
Expression, Functions and Potential Therapeutic Implications of CD157 in Acute Myeloid Leukemia
*Corresponding Author(s):
Yuliya YakymivLaboratory Of Immunogenetics, Department Of Medical Sciences, University Of Torino, 10126 Torino, Italy
Tel:+39 0116961734 ,
Email:yuliya.yakymiv@unito.it
Abstract
Acute Myeloid Leukemia (AML) is a heterogeneous disease characterized by the accumulation of immature myeloid blasts in the bone marrow and peripheral blood. Despite recent clinical advances, the outcome for most patients remains poor. Fewer than one-third of adult AML patients enjoy durable remission, indicating a need for different therapeutic approaches. Immunotherapy carries a promise to eradicate chemoresistant clone(s) and provide long-term disease control; however, suitable targets for AML immunotherapy are currently limited, so discovery of new targets would be highly beneficial to patients. Here we provide an overview of CD157 glycoprotein, an adhesion molecule that has recently attracted attention due to its unique expression on both leukemic cells and the local microenvironment and its implication in the biology of AML. Finally, we discuss strengths and weaknesses of CD157 as a potential therapeutic target.
Keywords
Acute myeloid leukemia; Bone marrow microenvironment; CD157; Immunotherapy; Stem cells; Stromal cells
INTRODUCTION
Acute Myeloid Leukemia (AML) is a genetically, epigenetically and clinically heterogeneous clonal disorder characterized by abnormal proliferation of undifferentiated myeloid progenitors, impaired hematopoiesis and aggressive clinical course [1]. AML has the highest mortality rate among leukemias, and its incidence increases with age, with a slight prevalence in male (54.97%) [2-4]. Despite advances in understanding the biology and genetics of AML, the standard of care for patients has only changed minimally over the past 40 years. Intensive induction chemotherapy with cytarabine plus anthracycline, also known as “3+7” regimen, followed by consolidation chemotherapy or allogeneic hematopoietic stem cell transplantation, in eligible patients, still remains the mainstay of treatment [5]. Despite recent advances in treatment options, the outcome of AML patients remains poor with five-year overall survival rates of approximately 24% for older patients, due to their diminished tolerance for intensive chemotherapy with increased risk of treatment-related toxicity. The dismal prognosis of AML is largely due to the acquisition of resistance to chemotherapy and leukemia relapse [2]. Hence, there is an urgent need to understand the mechanisms of resistance to conventional chemotherapy in order to develop new therapeutic approaches leading to improved outcome of AML patients.
In recent years, the advances in the understanding of the pathogenesis of AML revealed a highly heterogeneous genomic landscape and underlined the complex and dynamic architecture of the disease, eventually leading to the identification of novel diagnostic and prognostic markers and potential therapeutic target [4].
In this review we describe the expression of CD157 in the leukemic cells and in the Bone Marrow (BM) microenvironment, its emerging functional role in the biology of the disease and its potential clinical utility as target for the design of novel therapeutic strategies.
CD157 IN ACUTE MYELOID LEUKEMIA
CD157 is a 45kDa Glycosylphosphatidylinositol (GPI)-anchored glycoprotein first described in 1985 as Mo5 myelomonocytic differentiation antigen [6], and a decade later identified on the membrane of human Bone Marrow Stromal Cells (BMSCs) and hereafter named Bone Marrow Stromal antigen 1 (BST-1) [7]. In the VI Human White Cell Differentiation Antigen workshop BST-1 and Mo5 were grouped together in the Cluster of Differentiation 157 (CD157) [8]. CD157 exists both as membrane-anchored as well as soluble protein [9,10]. Along with its paralogous gene CD38, with which CD157 gene clusters in a head-to-tail manner on human chromosome 4, CD157 constitutes the NAD glycohydrolase (NADase)/ADP ribosyl cyclase mammalian gene family. These two genes share similarities in terms of sequence and structure and encode two proteins with similar functions [11,12]. CD157 and CD38 proteins exert both receptor as well as ectoenzymatic functions; however, CD157 is a much less efficient ADP-ribosyl cyclase than CD38, furthermore, CD157 enzymatic functions are pH-dependent and requires the presence of metal ions like Zn2+ and Mn2+[13].
