
Efficacy of Different Doses of Human Autologous Adult Bone Marrow Stem Cell Transplantation on Angiogenesis in an Immune Deficient Rat Model with Hind Limb Ischemia
*Corresponding Author(s):
Boris W KramerMaastricht University Medical Center, P. Debyelaan 20, 6222 HX, Maastricht, Netherlands
Email:b.kramer@maastrichtuniveristy.nl
Abstract
Stem cell transplantation has been implied to facilitate angiogenesis by direct paracrine effects on signaling pathways. There are many different sources, types, dosages and routes of administration of stem cells under investigation. In this study we tested the concomitant intramuscular and intravenous administration of human mesenchymal and hematopoietic stem cells from bone marrow aspirates in three different dosages in order to establish an optimal dose for angiogenesis. For this purpose a nude T cell deficient rat model with hind limb ischemia was used as a model for angiogenesis which did not warrant immunosuppressive drugs.
Methods and Results
Seven days after a surgical occlusion of the A. iliaca externa in the right hind limb, a baseline Digital Subtraction Angiography (DSA) was performed, and animals were intra arterially and intramuscularly injected with low, medium or high concentrations of human mesenchymal stem cells or with a saline 0.9% solution (vehicle). At day 35, DSA was repeated, and the images of both registrations were compared between the different concentration groups and the vehicle group.
DSA clearly demonstrated the presence of occlusions, resulting in moderate to severe ischemia 7 days after ligation. Comparing Mean Gray Values at day 35 DSA versus day 7 DSA, the difference did show a natural decrease in the Vehicle-treated group, whereas low- and medium human mesenchymal stem cell doses established a significant increase. High dose had a tendency to decreased values resulting in muscle damage and further occlusions. A dose response could not be detected.
Conclusion
This study showed that the centrifuged human bone marrow suspension containing low and medium concentrations of mesenchymal and hematopoietic stem cells significantly improved the hind limb ischemia as compared to vehicle-treated group in T-cell deficient rats, and that this effect is almost lost with higher doses, may be due to hyper viscosity.
Keywords
INTRODUCTION
We asked in this study whether we can determine an optimal dose for the administration of unfractionated human bone marrow derived cells, containing a mixture of mesenchymal, hematopoietic stem cells and angiotrophic factors after centrifugation, in an immunodeficient animal model in order to promote angiogenesis after hind limb ischemia.We combined intra-arterial with intramuscular administration of human cells to test for enhanced revascularization after 35 days in a dose dependent manner.
METHODS
After clinical examination, 35 female animals were anesthetized and the right A. iliaca externa was proximally occluded to establish a right hind limb ischemia. Rats were anesthetized with isoflurane, and the right hind limb was shaved with an electric clipper, and disinfected with ethanol. The animals then were subcutaneously injected with tramadol 2mg/kg Body Weight (BW) for analgesia. After an incision in the right hind limb, the A. iliaca externa was exposed and proximally occluded with Vycril 5-0. Subcutaneous tissue was then sutured with Vycril 3-0 and the skin was closed with Michel clamps. After the occlusion, the animals were kept one week in their cages for recovery before digital subtraction angiography.
Seven days after the occlusion, the animals were again anesthetized with isoflurane and then the ventral region of the neck was clipped and disinfected with ethanol. The animals received Tramadol 2mg/kg BW subcutaneously for analgesia. First, a conventional X-Ray was performed to document the anatomic locations for further analysis. Secondly, the Arteria Carotis Communis Sinistra (ACCS - left common carotid artery) was prepared and a catheter (1270.02 Umbilical Catheter, Vygon, 2,5 French 30 cm) was inserted into the ACCS and guided into the aorta, with the catheter tip ending directly in front of the bifurcation of the A. iliaca interna. Finally, a Digital Subtraction Angiography (DSA) was performed with a GE OEC 7700 X-ray device and using 0.3 to 0.5 ml of Ultravist® in a fast bolus to visualize the vessels of the rats. The complete DSA procedures were repeated at day 35 after the occlusion with the difference that the catheters were then inserted directly in the abdominal aorta. The DSA device (GE, OEC 7700) settings were 2 images per second at 1,1-1,2 mA and 52- 54mV. The analysis of the angiography was performed using ImageJ® software.
