
Full-Term Human Amniotic Fluid Mesenchymal Stem Cells Show Nephroprotective Effect in an Acute Kidney Injury Model
*Corresponding Author(s):
Cristina GrangeDepartment Of Medical Sciences, University Of Turin, Via Nizza 52, 10126 Torino, Italy
Tel:+39 0116706453,
Email:cristina.grange@unito.it
Abstract
Stem cells isolated from amniotic fluid of full-term pregnancies have been recently proposed as a new source of stem cells. The comparison with first trimester amniotic fluid stem cells is relevant for their application in regenerative medicine. We here isolated a population of human Amniotic Fluid Mesenchymal Stem Cells (AFMSC) from full-term pregnancies during caesarean section. Full-Term (FT)-AFMSCs expressed several stem/stromal cell markers, in common with bone marrow mesenchymal stromal cells and with mid-term Amniotic Fluid Stem Cells (AFSCs) commonly isolated after amniocentesis. The regenerative potential of FT-AFMSCs was investigated in a murine model of acute kidney injury induced by glycerol injection and compared with that of mid-term AFSCs, used as positive control. The administration of FT-AFMSCs accelerated the recovery of renal function, promoting the amelioration of blood urea nitrogen and serum creatinine levels. Moreover, treatment with FT-AFMSCs stimulated tubular cells proliferation. The beneficial effects generated by FT-AFMSCs in renal recovery were comparable to those obtained using AFSCs from amniocentesis.
The results of the present paper suggest that stem cells isolated from full term amniotic fluid are effective in the promotion of renal regeneration and can be an alternative stem cell source obtained at the final stage of pregnancy.
INTRODUCTION
Acute Kidney Injury (AKI) is a highly prevalent health condition characterised by rapid and progressive decrease in glomerular filtration rate, causing loss of renal function [1,2]. One in five adults and one in three children experienced AKI worldwide [3], showing an incidence rate of chronic kidney damage and kidney failure of 25.8% and 8.6% person/years, respectively [4]. It is clear the need for new approaches aimed at prevention and treatment of AKI [4,5].
Several studies in the last years demonstrated the successful use of different stem cell types in the treatment of AKI in distinct experimental animal models [6-11]. Administration of bone marrow-derived mesenchymal stem cells [8,10], bone-marrow derived hematopoietic stem cells [8,12], adipose-derived stem cells [13,14] and endothelial progenitor cells [15,16] have been successfully tested in preclinical models of AKI. Moreover, several studies demonstrated the potential effect of human Amniotic Fluid Stem Cells (AFSCs), obtained during mid-term pregnancy by c-kit/ sorting, for regenerative purposes in different disease models [7,17].
Amniotic fluid provides nutrients and mechanical protection to the developing embryo during gestation. Since it contains cells of fetal origin, it is normally analysed in prenatal diagnosis by amniocentesis to test for possible chromosomal aberrations of the embryo. Therefore, cells from AF can be easily collected by amniocentesis, isolated and expanded in culture [18]. The heterogeneous population isolated includes mesenchymal stem, progenitor and differentiated cells. Several groups have reported their subsequent differentiation into all three types of germ layer cells [19-22] and their potential to treat diseases of different organs including AKI [7,17]. In particular, intravenous injection of AFSCs into SCID mice with glycerol induced AKI ameliorated renal function compared with injection of mesenchymal stem cells, protecting tubular epithelial cells and modulating the inflammatory response [17]. Interestingly, an acute ischemia reperfusion rat model treated with AFSCs with renal progenitor phenotypes showed a reduction of fibrotic process demonstrating the nephroprotective effect of cell treatment [23]. In all these studies the stem cells were derived from mid-term amniotic fluid followed amniocentesis procedure considered invasive with a certain risk for both the mother and the fetus. To possibly exclude procedural risks and minimal ethical objections, isolation and characterisation of cells derived from full-term amniotic fluid have been recently proposed [24].
Based on plastic adherence of unselected populations of amniotic fluid cells, Amniotic Fluid Mesenchymal Stem Cells (AFMSCs) have been isolated from second and third trimester amniotic fluid. A recent study reported the feasibility of collecting big volumes of term amniotic fluid using a siphoning catheter system, allowing the isolation and characterisation of full-term AFMSCs (FT-AFMSCs) [25]. These cells were highly proliferative adherent progenitors with properties similar to those of bone marrow-derived Mesenchymal Stromal Cells (MSCs); interestingly, they could be reprogrammed to pluripotent stem cell fate and then differentiated into hematopoietic and neuronal cell lineages [25]. By using specific growth medium, AFMSCs with mesenchymal characteristics of renal origin have been isolated and characterised as renal progenitor on the basis of their cell morphology, cell surface marker expression, transcriptome and ability to differentiate into chondrocytes, osteoblasts and adipocytes [26]. However, the renoprotective ability demonstrated for mid-term AFSCs in AKI models has not been investigated in FT-AFMSCs.