Besides its functions as an ectoenzyme, CD157 is able of transducing intracellular signals although it lacks a cytoplasmatic domain. To behave as a receptor, CD157 establishes functional and structural crosstalk with β1 (CD29) and β2 (CD18) integrins [14]. Binding of CD157 by its ligand fibronectin or by means of specific monoclonal antibodies (mAbs) mimicking the natural ligand, induces tyrosine phosphorylation of Focal Adhesion Kinase (FAK) in human AML cell lines [15], regulates neutrophil polarization and calcium homeostasis [16], and mediates the activation of SRC, MAPK and AKT signalling pathways in neutrophils and monocytes [17]. Moreover, CD157 plays a crucial role in leukocyte adhesion to Extracellular Matrix (ECM) proteins and diapedesis across the vascular endothelium [16]. In selected epithelial tumors, such as epithelial ovarian cancer and malignant pleural mesothelioma, CD157 promotes metastatic diffusion and epithelial-mesenchymal transition [18,19].
CD157 glycoprotein is expressed at variable levels by leukemia blasts in 97% of AML patients, with no variation of expression at diagnosis or relapse [20,21]. Though at lower extent than in bulk AML blasts, CD157 was also found in CD34+CD38− leukemia-initiating cells, characterized by long-term repopulating potential, ability to propagate and maintain the AML phenotype, and are supposed to be critical for AML relapse [21,22]. A correlation has been reported between high CD157 expression levels and the adverse prognosis group of patients according to the European Leukemia Net (ELN) classification-2017 [23], while no correlation was found between NPM1 and FLT3-ITD mutational status and CD157 expression in a small AML patient cohort analysed [21]. Moreover, CD157 shows highest expression in myelomonocytic and monoblastic leukemia, then in the other AML subtypes [20,21].
CD157 IN THE BONE MARROW MICROENVIRONMENT
Over the last few years, several studies have recognised a key role of the BM microenvironment in the pathogenesis of AML [24]. First described by Schofield in 1987 [25], the BM microenvironment, also called BM niche, is a dynamic and multifaceted environment composed of the endosteal niche (which includes osteoblasts, osteoclasts, adipocytes and mesenchymal stromal cells) and the vascular niche (which includes endothelial cells, CXCR12-abundant reticular cells and megakaryocytes), as well as the surrounding supportive stromal cells and ECM proteins [24,26,27]. The two niches are closely related, both anatomically and functionally, and regulate survival, self-renewal, quiescence, differentiation, proliferation and migration of Hematopoietic Stem Cell (HSC) thought direct cell-cell contact and a variety of soluble factors [26,28]. In addition to regulating the normal hematopoiesis, the BM niche also hosts the leukemia initiating cells, which are responsible for disease initiation and progression, and are believed to be the main cause of relapse and resistance to chemotherapy in AML [22]. AML cells alter the microenvironment to facilitate their own growth and progression by interacting with both cellular and extracellular BM components [29]. Indeed, many leukemic blasts features are not only cell-intrinsic, but also environment-regulated and supported [30].
Cell–cell and cell–matrix interactions within the BM microenvironment contribute significantly to chemotherapy resistance and progression of AML. At the cellular level, this highly mutual interaction is granted by Cell Adhesion Molecules (CAMs) integrating differentiation, proliferation, and pro-survival signals from the surrounding microenvironment inside the cell, giving rise to the so-called cell adhesion-mediated drug resistance (CAM-DR) [31]. CAM-DR is mediated by soluble factors released by cells into the microenvironment and by adhesion molecules expressed on the surface of both AML blasts and stromal cells, like VLA-4 (α4β1 integrin) expressed by AML cells and its ligands expressed by BMSCs, including fibronectin and Vascular-Cell Adhesion Molecule (VCAM)-1 [29,32]. Deciphering the complex interactions between leukemic cells and BM niche may reveal new targets to improve AML therapy and prevent relapse [33]. Besides leukemia cells, CD157 is expressed by multiple cell populations in the BM niche, implicated in leukemia maintenance and progression (Figure 1).