CELL PREPARATION
|
Concentration group |
Mean number of viable CD34+ Cells administered intraarterially (I.A.) and intramuscularly (I.M.) per animal |
Total number of rats |
|
Low Concentration |
I.A.: 327,600 CD34+ Cells/kg
I.M.: 163,800 CD34+ Cells/kg |
7 |
|
Medium Concentration |
I.A.: 688,800 CD34+ Cells/kg
I.M.: 344,400 CD34+ Cells/kg |
6 |
|
High Concentration |
I.A.: 1,308,000 CD34+ Cells/kg
I.M.: 654,000 CD34+ Cells/kg |
5 |
The primary outcome of this study was the change in the vascular density of the blood vessels as an indicator of angiogenesis in the ischemic limbs after treatment with various doses of bone marrow cells versus treatment with the vehicle. Therefore, Regions of Interest (ROI) were set by conventional X-ray from the head of the femur to the distal end of the fibula of both the vascular occluded right and the intact left hind limbs of the individual rats. In these regions, the Mean Gray Value (MGV) was visualized in the digital images taken during the subtraction angiography (DSA) at day 7 and day 35 (MGV-7 and MGV-35). MGV was calculated using the pixel calculator of the ImageJ® Software.
The effect of the unilateral ligation of the (right) iliac artery was studied by comparing MGV-7 of both legs. Then, the effect of the vehicle on vascularization in the right hind limb after ligation was studied by comparing Vehicle MGV-7 and MGV-35 calculations in the right hind limb. The effect of bone marrow cells on the vascularization in the ischemic legs was studied by comparing their MGV-7 and MGV-35 calculations in the ligated right hind limbs with the vehicle MGVs.
STATISTICAL ANALYSIS
The individual changes between the MGV at day 35 and day 7 for every group were tested within the group using a pair wise T-test. At last, the positive changes in the MGV ratio’s for the occluded legs are grouped as 1. No changes or negative changes are grouped as 0. With a Chi-square non parametric test the bone marrow treated group was compared with the Vehicle group and tested for significance. Statistical significance was assumed with p<0.05 levels.
RESULTS
The MGV-7 and MGV-35 for both vascular occluded right and intact left legs were calculated for each animal. There was a significant decrease in MGV-7 in the occluded right legs as compared with the intact left legs in MGV-7 (Figure 1a), which showed about 40% loss of calculated vascular density as compared to the left MGV. During the follow-up, vehicle-treated animals showed significant progression up to roughly 60 % of the MGV at day 35 (Figure 1b) which is in line with results in immunocompetent animal models [8].
 Figure 1: Overview MGV-7 and MGV-35 values in the ischemic right hind leg (left) and in the intact left hind leg. (1a): MGV in intact left leg for Vehicle, low dose, medium dose and high dose group. (1b): MGV in occluded right leg for vehicle, low dose, medium dose and high dose group. Significance compared to MGV-7 with ANOVA marked with *p<0.01. (1c): Percentage of change between the MGV at day 7 and 35 for the occluded leg and the different groups. (*1) Deterioration of the limb ischemia in vehicle treated animals (Pair wise T-Test p = 0.003); (*2) Difference after low dose cell therapy to the vehicle treated group (ANOVA p < 0.001); (*3) Difference after medium dose cell therapy to the vehicle treated group (ANOVA p < 0.01). No improvement after high dose therapy.
Figure 1: Overview MGV-7 and MGV-35 values in the ischemic right hind leg (left) and in the intact left hind leg. (1a): MGV in intact left leg for Vehicle, low dose, medium dose and high dose group. (1b): MGV in occluded right leg for vehicle, low dose, medium dose and high dose group. Significance compared to MGV-7 with ANOVA marked with *p<0.01. (1c): Percentage of change between the MGV at day 7 and 35 for the occluded leg and the different groups. (*1) Deterioration of the limb ischemia in vehicle treated animals (Pair wise T-Test p = 0.003); (*2) Difference after low dose cell therapy to the vehicle treated group (ANOVA p < 0.001); (*3) Difference after medium dose cell therapy to the vehicle treated group (ANOVA p < 0.01). No improvement after high dose therapy.At day 35, a significant increase of vascular density was found in all other bone marrow cells treated groups compared to the vehicle treated group (Figure 1b) except in the high dose group. To set these changes into proportion to each other, figure 1c presents the percentages of change in MGV at day 35 for the occluded leg for the different ASCT01 and vehicle treated groups. Low and medium dose of unfractionated bone marrow cells improved mean gray values significantly which high dose treatment did not. The proportional improvement was highest in the low dose bone marrow cells group. We then asked the question whether there was a correlation between the number of CD34+ cells and the MGV improvement. Figure 2 represents the dose response curve which showed no clear correlation between the absolute number of CD34+ cells administered intraarterially and intramuscularly and the absolute change in MGV at day 35 compared with day 7.