In this study, we isolated cells from human full-term amniotic fluids obtained after caesarean section. FT-AFMSCs were tested in comparison with mid-term AFSCs in a glycerol-induced rhabdomyolysis AKI mouse model.
MATERIALS AND METHODS
Isolation and culture of cells from mid and term amniotic fluid
Cells were isolated from the human AF of twenty-five healthy women who underwent a caesarean delivery after informed consent was obtained according to ethical committee of Health and Science City Hospital (N. CS2/320). Samples were collected by puncturing the amniotic membranes after the myometrium incision during caesarean delivery. Samples were centrifuged at 1500 rpm for 10 minutes and all isolated cells were plated in 35mm2 Petri dishes containing α-MEM Medium (Gibco/BRL) supplemented with 20% Chang Medium B (Irvine Scientific, Santa Ana, California, USA) and 2% Chang Medium C (Irvine Scientific), 20% Fetal Calve Serum (Invitrogen, Carlsbad, CA, USA), 50 IU/mL penicillin, 50 g/mL streptomycin, 5mM glutamine (all from Sigma-Aldrich, St. Louis, MO, USA). The medium was removed after 5 days of incubation at 37°C with 5% CO2 incubator, removing the non-adhering cells. Afterwards, the medium was replaced twice weekly until the cells reached confluence. FT-AFMSCs were used between 1st-6th passages. AFSCs from mid-term amniotic fluid were generated as described [7] and used as positive control. Cells, collected during amniocentesis of healthy woman (as approved by the IRB of Children’s Hospital Los Angeles), were sorted using c-kit selection, cloned and cultured as above. AFSCs were used between 17th-26th passages.
Flow cytometry
FT-AFMSCs were characterized at passage 1 or 2 using flow cytometry analysis. Cells were detached using the non-enzymatic cell dissociation solution (Sigma-Aldrich), centrifuged at 1,200 rpm for 5 minutes and then solved in 0.1% Bovine Serum Albumin (BSA)-Phosphate Buffered Saline (PBS). For each staining, approximately 100,000 cells were incubated for 30 minutes at 4°C with either FITC, APC or PE-conjugated antibodies against CD24, CD29, CD73, CD90, CD133, CD146, CD166 (BD Bioscences, Franklin Lakes, NJ, USA), CD44 (BioLegend, San Diego, CA, USA), CD105, CD117 (MiltenyiBiotec, Aubum, CA, USA), SSEA4 (R&D Systems, Minneapolis, USA). Labelled cells were washed by centrifugation in 0.1% BSA-PBS and, finally, the pellet was resuspended in 200 µl of 0.1% BSA-PBS. Cells were then exposed to cytofluorimetric analysis (FACScan, Becton Dickinson), and the percentage of positive cells was measured.
RNA extraction and real time-PCR
Total RNA was extracted by Trizol (Life Technology, Carlsbad, CA) according to the manufacturer’s protocol. RNA quantification was performed using the Nanodrop spectrophotometer (ND-1000; Nanodrop, Wilmington, DE, USA) and 200 ng of RNA was retro transcribed adopting High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The mRNA expression was assessed by quantitative real-time PCR (RT-PCR). In brief, RT-PCR mix containing 5 ng of cDNA, 100 nM of each primer (Table 1), and 1x SYBR GREEN PCR Master Mix (Applied Biosystems) were assembled by a 96-well StepOne Real Time System (Applied Biosystems). Negative cDNA controls (no cDNA) were cycled in parallel with each run. Data were showed as relative quantification (2- ΔΔCt) using ΔΔCt method and adopting GAPDH as normalizer.
|
Target |
Forward Primer |
Reverse Primer |
|
GAPDH |
CCGCTTCGCTCTCTGCTC |
CGACCAAATCCGTTGACTCC |
|
OCT4 A |
AGCAGGAGTCGGGGTGG |
CTGGGACTCCTCCGGGTT |
|
PAX2 FOXD1 |
CCCAGCGTCTCTTCCATCA ACCCTGAGCACTGAGATGTC |
GGCGTTGGGTGGAAAGG CCACGTCGATGTCTGTTT |
|
VIMENTIN |
GGAACAGCATGTCCAAATCGAT |
CAGCAAAGTTGGATTTGTACCATT |
Table 1: RT-PCR primer sequences.