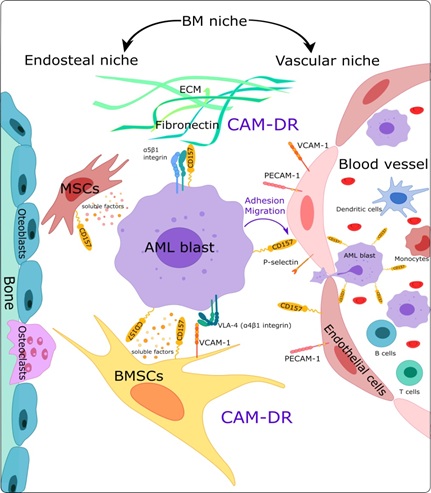 Figure 1: Schematic representation of the AML bone marrow endosteal and vascular niches. AML cells are surrounded by a complex microenvironment composed of extracellular matrix (ECM) proteins and several different cell types, including bone marrow stromal cells (BMSCs), endothelial cells, mesenchymal stromal cells (MSCs), dendritic cells, macrophages, and immune cells. The cell-cell and cell-ECM interactions taking place in the BM microenvironment activate signaling pathways protecting tumor cells from chemotherapy-mediated toxicity (CAM-DR).The expression of CD157 on different cell types is indicated.
Figure 1: Schematic representation of the AML bone marrow endosteal and vascular niches. AML cells are surrounded by a complex microenvironment composed of extracellular matrix (ECM) proteins and several different cell types, including bone marrow stromal cells (BMSCs), endothelial cells, mesenchymal stromal cells (MSCs), dendritic cells, macrophages, and immune cells. The cell-cell and cell-ECM interactions taking place in the BM microenvironment activate signaling pathways protecting tumor cells from chemotherapy-mediated toxicity (CAM-DR).The expression of CD157 on different cell types is indicated.
CD157 binds with high affinity to the heparin-binding domain 1 and 2 composing fibronectin and other selected ECM proteins [34]. The CD157-ECM interaction promotes the concomitant localization of CD157 with β1 and β2 integrins in lipid rafts, thus favouring the organisation of a multimolecular complex that strengthens leukemia cell adhesion through the activation of the MAPK/ERK1/2 and PI3K/Akt signaling pathways [17,34]. Indeed, experimental evidence indicated that genetic loss of CD157 reduced THP1 and U937 AML cell adhesion to fibronectin, collagen type I, and fibrinogen, which are CD157 ligands [34], and increased AML cell sensitivity to cytarabine (AraC). Moreover, fibronectin-mediated CAM-DR against AraC treatment proved to be stronger in CD157-positive U937 cells than in U937 cells devoid of CD157 [20]. Collectively, these data suggest that CD157 has an important role in facilitating leukemia cell interactions with selected ECM proteins and modulates the sensitivity of AML cells to chemotherapy. Preliminary experimental evidence inferred from in vitro models indicated an active role of CD157 expressed by BMSCs in the retention of AML blasts within the BM niche and in their protection against AraC toxicity [35]. The molecular mechanism through which CD157, expressed both by stromal cells and AML blasts, exerts its protective effects against chemotherapy deserves further investigation.
Endothelial Cells (ECs), together with CXCR12-abundant reticular cells, are critical components of BM vascular niche located within the sinusoidal vessels [24,36]. Their main function is to support the proliferation and differentiation of HSCs and lineage-committed progenitors, in addition to the maintenance of HSCs quiescence [37,38]. Normal HSCs are found near sinusoidal endothelium within the BM, and similar intercellular relationship is supposed to exist in leukemia [39]. An increasing number of studies suggested that there is a functional interaction between AML blasts and vascular endothelium [39,40].
