
Could A Regulable Expression of HGF from Mesenchymal Stem Cells Improve the Ischemic Stroke Treatment?
*Corresponding Author(s):
Estudillo EnriqueLaboratorio De Reprogramacion Celular, Instituto Nacional De Neurología Y Neurocirugía Manuel Velasco Suárez, Mexico City 14269, Mexico
Tel:+525556063822,
Email:jestudillo@innn.edu.mx
Abstract
The study of Mesenchymal Stem Cells (MSCs) as a therapeutic strategy to treat Ischemic Stroke (IS) has been improving through decades till the point of reaching clinical trials. One of their key features for their success is their versatility regarding to the different routes through which they can be administered; MSCs can be delivered by intravenous administration since these cells are able to reach injured brain regions usually impeded by the blood brain barrier. Although genetic engineering has allowed the MSCs modification in order to enhance their therapeutic effect on IS by introducing therapeutic genes in their genome, the control of their expression has not been considered in deep for their translation to clinics. Interestingly, a regulatory expression system has been introduced in MSCs to treat ischemic limbs by an inducible expression of HGF. Therefore, we hypothesize that MSCs with a regulable system for the expression of HGF could be administered intravenously to treat IS and avoid HGF secretion at undesired time points.
Mesenchymal Stem Cells in the Ischemic Stroke Treatment
Ischemic Stroke (IS) has become the second common cause of death worldwide and it is one of the leading causes of disability which is characterized by cognitive dysfunction, impaired mobility and medical comorbidities that compromise the quality of life [1]. Therefore, new strategies to promote restorative processes in the central nervous system are essential to treat this condition.
Mesenchymal Stem Cells (MSCs) are suitable candidates to promote regeneration in neural tissue damaged by IS. These cells promote regeneration and functional recovery in animal models of IS and several clinical studies have provided evidence of positive results that encourage further research of this therapeutic strategy to finally apply it in clinics [2]. Moreover, MSCs are capable of migrating from the peripheral milieu to ischemic regions inside the brain in response to factors such as the stromal cell-derived factor-1α (SDF-1α) [3]. This property allows their intravenous administration (IV) to treat stroke with a similar efficacy to other alternatives of delivery such as intracranial administration [2,4]. IV administration of MSCs has been tested in animal models of IS with positive results [5]. Although clinical trials have demonstrated the safety of autologous MSCs engraftment by IV route for the treatment of acute IS, no significant improvements of neurological outcome could be appreciated [6]. Therefore, new approaches must be developed in order to promote a therapeutical effect derived from MSCs.
Gene therapy is the transfer of genetic material to a patient and treats a specific disease; particularly, ex vivo gene therapy consists on transferring specific genes to cultured cells for subsequent transplantation in the host to promote a therapeutic effect [7]. MSCs have been genetically modified to express multiple trophic factors such as PIGF, GDNF, BDNF or HGF in order to increase their therapeutic effect on IS [8]. The delivery of genetically modified MSCs to neural tissue damaged by IS has been performed by intracranial and IV administration [4,8]; these strategies promote neurological recovery; however, there is a lack of modulation of the secreted therapeutical molecules as MSCs constitutively express the transgene. Recently, MSCs were modified with a regulable gene expression system to induce HGF in the presence of doxycycline (MSCs-HGF) [9]. When applied on hind limb ischemia, MSC-HGF were able to improve irrigation when compared with wild type MSCs, thus indicating that an inducible expression of therapeutic genes could be a feasible approach to apply in multiple diseases including IS in order to control the expression of a transgene [9].
Modified Mesenchymal Stem Cells Improve the Ischemic Stroke Treatment
In multiple studies, MSCs have been genetically modified to enhance their therapeutic effect at IS. By adenoviral transduction, MSCs were modified to constitutively express PIGF which enhanced their therapeutic effect on IS by reducing the infract area, cell death and increasing angiogenesis and behavioral performance. Remarkably, these cells were able to reach the injured zones when administered intravenously, thus indicating that a low invasive administration could be also viable in patients [5]. Other promising therapeutical molecules include BDNF and GDNF; by transducing MSCs with adenoviral vectors encoding for either BDNF or GDNF, MSCs were able to enhance their therapeutical effect after their IV administration in rats that were submitted to IS when compared with wild type cells. MSCs overexpressing BDNF and GDNF were also able to reach the injured regions, reduce the infarct area and improve the performance of rats in behavioral tests [10,11]. In another study, MSCs were transduced with a replication-incompetent herpes virus simplex to overexpress HGF after intracranial administration; this modification improved behavioral performance and reduced infarct area and apoptosis after IS [12]. Although successful, these strategies require a regulation system to control the release of the therapeutic molecules since they are related with tumorigenesis. The constitutive expression of PIGF could represent a potential risk for the surrounding tissues including those exposed to the secreted PIGF during the MSC migration toward the ischemic zone, since PIGF is upregulated in tumors and it may play a role in their progression [13]. Unfortunately, the constitutive expression of GDNF or BDNF also has the risk of promoting a malignant phenotype as both factors are expressed in different kind of tumors [14,15]. In a similar way, downstream effectors of the HGF signaling pathway are related with tumorigenesis [16].