Animal and experimental design
In vivo studies were approved and conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Italian Ministry of Health (274/2015-PR-26031.EXT24). To generate a model of rhabdomyolysis induced-AKI [27], male SCID mice of 7-8 weeks old (Charles River Laboratories) were treated with hypertonic glycerol (8 ml/kg body weight of 50% glycerol solution) by intramuscular injection into the inferior hind limb. The glycerol injection induces myolysis and haemolysis, leading to toxic and ischemic tubular damage. This model of AKI is characterized by a strong damage occurring at day 1 and a spontaneous recovery 5-8 days post injury [28-30]. Mice were treated the day after glycerol injection (peak of damage) with 350,000 cells in 120 µl PBS or PBS alone, as control, by intravenous injection in the tail vein. In each group (including three mice at least), mice were killed three days after glycerol administration. Blood samples were collected for Blood Urea Nitrogen (BUN) and creatinine determination.
Kidney tissues were processed for histology. The following groups were studied: 1) PBS group; 2) AFSC group; 3) FT-AFMSCs groups. Three different cell lines of FT-AFMSC were tested, using at least three mice for each cell line.
BUN and Creatinine assays
Blood samples were collected four days after glycerol treatment, immediately centrifuged at 3,000 rpm for 10 minutes at room temperature. Plasmatic phase was collected and freshly used for creatinine and BUN determination. Serum creatinine was evaluated using a colorimetric microplate assay based on the Jaffe reaction (Quantichrom Creatinine Assay, BioAssay Systems, Hayward, CA), while BUN was measured by direct quantification of serum urea using a colorimetric assay kit (Quantichrom Urea Assay, BioAssay Systems).
For renal histology, paraffin kidney sections of 5 µm thick were routinely stained with haematoxylin and eosin (Merck, Darmstadt, Germany). Luminal hyaline casts and denudation of tubular basement membrane were assessed in non-overlapping fields (up to 10 for each section) using a 20x objective (high power field, HPF). Number of casts and tubular necrosis was recorded in a single-blind fashion [28]. To assess the proliferation of tubular cells, immunohistochemical analysis for the detection of Proliferating Cell Nuclear Antigen (PCNA) positive cells was performed. In details, to retrieval antigens, kidney sections were boiled in citrate buffer for 10 minutes, followed by 20 minutes of blocking with a solution of 5% bovine serum albumin. Renal sections were stained with a monoclonal PCNA antibody (1:400, Santa Cruz Biotechnology, Santa Cruz, CA). For the immunoperoxidase staining, a secondary antibody, horseradish peroxidase-conjugated (1:300, Pierce, Rockford, IL) was used. Results were quantified by counting positive nuclei within tubule per field, selecting randomly 10 sections each renal cortex, using a 20x objective (high power field, HPF).
Statistical analysis
Results are generally expressed as mean ± SD or SEM. Statistical analysis was performed by Unpaired t-test between each mice group for BUN and creatinine evaluation. While ANOVA with Dunnett’s Multicomparison test was used in all other analysis. A P-value of <0.05 was considered significant.
RESULTS
Isolation of FT-AFMSCs
Eleven healthy women (age 30-35 years) undergoing scheduled term caesarean section for breech presentation (gestational age 37-40 weeks) were selected for this study. The median volume of collected AF was 13 mL (8.88-20mL) and all new-borns were vigorous with a median weight of 3,000 g (2,790-3,725 g) (Table 2). Cells were isolated and grown by using the Chang’s media, as described [7]. AFSCs, a well characterized source of multipotent stem cells obtained from mid-term amniotic fluid, were provided by Dr. Perin and used as positive control [7]. FT-AFMSCs grew until the 8th-10th passage while AFSCs were maintained in culture up to the 40th passage, maintaining their phenotype [7].
|
Patient |
Patient age (y) |
BMI |
Gestational week |
Neonate weight (g) |
Placenta wieght (g) |
AF volume (mL) |
|
1 |
35 |
20.5 |
39+1 |
3890 |
710 |
12 |
|
2 |
36 |
22 |
36+5 |
2375 |
450 |
14 |
|
3 |
33 |
22.2 |
39+1 |
2930 |
590 |
10 |
|
4 |
31 |
24.3 |
38+3 |
3000 |
620 |
5.5 |
|
5 |
35 |
21.3 |
39+1 |
3725 |
580 |
20 |
|
6 |
38 |
21.7 |
38+1 |
2840 |
500 |
22 |
|
7 |
27 |
22.3 |
37+6 |
3460 |
700 |
4 |
|
8 |
31 |
23.5 |
38+6 |
4060 |
920 |
_ |
|
9 |
30 |
18.3 |
39+1 |
2390 |
320 |
20 |
|
10 |
35 |
26 |
37+1 |
2790 |
920 |
19 |
|
11 |
30 |
25.6 |
39 |
3360 |
640 |
10 |
Table 2: Information associated with caesarean deliveries, new-borns and collection of the fluid. Data of the eleven patients analysed.