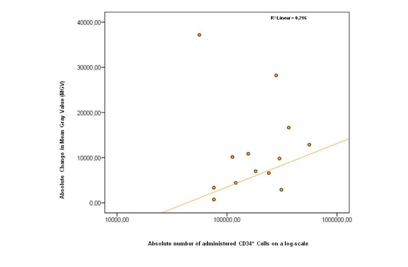 Figure 2: The dose-response graph describes the relationship between the total number of CD34+ cells in low and medium dose ASCT01 and the improvement on day 35 in Mean Gray Value (MGV). The values are weighted by dividing the expected value by the measured value for each point. The R2 is 0,216, which means that no correlation between the total CD34+ and the Mean Gray Value was found.
Figure 2: The dose-response graph describes the relationship between the total number of CD34+ cells in low and medium dose ASCT01 and the improvement on day 35 in Mean Gray Value (MGV). The values are weighted by dividing the expected value by the measured value for each point. The R2 is 0,216, which means that no correlation between the total CD34+ and the Mean Gray Value was found.The concomitant application of cells intraarterially and intramuscularly into the affected limb was done to optimize the re-vascularisation. Angiographical data, as obtained during this study, illustrated that the newly formed vessels were still very small. A representative study is shown in figure 3.
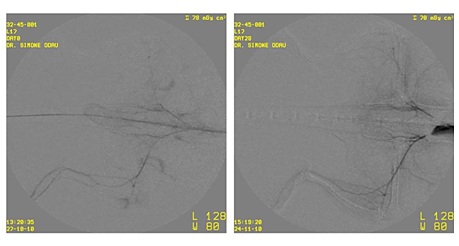 Figure 3: Example of a set of angiographic images of a with human bone marrow cells (ASCT01) treated T cell deficient rat. The left image is taken at day 7 (before treatment) and the right image is taken at day 35 after the treatment with low dose of human bone marrow cells. Despite concomitant application of cells intramuscularly and intravenously, the newly formed vessels are very small.
Figure 3: Example of a set of angiographic images of a with human bone marrow cells (ASCT01) treated T cell deficient rat. The left image is taken at day 7 (before treatment) and the right image is taken at day 35 after the treatment with low dose of human bone marrow cells. Despite concomitant application of cells intramuscularly and intravenously, the newly formed vessels are very small.DISCUSSION
Angiographical data illustrated that the newly perfused vessels were still very small (Figure 3). This could indicate that our intra-arterial and intramuscular approach did not generate an expedition of the underlying angiogenesis. The process of angiogenesis is starting up slowly, making the clinical improvements initially difficult to detect. Therefore, a longer follow up period after treatment would probably result in more and longer blood vessels formation and diameter in the treatment groups. This is most likely not possible in the placebo group due to the natural deterioration of the hind limb occlusion.
Our approach of intra-arterial plus intramuscular administration was very different from currently clinically tested intramuscular or intra-arterial approaches [6,7]. We chose the combined approach to bring the cells to the circulation and allow access from the endothelial side for cell to cell interaction and secretion of paracrine factors [19,20]. In addition, the allogenic cells have a short half-life irrespective of the immunosuppression of the animals [21].The chosen xenograft model, in which T-cell deficient rats were treated with human bone marrow stem cells without any further immune suppression, is useful in order to avoid the confounding effects of immunosuppression [22]. It has the advantages, such as better animal treatment conditions, and better mimicking the human clinical situation when compared with studies were immune suppressive drugs were used.
It would be interesting to also assess the effect of individual treatments (intra-arterial and intra-muscular administration) to clarify the contribution of each route of stem cells administration. Since clinical trials have shown equipoise between the two administration routes we did not include these groups in order to reduce the number of experimental animals.