As already described, the therapeutic genes proposed for neuroregeneration usually carry with them a potential risk of inducing malignant phenotypes, therefore, a regulable system must be implemented to control their expression and avoid any possible issue related with tumorigenesis. The inducible expression of HGF by MSCs in the presence of doxycycline successfully treated hind limb ischemia [9] and provided the first evidence that a regulable system for the secretion of trophic factors to treat ischemia disorders is feasible. Taking together all this information, it is possible that genetically modified MSCs with a regulable expression system for HGF, could reach the injured area after stroke when administered intravenously and promote recovery after the induction of HGF expression. This strategy brings a modulatory advantage on the trophic factor expression since it can be triggered only when required, thus avoiding the risk factor for tumorigenesis as many therapeutic molecules have been related with this process due to their trophic effects.
Designing A Regulable System Of Gene Expression For Mesenchymal Stem Cells
MSCs can be genetically modified through several tools such as viral or plasmidic vectors (Figure 1A). These strategies have allowed the insertion of different genes in MSCs to test their biomedical potential. Therefore, the insertion of a regulable system on MSCs to modulate the expression of a therapeutic gene is a feasible goal that has been already achieved in previous studies [9]. Chang and collaborators have paved the way to insert a regulable system of gene expression in MSCs by using the TALEN-mediated genome editing tool which allowed the expression of HGF under the presence of doxycycline [9]. Besides the TALEN-mediated genome editing technology, MSCs could also be modified by viral vectors in order to introduce a regulable system as the tetracycline-inducible expression system [17,18], however, viral transduction is based on unspecific insertion of the sequence of interest in the cell genome that could represent a risk of developing a malignant phenotype. Although new generation viral vectors are much safer than those previously engineered [7], a rigorous screening for potential alterations of modified MSCs should be performed in order to minimize any potential issue that this strategy could bring.
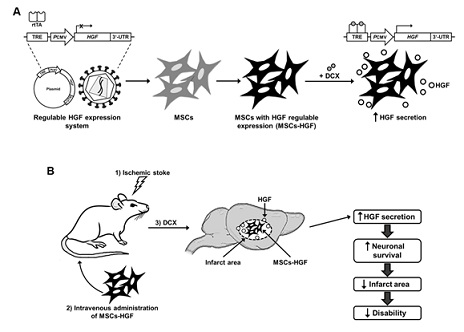 Figure 1: Hypothesized effect of regulable expression of HGF in ischemic stroke. A) Controlled HGF expression in mesenchymal stems cells (MSCs) can be assessed by employing the Tet on system to generate transformed MSCs (MSCs-HGF) to express HGF in presence doxycycline (DCX) increasing the affinity of the reverse tetracycline-controlled transactivator (rtTA) with the tetracycline response element (TRE) in the exogenous DNA to activate the transcription of HGF and finally promote its secretion. B) The MSCs-HGF reaching the infarct area in brain after the intravenous administration in rats with ischemic stroke increases HGF secretion in the injured brain and improves disability by supporting neuronal survival and reduces infarct area.
Figure 1: Hypothesized effect of regulable expression of HGF in ischemic stroke. A) Controlled HGF expression in mesenchymal stems cells (MSCs) can be assessed by employing the Tet on system to generate transformed MSCs (MSCs-HGF) to express HGF in presence doxycycline (DCX) increasing the affinity of the reverse tetracycline-controlled transactivator (rtTA) with the tetracycline response element (TRE) in the exogenous DNA to activate the transcription of HGF and finally promote its secretion. B) The MSCs-HGF reaching the infarct area in brain after the intravenous administration in rats with ischemic stroke increases HGF secretion in the injured brain and improves disability by supporting neuronal survival and reduces infarct area.
As previously described, IV delivery of MSCs with or without genetic modifications represents a viable strategy since it has been proven their safety on preclinical and clinical models respectively [5,6]. IV administration is less invasive than either intracranial or intraarterial administrations and represents a safe method of MSCs administration. Therefore, the effect of MSCs with a regulable system for the expression of HGF could be tested in a rodent model of IS by administering them intravenously, expecting that MSCs could reach the target regions and express the therapeutic gene only after doxycycline administration (Figure 1B). The controlled expression of the HGF would ensure a precise release of the therapeutic molecule and minimize unspecific effects at undesired time points.