Characterization of FT-AFMSCs
Isolated cell lines from amniotic fluid were immediately phenotypically characterized at the first passages [11,19,31]. Cytofluorimetric analysis showed that cells were positive for mesenchymal surface markers CD73, CD44, CD90, CD166 but not for CD105, at variance of mid-term AFSCs. Moreover, they were positive for CD146 and CD29, similarly to AFSCs, while CD24 expression was higher than mid-term AFSCs (Table 3, Figure 1A). Marker expression did not substantially change during passages (not shown). Moreover, FT-AFMSC lines resulted positive for the stem cell marker SSEA4, with variable expression of the renal progenitor CD133 marker. By RT-PCR analysis, the expression of Vimentin and PAX2 in FT-AFMSCs was similar to AFSCs while the expression of the pluripotent marker OCT4 was higher in FT-AFMSCs (Figure 1B). On the contrary, the expression of Forkhead box D1 (FOXD1), a gene involved in kidney development, was markedly higher in AFSCs compared with FT-AFMSCs (Figure 1B).
|
Markers |
AFSCs |
FT-AFMSCs 1 |
FT-AFMSCs 2 |
FT-AFMSCs 3 |
FT-AFMSCs 4 |
FT-AFMSCs 5 |
FT-AFMSCs 6 |
FT-AFMSCs 7 |
FT-AFMSCs 8 |
|
CD24 |
0.80 |
85.99 |
97.03 |
15.09 |
6.00 |
21.78 |
78.85 |
35.49 |
88.58 |
|
CD29 |
98.20 |
97.31 |
99.38 |
99.61 |
90.00 |
99.14 |
93.66 |
90.21 |
96.29 |
|
CD44 |
98.50 |
98.04 |
99.76 |
97.77 |
89.00 |
99.44 |
96.62 |
89.00 |
94.37 |
|
CD73 |
97.70 |
97.98 |
98.63 |
59.19 |
28.00 |
18.38 |
87.20 |
15.74 |
93.62 |
|
CD90 |
96.70 |
26.42 |
40.15 |
88.21 |
- |
76.55 |
57.18 |
70.13 |
- |
|
CD105 |
94.00 |
48.85 |
0.68 |
0.31 |
0.00 |
0.00 |
1.40 |
0.87 |
0.85 |
|
CD133 |
- |
29.61 |
88.41 |
0.64 |
- |
23.8 |
19.47 |
1.05 |
46.99 |
|
CD146 |
95.19 |
97.35 |
87.24 |
37.60 |
39.00 |
88.77 |
93.44 |
40.12 |
82.10 |
|
CD166 |
98.33 |
98.25 |
98.26 |
99.43 |
13.00 |
78.79 |
96.52 |
12.65 |
93.79 |
|
SSEA4 |
32.96 |
96.62 |
98.00 |
38.28 |
42.00 |
97.93 |
96.56 |
10.96 |
94.51 |
Table 3: FACS analysis.
Cytofluorimetric analysis of the expression of the mesenchymal markers (CD73,CD44, CD90, CD166, CD105), CD146, CD24, CD29, the stem markers SSEA4 and the renal progenitor marker CD133 in AFSCs and FT-AFMSCs. Cells were tested between the first and the third culture passage. Data are expressed as % positive cells and are mean of seven different FT-AFMSC lines compared to control AFSCs.