CD157 is constitutively expressed on the surface of ECs, mostly at the inter-endothelial junctions where it controls leukocyte diapedesis in inflammatory conditions [41]. Moreover, it has recently been described as a marker of the tissue-resident vascular endothelial stem cells population, located in different parts of the adult mouse blood vascular system and capable of clonal expansion and blood vessel generation [42]. Our studies demonstrated that CD157 ligation on Human Umbilical Vein Endothelial Cells (HUVEC) induced a rapid release of cytosolic Ca2+ and promoted reorganisation of the cytoskeleton with rearrangement of actin filaments and formation of stress fibres in cytokine activated HUVEC cells [41], which is instrumental to the formation of inter-endothelial gap favoring leukocyte transmigration and homing [43].
Mesenchymal Stem Cells (MSCs) are non-hematopoietic stromal cells that provide support to both HSCs [44] and leukemic cells [24], and retain the ability to differentiate into mesenchymal lineages such as osteoblasts, osteoclasts, chondrocytes and adipocytes [45]. MSCs support primary AML blasts proliferation and survival in vitro through the release of specific soluble mediators [46]. In addition, MSCs exert a unique immune-modulatory effect by upregulating indoleamine 2,3-dioxygenases (IDO1) enzyme that is implicated in immune suppression and tumor progression [47]. CD157 is strongly expressed on the surface of undifferentiated MSCs [7], but its expression decreases during osteoblasts differentiation [48], suggesting its role in the undifferentiated stage. Moreover, CD157 has been found to regulate self-renewal, stimulate migration and osteogenic differentiation potential of human MSCs through the interaction with the Scrapie-responsive gene 1 (SCRG1) during tissue and bone regeneration [49,50]. However, functional implication of CD157/SCRG1 complex in MSCs on the physiopathogenesis of leukemia remains so far unexplored.
CD157 AS A TARGET FOR THERAPY IN AML
In recent years, monoclonal antibodies-mediated immunotherapy has achieved considerable success for patients with solid tumors and hematological malignances, including AML [51]. However, AML represents a challenging malignancy to treat, indeed, besides its biological heterogeneity, the complexity of its clonal composition and propensity to change with disease progression further complicates the identification of an optimal target [52]. Indeed, integrated transcriptomic and proteomic analysis of AML surfaceome failed to identify a single surface antigen that meets the requirementsof a good therapeutic target [53]. Moreover, antigens expressed by AML cells are usually shared by normal myeloid progenitors and differentiated myeloid cells; hence, their utility as immunotherapeutic targets is mostly counteracted by on-target toxicity. Nevertheless, several AML tumor-associated antigens are at the forefront of targeted therapy development, which include CD33, CD123, CD13, CLL-1 and CD38 that may be present on both AML blasts and leukemic stem cells [54]. Ongoing clinical studies are investigating the suitability of CD157 as AML target candidate [28].
MEN1112/OBT357 is a first humanized, de-fucosylated IgG1 mAb targeting CD157 with high affinity, designed to optimize the interaction with the FcγRIIIa receptor (CD16) on the NK cells causing antibody-dependent cell-mediated cytotoxicity (ADCC) against AML blasts [20]. Despite the large use of mice animal models for the study of human AML cells in vivo, and for the pre-clinical evaluation of efficacy and safety of novel anti-cancer agents, to date none of the currently available models faithfully reproduce the clonal heterogeneity, initiation and progression of human AML, therefore, they are not considered informative [55]. Consequently, the ex vivo and in vitro experiments were preferred to evaluate the pre-clinical efficacy of MEN1112/OBS357. In an ex vivo experimental setting, MEN1112/OBT357 demonstrated a potent ADCC activity on AML cell lines and primary AML cells in an allogenic system, and to a lesser extent using autologous NK cells from AML patients. As CD157 is expressed on CD34+ BM progenitor cells, as well as on monocytes, the cytotoxic effects of MEN1112/OBT357 were evaluated on these cell populations. Treatment of healthy human Peripheral Blood Mononuclear Cells (PBMCs) with MEN1112/OBT357 resulted in a dose-dependent depletion of monocytes, however, a direct comparison of ADCC on AML cells, monocytes and CD34+ BM progenitor cells showed a slightly higher cytotoxicity against leukemic cells [21]. In addition, AML blast depletion was independent by FcγRIIIa polymorphism [56]. Moreover, safety and pharmacokinetics studies of MEN1112/OBT357 performed in non-human primates, considered the most relevant toxicology species for the in vivo investigations, established a half-life of approximately two weeks and an acceptable toxicological profile [57].