Conclusion
IS represents a major cause of disability that compromises the quality of life of either survivors or their families, therefore, new strategies to treat the disability consequences of IS must be developed. Gene therapy is a strategy that has been improving through many years in order to develop safer and more efficient methods to treat diseases through genetic modification and its clinical application is now viable. The optimization of the therapeutic effect of MSCs through genetic engineering has already been tested and a regulable system of gene expression will provide a safer use of these cells in IS when administered intravenously. This hypothesis builds on previous studies that have already achieved a regulable system to modulate the HGF expression and treat ischemic limbs in a preclinical model. Therefore, its implementation on the IS treatment is plausible due to the properties of MSCs that allow them to migrate from the peripheral milieu to the injured neural tissue. Although we focused on HGF, virtually every therapeutic gene studied to treat IS can be used with an inducible expression system, namely PIGF, GDNF or BDNF. Hence a wide number of options to treat IS by engineered MSCs with regulable gene expression should be explored in the near future to improve its treatment.
Acknowledgements
This research was supported by Instituto Nacional de Neurología y Neurocirugía Manuel Velasco Suárez (Grant number 153/18) and Escuela Militar de Graduados de Sanidad, SEDENA (Program A022).
Conflict of Interest
The authors declare no conflict of interest.
References
- Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, et al. (2019) Ischaemic stroke. Nat Rev Dis Primers 5: 70.
- Tang Y, Yasuhara T, Hara K, Matsukawa N, Maki M, et al. (2007) Transplantation of bone marrow-derived stem cells: a promising therapy for stroke. Cell Transplant 16: 159-169.
- Wang Y, Deng Y, Zhou GQ (2008) SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res 1195: 104-112.
- Jiang J, Wang Y, Liu B, Chen X, Zhang S (2018) Challenges and research progress of the use of mesenchymal stem cells in the treatment of ischemic stroke. Brain Dev 40: 612-626.
- Liu H, Honmou O, Harada K, Nakamura K, Houkin K, et al. (2006) Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain 129: 2734-2745.
- Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, et al. (2014) Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke 45: 3618-3624.
- Anguela XM, High KA (2019) Entering the Modern Era of Gene Therapy. Annu Rev Med 70: 273-288.
- van Velthoven CTJ, Kavelaars A, van Bel F, Heijnen CJ (2011) Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behav Immun 25: 1342-1348.
- Chang HK, Kim PH, Cho HM, Yum SY, Choi YJ, et al. (2016) Inducible HGF-secreting Human Umbilical Cord Blood-derived MSCs Produced via TALEN-mediated Genome Editing Promoted Angiogenesis. Mol Ther 24: 1644-1654.
- Horita Y, Honmou O, Harada K, Houkin K, Hamada H, et al. (2006) Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res 84: 1495-1504.
- Nomura T, Honmou O, Harada K, Houkin K, Hamada H, et al. (2005) I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 136: 161-169.
- Zhao MZ, Nonoguchi N, Ikeda N, Watanabe T, Furutama D, et al. (2006) Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab 26: 1176-1188.
- Kim KJ, Cho CS, Kim WU (2012) Role of placenta growth factor in cancer and inflammation. Exp Mol Med 44: 10-19.
- Fielder GC, Yang TW, Razdan M, Li Y, Lu J, et al. (2018) The GDNF Family: A Role in Cancer? Neoplasia 20: 99-117.
- Lam CT, Yang ZF, Lau CK, Tam KH, Fan ST, et al. (2011) Brain-derived neurotrophic factor promotes tumorigenesis via induction of neovascularization: implication in hepatocellular carcinoma. Clin Cancer Res 17: 3123-3133.
- Zhang YW, Wang LM, Jove R, Vande Woude GF (2022) Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene 21: 217-226.
- Lopez-Ornelas A, Vergara P, Segovia J (2014) Neural stem cells producing an inducible and soluble form of Gas1 target and inhibit intracranial glioma growth. Cytotherapy 16: 1011-1023.
- Jiménez A, López-Ornelas A, Estudillo E, González-Mariscal L, González RO, et al. (2014) A soluble form of GAS1 inhibits tumor growth and angiogenesis in a triple negative breast cancer model. Exp Cell Res 327: 307-317.
Citation: Adolfo LO, Adriana J, de la Cruz Neptali G, Carolina OF, Michelle HSA, et al. (2022) Could A Regulable Expression of HGF from Mesenchymal Stem Cells Improve the Ischemic Stroke Treatment? J Stem Cell Res Dev Ther 8: 101.
Copyright: © 2022 López-Ornelas Adolfo, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