We faced serious limitation due to the high viscosity which makes this approach less promising [4]. Our study has additional limitations with respect to the time period of follow up and the mode of administration. The approach to test cell-based or cell-derived preparations in T-cell deficient rats is however possible and allows the assessment of cell-based or cell-derived preparations without additional drugs administration since the quest for the best cell preparation is still ongoing [21]. The different properties of the cell mixture of bone marrow versus mesenchymal stromal cells offer opportunities in particular with respect to immunomodulation and/or regeneration [23]. Despite the evolving evidence for clinical effectiveness of autologous bone marrow derived treatment [7], the preparation of bone marrow and the selection of subgroups of cells from bone marrow warrants further studies [4].
CONCLUSION
ACKNOWLEDGEMENT
REFERENCES
- Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, et al. (2002) Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 360: 427-435.
- Klepanec A, Mistrik M, Altaner C, Valachovicova M, Olejarova I, et al.(2012) No difference in intra-arterial and intramuscular delivery of autologous bone marrow cells in patients with advanced critical limb ischemia. Cell Transplant 21: 1909-1918.
- Wang ZX, Li D, Cao JX, Liu YS, Wang M, et al. (2014) Efficacy of autologous bone marrow mononuclear cell therapy in patients with peripheral arterial disease. J Atheroscler Thromb 21: 1183-96.
- Fadini GP, Agostini C, Avogaro A (2010) Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis 209: 10-17.
- Scheubel RJ, Holtz J, Friedrich I, Borgermann J, Kahrstedt S, et al (2010) Paracrine effects of CD34 progenitor cells on angiogenic endothelial sprouting. Int J Cardiol 139:134-41.
- Abdul Wahid SF, Ismail NA, Wan Jamaludin WF, Muhamad NA, Abdul Hamid MKA, et al. (2018) Autologous cells derived from different sources and administered using different regimens for ‘no-option’ critical lower limb ischaemia patients. Cochrane Database Syst Rev 8: CD010747.
- Ponemone V, Gupta S, Sethi D, Suthar M, Sharma M, et al. (2017) Safety and Effectiveness of Bone Marrow Cell Concentrate in the Treatment of Chronic Critical Limb Ischemia Utilizing a Rapid Point-of-Care System. Stem Cells Int 2017: 4137626.
- Kinnaird T, Stabile E, Burnett MS, Epstein SE (2004) Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ Res 95: 354-363.
- Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA (2000) Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest 106: 571-578.
- Pesce M, Orlandi A, Iachininoto MG, Straino S, Torella AR, et al. (2003) Myoendothelial differentiation of human umbilical cord blood-derived stem cells in ischemic limb tissues. Circ Res 93: 51-62.
- Hirata K, Li TS, Nishida M, Ito H, Matsuzaki M, et al. (2003) Autologous bone marrow cell implantation as therapeutic angiogenesis for ischemic hindlimb in diabetic rat model. Am J Physiol Heart Circ Physiol 284: 66-70.
- Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, et al. (2004) Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 94: 678-685.
- Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, et al. (2010) Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 121: 1113-1123.
- Lawall H, Bramlage P, Amann B (2010) Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb Haemost 103: 696-709.
- Urbich C, Dimmeler S (2004) Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res 95: 343-53.
- Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, et al. (2001) Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 103: 2776-2779.
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, et al. (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434-8.
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, et al. (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221-228.
- Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, et al. (2004) Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation109: 1543-1549.
- Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, et al. (2011) Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res 109: 724-728.
- Schiattarella GG, Perrino C, Magliulo F, Carbone A, Bruno AG, et al. (2014) Physical activity in the prevention of peripheral artery disease in the elderly. Front Physiol 5: 12.
- Amann B, Luedemann C, Ratei R, Schmidt-Lucke JA (2009) Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant 18: 371-380.
- Aurora AB, Olson EN (2014) Immune modulation of stem cells and regeneration. Cell Stem Cell 15: 14-25.
Citation: Beugels J, De Munter JPJM, Van der Hulst R, Kramer BW, Wolters ECH (2019) Efficacy of Different Doses of Human Autologous Adult Bone Marrow Stem Cell Transplantation on Angiogenesis in an Immune Deficient Rat Model with Hind Limb Ischemia. J Stem Cell Res Dev Ther: S1002.
Copyright: © 2019 Jip Beugels, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