Altogether, the pre-clinical studies supported the rational for further clinical development of MEN1112/OBT357. The ARMY-1 open-label Phase I clinical trial is currently ongoing (NCT02353143) for relapsed/refractory Acute Myeloid Leukemia and has recently completed the dose escalation step [57].
CONCLUSION AND PERSPECTIVES
AML remains a difficult disease to treat despite substantial improvements in understanding its pathophysiology and the emergence of novel therapies. A number of hurdles halt the successful implementation of targeted therapy approaches in AML. Unlike B-ALL, where CD19 and CD20 antigens are relatively restricted to B lymphoblasts [58], AML antigens are nonexclusive, causing a high risk of undesired on-target side effects due to their expression on normal hematopoietic tissues.
In this review, we summarized the current knowledge of the emerging biological role of CD157 in AML by addressing our attention on its unique pattern of expression in both leukemia and BM stromal cells and its emerging functional implication in leukemia maintenance and drug sensitivity. These findings prompt us to believe that CD157 can be a promising candidate to develop therapies that co-target several signalling pathways simultaneously. Anyhow, a note of caution is required, indeed, like other targets for immunotherapy in AML, CD157 expression is not restricted to leukemic cells, hinting that optimization of the clinical use of CD157-specific therapies could be challenging.
The results of MEN1112/OBT357 Phase I clinical trial expected with great interest will shed light on side effects of therapy. Based on lessons learned from other antibodies, it is tempting to predict that the use of low doses of MEN1112/OBT357 in combination with chemotherapy may have an acceptable toxicity profile. Moreover, as personalized therapeutic approaches based on molecular characterization of disease become routine, CD157-directed therapies may play a more prominent role in the treatment of defined subpopulations of AML patients. Gemtuzumab ozogamicin, the best studied anti-CD33 therapeutic antibody in AML [59], has been tested, examined, and modified numerous times in an attempt to maximize its safety and efficacy, and has provided first evidence that monoclonal antibodies could find a niche in the AML treatment armamentarium and should have a role in the clinical care of AML.
FUNDING
This work was supported by grants from the Italian Association for Cancer Research (AIRC, IG. 15968 to A.F.), from the Italian Ministry for University and Scientific Research (60% Projects 2015-2017 to A.F. and E.O.), and by Fondazione CRT-2017, Torino, Italy (to E.O.). Y.Y. is a student of the PhD Program in Biomedical Sciences and Oncology, University of Torino, Italy.
COMPETING INTERESTS
A.F. has received research support from Menarini Ricerche S.p.A. The other authors declare they have no competing interests.
REFERENCES
- De Kouchkovsky I, Abdul-Hay M (2016) ‘Acute myeloid leukemia: A comprehensive review and 2016 update’. Blood Cancer J 6: 441.
- Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM (2019) Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev 36: 70-87.
- Song X, Peng Y, Wang X, Chen Y, Jin L, et al. (2018) Incidence, Survival, and Risk Factors for Adults with Acute Myeloid Leukemia Not Otherwise Specified and Acute Myeloid Leukemia with Recurrent Genetic Abnormalities: Analysis of the Surveillance, Epidemiology, and End Results (SEER) Database, 2001-2013. Acta Haematol 139: 115-127.