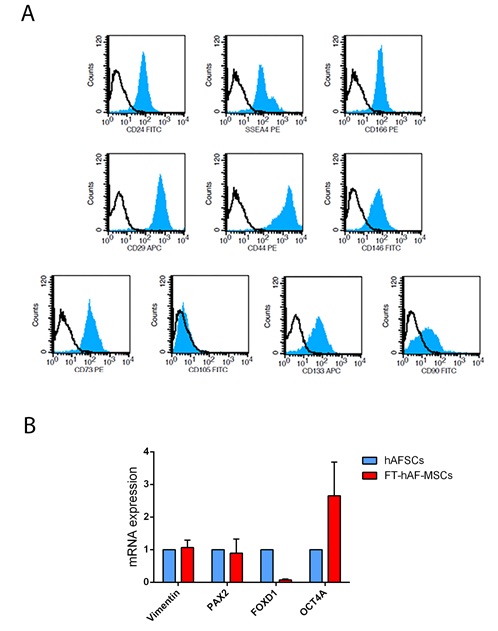
Figure 1: Phenotypic characteristics of FT-AFMSCs. (A) Representative FACS analysis of expression of mesenchymal, stem and renal markers by FT-AFMSCs. In each histogram, the blue filled area shows binding of the specific antibody and the black line shows the isotypic control. FT-AFMSC lines isolated (n=7) were characterized and showed similar marker expression. (B) By real time PCR, the expression of Vimentin, Pax2, FOXD1 and OCT4A was measured in FT-AFMSC lines (n=4) and control mid-term AFSCs. Data are expressed as relative quantification (RQ) of the mean of FT-AFMSC lines normalized to GAPDH and control mid-term AFSCs.
Effect of FT-AFMSCs on the recovery of glycerol-induced AKI in SCID mice
To test whether FT-AFMSCs may have a nephroprotective effect on AKI damage, experimental AKI was induced in male SCID mice by intramuscular injection of glycerol. Since creatinine and BUN reached their maximum peak 24 hours after injury [32], mice were treated at day 1 by intravenous injection of 350,000 cells from mid-term or full-term amniotic fluidin 120 µl PBS or of PBS alone. Animals were sacrificed at day 3 after cell injection. FT-AFMSCs promoted renal recovery and ameliorated the renal function, as shown by reduced creatinine and BUN levels (Figure 2A, 2B). The effects were consistent in all three FT-AFMSC lines used. As expected, mice treated with mid-term AFSCs had a similar protection from damage (Figure 2A, 2B).
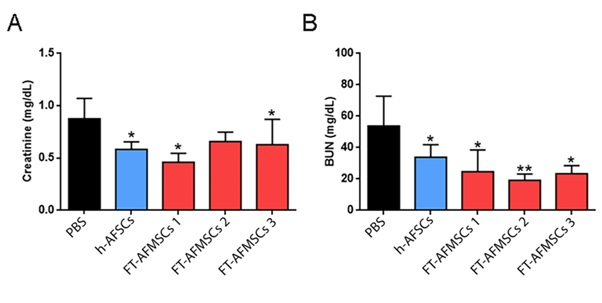
Figure 2: Effect of FT-AFMSC lines on renal function in AKI mice. (A-B) Changes in renal function were measured by (A) BUN and (B) creatinine. Glycerol injection resulted in elevated BUN and creatinine levels at day 4. In animals injected with control mid-term AFSCs and FT-AFMSC lines BUN and creatinine levels showed a significant reduction compared to PBS. Data are mean ± SD of mice for each group. PBS group (n=8); AFSC group (n=7); FT-AFMSCs 1 group (n=3); FT-AFMSCs 2 group (n=4); FT-AFMSCs 3 (n=5). *=p value < 0.05; **=p value < 0.01.
On day 3 after cell injection, the histological assessment of the renal tissue of mice treated with PBS, showed tubular epithelial cell necrosis and vacuolization with accumulation of intratubular protein casts (Figure 3A, PBS). At variance, animals treated with mid-term AFSCs or FT-AFMSC lines presented reduced tissue damage compared to PBS mice. In fact, morphometric evaluation clearly demonstrated a significant reduction of number of necrotic tubular cells (Figure 3B) and casts (Figure 3C). Moreover, administration of mid-term AFSCs or FT-AFMSC lines stimulated tubular cell proliferation. In fact, the quantification of PCNA positive nuclei revealed a significantly higher number of proliferating cells in mice treated with mid-term AFSCs or FT-AFMSCs compared to the one of PBS group (Figure 4).
