusion
Even if caesarean section is not always a complex surgical procedure, a complex set of elements and circumstances may compromise the outcome.Surgical technique combined with obstetric setting, clinical condition and pregnancy pathology are factor to take in account before performing a cesarean section, to avoid major complications. Medical formation and education on obstetric pathology is an important element that can change the clinical outcome.
References
- Allen VM, O’Connell CM, Baskett TF (2005) Maternal and perinatal morbidity of caesarean delivery at full cervical dilatation compared with caesarean delivery in the first stage of labour. BJOG 112: 986-990.
- Selo-Ojeme D, Sathiyathasan S, Fayyaz M (2008) Caesarean delivery at full cervical dilatation versus caesarean delivery in the first stage of labour: Comparison of maternal and perinatal morbidity. Arch Gynecol Obstet 278: 245-249.
- Radha P, Tagore S, Rahman MF, Tee J (2012) Maternal and perinatal morbidity after Caesarean delivery at full cervical dilatation. Singapore Med J 53: 655-658.
- Ayres AW, Johnson TR, Hayashi R (2001) Characteristics of fetal heart rate tracings prior to uterine rupture. Int J Gynaecol Obstet 74: 235-240.
- Leung AS, Leung EK, Paul RH (1993) Uterine rupture after previous cesarean delivery: Maternal and fetal consequences. Am J Obstet Gynecol 169: 945-950.
- Landon MB, Hauth JC, Leveno KJ, Spong CY, Leindecker S, et al. (2004) Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med 351: 2581-2589.
- Smith G, Pell J, Cameron A, Dobbie R (2002) Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA 287: 2684-2690.
- ACOG (2010) American College of Obstetricians and Gynecologists ACOG Practice Bulletin No. 115: Vaginal birth after previous cesarean delivery. Obstet Gynecol 116: 450-463.
- Akaba GO, Onafowokan O, Offiong RA, Omonua K, Ekele BA (2013) Uterine rupture: Trends and feto-maternal outcome in a Nigerian teaching hospital. Niger J Med 22: 304-308.
- https://www.who.int/health-topics/obesity#tab=tab_1
- https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index
- Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, et al. (2004) Obesity, obstetric complications and cesarean delivery rate: A population-based screening study. Am J Obstet Gynecol 190: 1091-1097.
- Slavin VJ, Fenwick J, Gamble J (2013) Pregnancy care and birth outcomes for women with moderate to super-extreme obesity. Women Birth 26: 179-184.
- Chelmow D, Rodriguez EJ, Sabatini MM (2004) Suture closure of subcutaneous fat and wound disruption after cesarean delivery: A meta-analysis. Obstet Gynecol 103: 974 -980.
- Coronado G, Marshall L, Schwartz S (2000) Complications in pregnancy, labor, and delivery with uterine leiomyomas: A population-based study. Obstet Gynecol 95: 764-769.
- Klatsky PC, Tran ND, Caughey AB, Fujimoto VY (2008) Fibroids and reproductive outcomes: A systematic literature review from conception to delivery. Am J Obstet Gynecol 198: 357-366.
- Kaymak O, Ustunyurt E, Okyay R, Kalyoncu S, Mollamahmutoglu L (2005) Myomectomy during cesarean section. Int J Gynaecol Obstet 89: 90-93.
- Li H, Du J, Jin L, Shi Z, Liu M (2009) Myomectomy during cesarean section. Acta Obstet Gynecol Scand 88: 183-186.
- Morales KJ, Gordon MC, Bates GW (2007) Postcesarean delivery adhesions associated with delayed delivery of infant. Am J Obstet Gynecol 196: e461-466.
- Tulandi T, Al-Sannan B, Akbar G, Ziegler C, Miner L (2011) Prospective study of intraabdominal adhesions among women of different races with or without keloids. Am J Obstet Gynecol 204: e131-134.
- Malvasi A, Tinelli A, Guido M, Cavallotti C, Dell'Edera D, et al. (2011) Effect of avoiding bladder flap formation in caesarean section on repeat caesarean delivery. Eur J Obstet Gynecol Reprod Biol 159: 300-304.
- Malvasi A, Tinelli A, Farine D, Rahimi S, Cavallotti C, et al. (2009) Effects of visceral peritoneal closure on scar formation at cesarean delivery. Int J Gynaecol Obstet 105: 131-135.
- Lyell DJ, Caughey AB, Hu E, Blumenfeld Y, El-Sayed YY, et al. (2012) Rectus muscle and visceral peritoneum closure at cesarean delivery and intraabdominal adhesions. Am J Obstet Gynecol 206: e511-515.