- Gurnari C, Voso MT, Maciejewski JP, Visconte V (2020) From bench to bedside and beyond: Therapeutic scenario in acute myeloid leukemia. Cancers (Basel) 12: 1-20.
- Lichtman MA (2013) A historical perspective on the development of the cytarabine (7days) and daunorubicin (3days) treatment regimen for acute myelogenous leukemia: 2013 the 40th anniversary of 7+3. Blood Cells, Mol Dis 50: 119-130.
- Todd RF, Roach JA, Arnaout MA (1985) The modulated expression of Mo5, a human myelomonocytic plasma membrane antigen. Blood 65: 964-973.
- Kaisho T, Ishikawa J, Oritani K, Tomizawa H, Muraoka O, et al. (1994) BST-1, a surface molecule of bone marrow stromal cell lines that facilitates pre-B-cell growth. Proc Natl Acad Sci U S A 91: 5325-5329.
- Hishihara K, Okuyama Y, Lee BOK, Itoh M, Nishikawa K, et al. (1997) CD157 (BST-1) workshop panel report. In: Kishimoto T, Kikutani H, van dem Borne AEGK, Goyert SM (eds) Leucocyte typing VI?: white cell differentiation antigens. Garland Publishing, New York, NY, USA, 1997, pp. 1086-1089.
- Augeri S, Capano S, Morone S, Fissolo G, Giacomino A, et al (2018) Soluble CD157 in pleural effusions: A complementary tool for the diagnosis of malignant mesothelioma. Oncotarget 9: 22785-22801.
- Lee BO, Ishihara K, Denno K, Kobune, K, Itoh M, et al. (1996) Elevated levels of the soluble form of bone marrow stromal cell antigen 1 in the sera of patients with severe rheumatoid arthritis. Arthritis Rheum 39: 629-637.
- Malavasi F, Funaro A, Roggero S, et al (1994) Human CD38: a glycoprotein in search of a function. Immunol Today 15: 95-97.
- Ortolan E, Vacca P, Capobianco A, Horenstein A, Calosso A, et al. (2002) CD157, the Janus of CD38 but with a unique personality. Cell Biochem Funct 20: 309-322.
- Hirata Y, Kimura N, Sato K, Ohsugi Y, Takasawa S, et al. (1994) ADP ribosyl cyclase activity of a novel bone marrow stromal cell surface molecule, BST-1. FEBS Lett 356: 244-248.
- Lavagno L, Ferrero E, Ortolan E, Malavasi F, Funaro A (2007) CD157 is part of a supramolecular complex with CD11b/CD18 on the human neutrophil cell surface. J Biol Regul Homeost Agents 21: 5-11.
- Liang F, Chang CF (2001) Signalling of GPI-anchored CD157 via focal adhesion kinase in MCA102 fibroblasts. FEBS Lett 506: 207-210.
- Funaro A, Ortolan E, Ferranti B, Gargiulo L, Notaro R, et al. (2004) CD157 is an important mediator of neutrophil adhesion and migration. Blood 104: 4269-4278.
- Lo Buono N, Parrotta R, Morone S, Bovino P, Nacci G, et al. (2011) The CD157-integrin partnership controls transendothelial migration and adhesion of human monocytes. J Biol Chem 286: 18681-18691.
- Morone S, Lo-Buono N, Parrotta R, Giacomino A, Nacci G, et al. (2012) Overexpression of CD157 contributes to epithelial ovarian cancer progression by promoting mesenchymal differentiation. PLoS One 7: 43649.
- Ortolan E, Giacomino A, Martinetto F, Morone S, Buono NL, et al. (2014) CD157 enhances malignant pleural mesothelioma aggressiveness and predicts poor clinical outcome. Oncotarget 5: 6191-6205.
- Yakymiv Y, Augeri S, Fissolo G, Peola S, Bracci C, et al. (2019) CD157: From Myeloid Cell Differentiation Marker to Therapeutic Target in Acute Myeloid Leukemia. Cells 8: 1580.