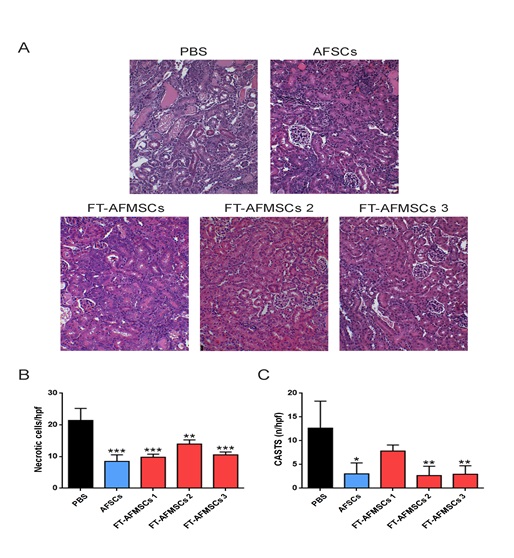
Figure 3: Effect of FT-AFMSC lines on morphology in AKI mice. (A) Representative micrographs of renal tissue from AKI mice on day 3 after cell injection showing tubular necrosis and tubular protein casts. Kidneys from mice treated with PBS showed tubular necrosis and cast accumulation. Contrary, FT-AFMSC lines showed signs of recovery of tissue injury as mice injected with mid-term AFSCs. Original magnification 200x. (B-C) Count of tubular (B) necrosis and (C) tubular casts at day 4 after damage. Data are mean ± SD of the count of 10 fields/section in two sections/mouse. n = 3-9 mice for each group were evaluated. ANOVA with Dunnett’s Multicomparison test was performed. PBS group (n=8); AFSC group (n=4); FT-AFMSCs 1 group (n=3); FT-AFMSCs 2 group (n=4); FT-AFMSCs 3 (n=6). *=p value < 0.05; **=p value < 0.01; ***=p value < 0.001.
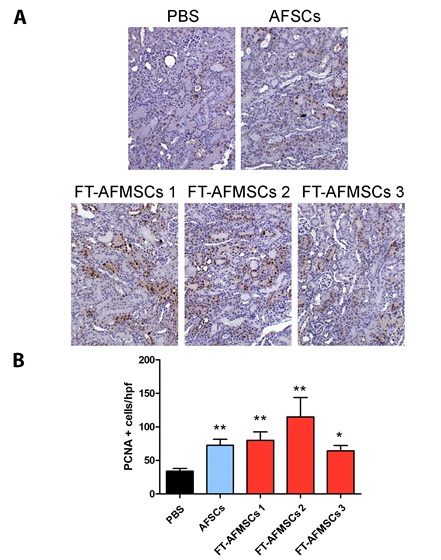
Figure 4: Cell proliferation in AKI untreated or treated with AFSCs or FT-AFMSCs. (A) Representative micrographs showing PCNA (Proliferating Cell Nuclear Antigen) positive cells in kidney tissues from AKI mice on day 3 after cell or PBS injection. Original magnification x200. (B) Quantification of PCNA positive cells in AKI mice treated with cell or PBS injection. Data are expressed as the mean ± SEM of the count of 10 fields/section (n=at least 3 mice for each group). ANOVA with Dunnet’s Multicomparison test was performed: *p<0.05, **p<0.001 versus Vehicle.
DISCUSSION
In this present study, we isolated FT-AFMSCs from full-term pregnancies with the aim of investigating their regenerative potential after injection in a murine model of AKI. We found that FT-AFMSCs lines ameliorate the AKI recovery, preventing tubular cell necrosis and cast accumulation, with a comparable effect to mid-term AFSCs.
It is well established that amniotic fluid contains a population of stromal cells with similar characteristics to MSCs isolated from bone marrow. AFMSC from mid-term pregnancies have been extensively characterized, they exhibit a broad differentiation potential toward mesenchymal lineages, and extensive regenerative ability [33]. At variance, only a limited number of articles characterize FT-AFMSCs collected during 38-40 weeks of gestation [34-36], showing characteristics that are similar to that of mid-term AFMSCs [17]. We here isolated eleven lines of FT-AFMSCs. FT-AFMSCs were positive for mesenchymal markers including CD29, CD44, CD73 and CD90 as previous described [34,37,38], but lacking CD105 marker whose expression is detected only partially in other articles [25,39]. The absence of CD105 expression could be consequent of short-term expansion of our cultures; in fact, Wang et al. demonstrated that CD105 expression increased following long-term culture since this condition is permissive for mesenchymal cell growth or for mesenchymal progenitor cells to differentiate into CD105 positive cells [32]. As Phermthai et al., showed for amniotic fluid colony forming cells, our FT-AFMSCs expressed OCT4, vimentin, SSEA4 and CD133 [26,40].
We previously showed that mid-term AFMSCs injected in a murine glycerol-induced AKI model accelerate tubular recovery [17]. The novelty introduced by this study is the demonstration of a potential protective effect of FT-AFMSCs in the same AKI model. The FT-AFMSCs were able to ameliorate renal function and histology, limiting cell necrosis and promoting proliferation. A similar pro-regenerative effect has been previously described in a rat renal damage due to cisplatin injection [31]. Moreover, we compared the effect of FT-AFMSCs with that c-kit isolated AFSCs. Compared to FT-AFMSCs, AFSCs isolated from mid-term pregnancies, selected and cloned showed higher expansion abilities and broad multipotency [7]. AFSCs have been described to growth at a very high number of passages, further exceeding that of MSCs. Nevertheless, the nephroprotective effect of full-term AFMSCs in murine glycerol-induced AKI model was similar.