- Cakir Gungor AN, Oguz S, Hacivelioglu S, Isik S, Uysal A, et al. (2013) Predictive value of striae gravidarum severity for intraperitoneal adhesions or uterine scar healing in patients with previous caesarean delivery. J Matern Fetal Neonatal Med 27: 1312-1315.
- Pandit SN, Khan RJ (2013) Surgical techniques for performing caesarean section including CS at full dilatation. Best Pract Res Clin Obstet Gynaecol 27: 179-195.
- Mhyre JM, Tsen LC, Einav S, Kuklina EV, Leffert LR, et al. (2014) Cardiac arrest during hospitalization for delivery in the United States, 1998-2011. Anesthesiology 120: 810-818.
- Jeejeebhoy FM, Zelop CM, Windrim R, Carvalho JC, Dorian P, et al. (2011) Management of cardiac arrest in pregnancy: A systematic review. Resuscitation 82: 801-819.
- Morrison LJ, Deakin CD, Morley PT, Callaway CW, Kerber RE, et al. (2010) Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 122: S345-421.
- Katz VL (2012) Perimortem cesarean delivery: Its role in maternal mortality. Semin Perinatol 36: 68-72.
- O’Leary JL, O’Leary JA (1966) Uterine artery ligation in the control of intractable postpartum hemorrhage. Am J Obstet Gynecol 94: 920-924.
- AbdRabbo SA (1994) Stepwise uterine devascularization: A novel technique for management of uncontrolled postpartum hemorrhage with preservation of the uterus. Am J Obstet Gynecol 171: 694-700.
- Sovik E, Stokkeland P, Storm BS, Asheim P, Bolas O (2012) The use of aortic occlusion balloon catheter without fluoroscopy for life-threatening post-partum haemorrhage. Acta Anaesthesiol Scand 56: 388-393.
- Belfort MA, Zimmerman J, Schemmer G, Oldroyd R, Smilanich R, et al.(2011) Aortic compression and cross clamping in a case of placenta percreta and amniotic fluid embolism: A case report. AJP Rep 1: 33-36.
- Chandraharan E, Rao S, Belli AM, Arulkumaran S (2012) The Triple-P procedure as a conservative surgical alternative to peripartum hysterectomy for placenta percreta. Int J Gynaecol Obstet 117: 191-194.
- Dildy GA, Scott JR, Saffer CS, Belfort MA (2022) An effective pressure pack for severe pelvic hemorrhage. Obstet Gynecol 108: 1222-1226.
- Charoenkwan K (2014) Effective use of the Bakri postpartum balloon for posthysterectomy pelvic floor hemorrhage. Am J Obstet Gynecol 210: e581-583.
- Lawson J (1974) Embryotomy for obstructed labour. Trop Doct 4: 188-191.
- Smale LE (1974) Destructive operations on the fetus: Review of literature and application in 10 cases of neglected dystocia. Am J Obstet Gynecol 119: 369-374.
- Belfort M (1988) A new instrument for the delivery of the impacted dead fetus. Trop Doct 18: 180-182.
- Belfort M, Moore P (1990) The use of a cephalic perforator for delivery of the dead fetus in cases of severe abruptio placentae. SAMJ 77: 80-82.
- Creanga AA, Genadry RR (2007) Obstetric fistulas: A clinical review. Int J Gynaecol Obstet 99: S40-46.
- Monjok E, Okokon IB, Opiah MM, Ingwu JA, Ekabua JE (2012)Obstructed labour in resource-poor settings: The need for revival of symphysiotomy in Nigeria. Afr J Reprod Health 16: 94-101.
- Hofmeyr GJ, Shweni PM (2012) Symphysiotomy for feto-pelvic disproportion. Cochrane Database Syst Rev10: CD005299.
- Okafor UV, Efetie ER, Nwoke O, Okezie O, Umeh U (2012) Anaesthetic and obstetric challenges of morbid obesity in caesarean deliveries: A study in South-eastern Nigeria. Afr Health Sci 12: 54-57.
- Kwawukume E (2002) Myomectomy during cesarean section. Int J Gynaecol Obstet 76: 183-184.
- Song D, Zhang W, Chames MC, Guo J (2013) Myomectomy during cesarean delivery. Int J Gynaecol Obstet 121: 208-213.
Citation: Felis S, Belmartino S, Bertoni M, Carrucciu F, Loddo S, et al. (2023) Trends and Factors Associated with Intentional and Unintentional Injuries Leading to Emergency Department Visits During the COVID-19 Pandemic. J Emerg Med Trauma Surg Care 9: 064.
Copyright: © 2023 Salvatore Felis, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

