
Human Neural Stem Cells in Space Proliferate more than Ground Control Cells: Implications for Long-Term Space Travel
*Corresponding Author(s):
Araceli Espinosa-JeffreyDepartment Of Psychiatry, Semel Institute For Neuroscience And Human Behavior At University Of California Los Angeles, Los Angeles, United States
Tel:+1 3108251747,
Email:aespinosa@mednet.ucla.edu
Abstract
Long-term travel and lengthy stays for astronauts in outer space are imminent. To date, more than 500 astronauts have experienced the extreme conditions of space flight including microgravity and radiation. For the past decade, many studies associated with long-duration spaceflight have shown the recurring occurrence of ophthalmic abnormalities. The reasons of the observed changes in some astronauts remained unclear. However, factors such as the increase in intracranial pressure and fluid shifts are among the top potential contributing elements. Here we report a study that specifically looked at the effect of space environment on the proliferation and physiology of human Neural Stem Cells (NSCs) onboard the International Space Station (ISS) as compared to ground controls. The study revealed that human NSCs proliferated seven times more while in space (SPC) when compared to on Earth (1G) control cultures. We also examined by continuous live imaging the behavior of space flown NSCs upon return to Earth. We found that after space flight, they continued proliferating at the same pace as 1G controls. Interestingly, NSCs flown to space displayed a larger diameter than control cells. These phenomena, increased proliferation while in space and larger cell soma may contribute to intracranial hypertension found in astronauts, representing a risk factor and potential limitation to long duration space missions such as travelling to the Moon or Mars. In addition, NSCs are essential to maintain Central Nervous System (CNS) function, as they are the basis for the regeneration of CNS cell populations in health and disease.
Keywords
Cell fate choice; Cell proliferation; Differentiation; Human neural stem cells; Intracranial hypertension; Long-term space exploration; Microgravity; Space flight associated neuro-ocular syndrome
Graphical Abstract
Abbreviations
BrdU: 5-bromo-2'-deoxyuridine
CDKs: Cyclin-Dependent Kinases
CDKIs: Cyclin-Dependent Kinase Inhibitors
CNS: Central Nervous System
DDREF: Dose-Rate Reduction Effectiveness Factor
DNA: Deoxyribonucleic Acid
ES: Embryonic stem cells
hips: Human Induced Pluripotent Stem Cells
HNSCs: Human Neural Stem Cells
HNSC.100: Human Neural Stem Cell Line
HZE: High Atomic Number Energy
iPS: Induced Pluripotent Stem Cells
ISS: International Space Station
miRNA: Micro RNA
MSCs: Mesenchymal Stem Cells
NSCs: Neural Stem Cells
OLs: Oligodendrocytes
OLPs: Oligodendrocyte Progenitors
PSA-NCAM: Polysialylated-Neural Cell Adhesion Molecule
QF: Radiation Quality Factor
SANS: Space Flight Associated Neuro-Ocular Syndrome
sim-µG: Simulated Microgravity
SPC: Space
SPC-µG: Space Microgravity
STM: Stem Cells Medium
1G: Terrestrial Gravity
0G: Zero Gravity
Introduction
In human embryonic life, pluripotent cells proliferate and commit into distinctive cell lineages. During development, in vivo lineage commitment occurs and is maintained by the epigenetic programming of gene expression profiles in which methylation plays a prominent role [1]. Cell renewal and the ability to specify into a particular phenotype are the two main characteristics of stem cells and organ-related stem cells such as neural stem cells (NSCs) that can differentiate into neurons, astrocytes, and oligodendrocytes. For these reasons, NSCs play key roles in development and in adulthood when they maintain the homeostasis of the central nervous system (CNS) [2]. Moreover, these cells are a promising source for cell replacement therapies for people with neurological disorders like multiple sclerosis, or developmental disabilities such as cerebral palsy. In vivo, these cells are influenced by intrinsic and environmental signals within their niche to regulate self-renewal and differentiation as described in the literature [3].