Altogether, the results of this study underline a potential interest for FT-AFMSCs. In fact, although they are surely less proliferative as compared to mid-term AFSCs, they show similar renal regenerative capacity and can be easily obtained from the AF without ethical concerns. Moreover, the collection does not involve invasive procedures, such as amniocentesis, necessary for the mid-term AFSCs isolation that could impose certain level of risks to the mother and the fetus.
ACKNOWLEDGEMENT
The authors thank Federica Antico for the histological assistance.
SOURCES OF SUPPORT
This study was supported by MIUR (Ministry of Health and Research), ex60% to BB.
AUTHOR CONTRIBUTIONS
CI: Cell isolation, culture and characterization; in vivo studies and tissue analyses. Interpretation of results and manuscript writing. CG: In vivo studies and tissue analyses. Interpretation of results and manuscript writing. LM: Sample collection, clinical data analysis; LP: AFSC generation, interpretation of results; BB: Study design and coordination, data interpretation and manuscript writing.
REFERENCES
- Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, et al. (2013) KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 61: 649-672.
- Pozzoli S, Simonini M, Manunta P (2018) Predicting acute kidney injury: current status and future challenge J Nephrol 31: 209-223.
- Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, et al. (2013) World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 8: 1482-93.
- Coca SG, Singanamala S, Parikh CR (2012) Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81: 442-448.
- Humphreys BD, Cantaluppi V, Portilla D, Singbartl K, Yang L, et al. (2016) Targeting Endogenous Repair Pathways after AKI. J Am Soc Nephrol 27: 990-998.
- Chen J, Park HC, Addabbo F, Ni J, Pelger E, et al. (2008) Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int 74: 879-889.
- Perin L, Sedrakyan S, Giuliani S, Da Sacco S, Carraro G, et al. (2010) Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis. PLoS One 5: 9357.
- Morigi M, Introna M, Imberti B, Corna D, Abbate M, et al. (2008) Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells 2008 26: 2075-2082.
- Lin F, Cordes K, Li L, Hood L, Couser WG, et al. (2003) Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 14: 1188-1199.
- Duffield JS, Bonventre JV (2005) Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int 68: 1956-1961.
- Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG (2007) Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 18: 2486-2496.
- Li L, Black R, Ma Z, Yang Q, Wang A, et al. (2012) Use of mouse hematopoietic stem and progenitor cells to treat acute kidney injury. Am J Physiol Renal Physiol 302: 9-19.
- Feng Z, Ting J, Alfonso Z, Strem BM, Fraser JK, et al. (2010) Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transplant 25: 3874-3884.
- Sheashaa H, Lotfy A, Elhusseini F, Aziz AA, Baiomy A, et al. (2016) Protective effect of adipose-derived mesenchymal stem cells against acute kidney injury induced by ischemia-reperfusion in Sprague-Dawley rats. Exp Ther Med 11: 1573-1580.
- Chen B, Bo CJ, Jia RP, Liu H, Wu R, et al. (2013) The renoprotective effect of bone marrow-derived endothelial progenitor cell transplantation on acute ischemia-reperfusion injury in rats. Transplant Proc 45: 2034-2039.
- Liang CJ, Shen WC, Chang FB, Wu VC, Wang SH, et al. (2015) Endothelial Progenitor Cells Derived From Wharton's Jelly of Human Umbilical Cord Attenuate Ischemic Acute Kidney Injury by Increasing Vascularization and Decreasing Apoptosis, Inflammation, and Fibrosis. Cell Transplant 24: 1363-1377.
- Hauser PV, De Fazio R, Bruno S, Sdei S, Grange C, et al. (2010) Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery. Am J Pathol 177: 2011-2021.
- Da Sacco S, Sedrakyan S, Boldrin F, Giuliani S, Parnigotto P, et al. (2010) Human amniotic fluid as a potential new source of organ specific precursor cells for future regenerative medicine applications. J Urol 183: 1193-1200.
- Antonucci I, Iezzi I, Morizio E, Mastrangelo F, Pantalone A, et al. (2009) Isolation of Osteogenic Progenitors From Human Amniotic Fluid Using a Single Step Culture Protocol. BMC Biotechnol 9: 9.
- Gosden CM (1983) Amniotic fluid cell types and culture. Br Med Bull 39: 348-354.