- Krupka C, Lichtenegger FS, Köhnke T, Bögeholz G, Bücklein V, et al. (2017) Targeting CD157 in AML using a novel, Fc-engineered antibody construct. Oncotarget 8: 35707-35717.
- Huntly BJP, Gilliland DG (2005) Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer 5: 311-321.
- Döhner H, Estey E, Grimwade D, Amadori S, Frederick R. Appelbaum et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129: 424-447.
- Ladikou EE, Sivaloganathan H, Pepper A, Chevassut T (2020) Acute Myeloid Leukaemia in Its Niche: the Bone Marrow Microenvironment in Acute Myeloid Leukaemia. Curr Oncol Rep 22: 1-9.
- Schofield R (1978) The relationship between the spleen colony-forming cell and the haemopoietic stem cell. A hypothesis. Blood Cells 4: 7-25.
- Shafat MS, Gnaneswaran B, Bowles KM, Rushworth SA (2017) The Bone Marrow Microenvironment - Home of the Leukemic Blasts. Blood Rev 31: 277-286.
- Nehrbas J, Butler JT, Chen D-W, Kurre P (2020) Extracellular Vesicles and Chemotherapy Resistance in the AML Microenvironment. Front Oncol 10: 90.
- Schürch CM (2018) Therapeutic antibodies for myeloid neoplasms-Current developments and future directions. Front Oncol 8: 152.
- Wang A, Zhong H (2018) Roles of the bone marrow niche in hematopoiesis, leukemogenesis, and chemotherapy resistance in acute myeloid leukemia. Hematology 23: 729-739.
- Behrmann L, Wellbrock J, Fiedler W (2018) Acute Myeloid Leukemia and the Bone Marrow Niche-Take a Closer Look. Front Oncol 8: 444.
- Meads MB, Gatenby RA, Dalton WS (2009) Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat Rev Cancer 9: 665-674.
- Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, et al. (2003) Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med 9: 1158-1165.
- Karantanou C, Godavarthy PS, Krause DS (2018) Targeting the bone marrow microenvironment in acute leukemia. Leuk Lymphoma 59: 2535-2545.
- Morone S, Augeri S, Cuccioloni M, Mozzicafreddo M, Angeletti M, et al. (2014) Binding of CD157 protein to fibronectin regulates cell adhesion and spreading. J Biol Chem 289: 15588-15601.
- Ortolan E, Augeri S, Fissolo G, Musso I, Funaro A (2019) CD157: From immunoregulatory protein to potential therapeutic target. Immunol Lett 205: 59-64.
- Eltoukhy HS, Sinha G, Moore C, Guiro K, Rameshwar P (2016) CXCL12-Abundant Reticular Cells (CAR) Cells: A Review of theLiterature with Relevance to Cancer Stem Cell Survival. J Cancer Stem Cell Res 4: 1004.
- Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, et al. (2015) Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526: 126-130.
- Konopleva MY, Jordan CT (2011) Leukemia stem cells and microenvironment: Biology and therapeutic targeting. J Clin Oncol 29: 591-599.
- Pezeshkian B, Donnelly C, Tamburo K, Geddes T, Madlambayan GJ (2013) Leukemia Mediated Endothelial Cell Activation Modulates Leukemia Cell Susceptibility to Chemotherapy through a Positive Feedback Loop Mechanism. PLoS One 8: 60823.
- Cogle CR, Goldman DC, Madlambayan GJ, Leon RP, Masri AA, et al. (2014) Functional integration of acute myeloid leukemia into the vascular niche. Leukemia 28: 1978-1987.
- Funaro A, Ortolan E, Bovino P, Buono NL, Giulia Nacci, et al. (2009) Ectoenzymes and innate immunity: The role of human CD157 in leukocyte trafficking. Front Biosci 14: 929-943.