- Hoehn H, Salk D (1982) Morphological and biochemical heterogeneity of amniotic fluid cells in culture. Methods Cell Biol 26: 11-34.
- Orciani M, Emanuelli M, Martino C, Pugnaloni A, Tranquilli AL, et al. (2008) Potential role of culture mediums for successful isolation and neuronal differentiation of amniotic fluid stem cells. Int J Immunopathol Pharmacol 21: 595-602.
- Mori da Cunha MGMC, Zia S, Arcolino FO, Carlon MS, Beckmann DV, et al. (2015) Amniotic Fluid Derived Stem Cells with a Renal Progenitor Phenotype Inhibit Interstitial Fibrosis in Renal Ischemia and Reperfusion Injury in Rats. PLoS One 10: 0136145.
- Hamid AA, Joharry MK, Mun-Fun H, Hamzah SN, Rejali Z, et al. (2017) Highly potent stem cells from full-term amniotic fluid: A realistic perspective. Reprod Biol 17: 9-18.
- Moraghebi R, Kirkeby A, Chaves P, Rönn RE, Sitnicka E, et al. (2017) Term amniotic fluid: an unexploited reserve of mesenchymal stromal cells for reprogramming and potential cell therapy applications. Stem Cell Res Ther 8: 190.
- Rahman MS, Spitzhorn LS, Wruck W, Hagenbeck C, Balan P, et al. (2018) The presence of human mesenchymal stem cells of renal origin in amniotic fluid increases with gestational time. Stem Cell Res Ther. 9: 113.
- Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, et al. (2004) Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med 14: 1035-1041.
- Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, et al. (2009) Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20: 1053-1067.
- Grange C, Moggio A, Tapparo M, Porta S, Camussi G, et al. (2014) Protective effect and localization by optical imaging of human renal CD133+ progenitor cells in an acute kidney injury model. Physiol Rep 2: 12009.
- Moggio A, Geraci S, Boido A, Sticht C, Gretz N, et al. (2017) Assessment of Acute Kidney Injury in Rhabdomyolytic Mice by Transcutaneous Measurement of Sinistrin Excretion. Nephrol Dial Transplant 32: 1167-1175.
- Al-Husseiny F, Sobh MA, Ashour RH, Foud S, Medhat T, et al. (2016) Amniotic Fluid-Derived Mesenchymal Stem Cells Cut Short the Acuteness of Cisplatin-Induced Nephrotoxicity in Sprague-Dawley Rats. Int J Stem Cells 9: 70-78.
- Wang D, Chen R, Zhong X, Fan Y, Lai W, et al. (2014) Levels of CD105(+) cells increase and cell proliferation decreases during S-phase arrest of amniotic fluid cells in long-term culture. Exp Ther Med 8: 1604-1610.
- Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, et al. (2007) Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev 16: 931-952.
- You Q, Cai L, Zheng J, Tong X, Zhang D, et al. (2008) Isolation of human mesenchymal stem cells from third-trimester amniotic fluid. Int J Gynaecol Obstet 103: 149-152.
- Bottai D, Cigognini D, Nicora E, Moro M, Grimoldi MG, et al. (2012) Third trimester amniotic fluid cells with the capacity to develop neural phenotypes and with heterogeneity among sub-population Restor Neurol Neurosci 30: 55-68.
- Bottai D, Scesa G, Cigognini D, Adami R, Nicora E, et al. (2014) Third trimester NG2-positive amniotic fluid cells are effective in improving repair in spinal cord injury. Exp Neurol 254: 121-133.
- Gao L, Zhao M, Ye W, Huang J, Chu J, et al. (2016) Inhibition of glycogen synthase kinase-3 (GSK3) promotes the neural differentiation of full-term amniotic fluid-derived stem cells towards neural progenitor cells.Tissue Cell 48: 312-320.
- De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, et al. (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25: 100-106.
- Tsai MS, Lee JL, Chang YJ, Hwang SM (2004) Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 19: 1450-1456.
- Phermthai T, Odglun Y, Julavijitphong S, Titapant V, Chuenwattana P, et al. (2010) A novel method to derive amniotic fluid stem cells for therapeutic purposes. BMC Cell Biol 11: 79.
Citation: Iampietro C, Grange C, Marozio L, Benedetto C, Perin L, et al. (2020) Full-Term Human Amniotic Fluid Mesenchymal Stem Cells Show Nephroprotective Effect in an Acute Kidney Injury Model. J Stem Cell Res Dev Ther 6: 041.
Copyright: © 2020 Corinne Iampietro, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