- Wakabayashi T, Naito H, Suehiro JI, Lin Y, Kawaji H, et al. (2018) CD157 Marks Tissue-Resident Endothelial Stem Cells with Homeostatic and Regenerative Properties. Cell Stem Cell 22: 384-397.
- Vicente-Manzanares M, Sánchez-Madrid F (2004) Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol 4: 110-122.
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, et al. (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466: 829-834.
- Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726-736.
- Brenner AK, Nepstad I, Bruserud Ø (2017) Mesenchymal stem cells support survival and proliferation of primary human acute myeloid leukemia cells through heterogeneous molecular mechanisms. Front Immunol 8: 106.
- Isidori A, Salvestrini V, Ciciarello M, Loscocco F, Visani G, et al. (2014) The role of the immunosuppressive microenvironment in acute myeloid leukemia development and treatment. Expert Rev Hematol 7: 807-818.
- Quarona V, Ferri V, Chillemi A, Bolzoni M, Mancini C, et al. (2015) Unraveling the contribution of ectoenzymes to myeloma life and survival in the bone marrow niche. Ann N Y Acad Sci 1335: 10-22.
- Aomatsu E, Takahashi N, Sawada S, Okubo N, Hasegawa T, et al. (2014) Novel SCRG1/BST1 axis regulates self-renewal, migration, and osteogenic differentiation potential in mesenchymal stem cells. Sci Rep 4: 3652.
- Chosa N, Ishisaki A (2018) Two novel mechanisms for maintenance of stemness in mesenchymal stem cells: SCRG1/BST1 axis and cell–cell adhesion through N-cadherin. Jpn Dent Sci Rev 54: 37-44.
- Scott AM, Wolchok JD, Old LJ (2012) Antibody therapy of cancer. Nat Rev Cancer 12: 278-287.
- Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, et al. (2016) Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374: 2209-2221.
- Perna F, Berman SH, Soni RK, Mansilla-Soto J, Eyquem J, et al. (2017) Integrating Proteomics and Transcriptomics for Systematic Combinatorial Chimeric Antigen Receptor Therapy of AML. Cancer Cell 32: 506-519.
- Williams BA, Law A, Hunyadkurti J, Desilets S, Leyton JV, et al. (2019) Antibody Therapies for Acute Myeloid Leukemia: Unconjugated, Toxin-Conjugated, Radio-Conjugated and Multivalent Formats. J Clin Med 8: 1261.
- Almosailleakh M, Schwaller J (2019) Murine models of acute Myeloid Leukaemia. Int J Mol Sci 20: 453.
- Venditti A, Brucisano F, Maurillo L, Principe MID, Coppola A, et al. (2015) MEN1112/OBT357, an anti BST1/CD157 humanized antibody inducing Acute Myelogenous Leukemia (AML) blast depletion in an autologous ex vivo assay: A potential new targeted therapy for AML. Blood 126: 788.
- Venditti A, Breems D, Havelange V, Martinelli G, Baldini S, et al. (2015) “ARMY”: First-in-human study of the humanized, defucosylated monoclonal antibody (mAb) MEN1112/OBT357 targeting CD157 antigen, in relapsed or refractory (R/R) acute myeloid leukemia (AML). J Clin Oncol 33: TPS3100.
- Craddock C, Hoelzer D, Komanduri K V (2019) Current status and future clinical directions in the prevention and treatment of relapse following hematopoietic transplantation for acute myeloid and lymphoblastic leukemia. Bone Marrow Transplant 54: 6-16.
- Yu B, Liu D (2019) Gemtuzumab ozogamicin and novel antibody-drug conjugates in clinical trials for acute myeloid leukemia. Biomark Res 7: 24.
Citation: Yakymiv Y, Augeri S, Bracci C, Ortolan E, Funaro A (2020) Expression, Functions and Potential Therapeutic Implications of CD157 in Acute Myeloid Leukemia. J Stem Cell Res Dev Ther 6: 038.
Copyright: © 2020 Yuliya Yakymiv, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