Recently, following clinical issues associated with the eyes of many astronauts upon their return to Earth, space agencies are diligently working on addressing the mechanisms related to the space flight associated neuro-ocular syndrome (SANS) [4]. Many elements are potential contributing factors, but an increased volume of the cerebrospinal fluid in the brain and the optic nerve sheaths seemed to play an important role [5].There is strong evidence that changes in gravity loads during long-duration spaceflight will have an impact on the CNS and as a result on astronaut’s health and performance [6]. The environment determines what happens with NSCs, their fate to remain as such or differentiate [7]. Here we examined a novel effector on these cells, “space microgravity”, to determine if they keep their multi-lineage differentiation potential and regenerative capabilities. Recently, we have shown that human NSCs grown in space for over 5 weeks upon their return, expressed specific biomarkers, and displayed both higher oxygen consumption and glycolysis as compared to ground controls [8]. This study consists of both, data obtained while NSCs were in flight and data obtained in our laboratory after space flight. We report that human NSCs flown to the International Space Station (ISS) proliferated more while in space (SPC) when compared to 1G control cultures. Through continuous live imaging, we also ascertained the behavior of NSCs after the space flight, showing some “memory” of the effects of microgravity. NSCs preserved their well-known properties; they exhibited their typical self-renewal capacity and their fate choice properties when placed in a culture medium known to induce the astrocyte phenotype. A novel feature was noticed and is related to the observation that the NSCs were larger than ground control cells. Finally, we also tested the potential paracrine effects of NSCs flown to space, by using their secretome on naïve cells. It was demonstrated that NSCs generated in 1G proliferated more than the control counterparts did.
Materials And Methods
Cells
Prior to the space flight, a homogeneous population of NSCs was obtained from human induced pluripotent stem cells (hiPS). The original cells, known as “CS83iCTR-33nxx” (such as skin cells), were “reprogrammed” and provided to us by Cedars-Sinai Medical Center via a material transfer agreement. These cells were converted to “neural stem cells” by Dr. Espinosa using the culture medium “NSM” (neural specification medium with hepes to maintain the pH throughout the experiment) that she designed for neural specification [9]. Control cells were grown in the same types of hardware and pre-seeded onto mesh carriers just like those cells that travelled to space. Six carriers 2mm × 3mm were placed in the cell chamber. For the “in flight” proliferation study, we selected Type IV units because they offer the advantage of refreshing the culture medium twice by collecting the old medium into the first tank and replacing it with fresh medium contained in the second tank. The first tank contained medium with bromodeoxyuridine (BrdU) whereas the second did not. Before the flight implementation, the culture medium was equilibrated overnight at 5% CO2 in the incubator where cells were maintained. BrdU is a synthetic “thymidine analog” that incorporates into newly synthesized DNA during the S phase of the cell cycle [10, 11].
For space flight, NSCs were seeded onto mesh carriers and placed “free floating” using the pre-equilibrated culture medium in the cell chamber of Automated Type IV units from Yuri (Meckenbeuren, Ger). Because the incubator used onboard the ISS, the Space Technology and Advanced Research System Experiment Facility-1 (STaARS EF-1) does not provide CO2 capabilities, the culture medium was pre-equilibrated at 5% CO2 as previously shown [8]. The automated Type IV unit used during the in-flight proliferation experiment contains one Culture Chamber with a volume of 11.5ml ± 0.3ml Two Media Exchanges: Refreshment Medium and Fixative, Two Tanks with a volume of 11ml ± 0.3 ml each. The time needed for automated media exchange is approximately 5 to 7 min. This hardware is flight-proven and entails the Outer Shell covers, and the inner shell consisting of the cell culture chamber and integrated tanks containing the fresh culture media. This hardware from Yuri (formerly known as Kiwi, and prior to it, Airbus) was leased by STaARS for this study. We used it for flight and ground control. These hardware-units containing the experiment clip-in-place onto the Space Technology and Advanced Research System-1 Experiment Facility (STaARS EF-1) and interface mechanically and electrically with it (Figure 1). (For details on the hardware see: https://www.yurigravity.com/our-service). Our cells were flown aboard SpaceX-16.The units were placed in their self-contained black cover and then placed insidea 37°C pre-conditioned double bag during ascent and descent.
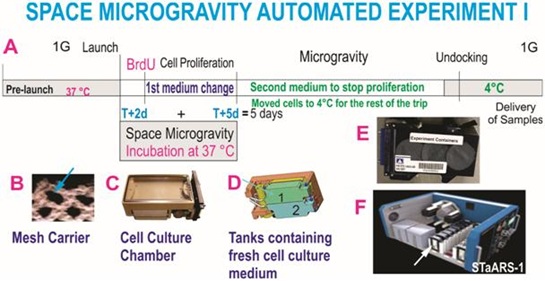 Figure 1: Automated Experiments. A) Shows the synopsis of the experiment and hardware used for the in-flight proliferation study. T indicates the time when cells started being in space microgravity or time 0days (d), T+2d (means time from berth plus 2days). B) To prevent cells from detaching from the surface of the hardware, during ascent and descent, cells were pre-seeded onto mesh carriers. These carriers were previously tested for this purpose, the arrow shows the mesh where cells grew freely forming tridimensional structures, mesh carriers used in these units were 2mm × 3mm. C) View of the Type IV chamber where cells were seeded (Airbus-Yuri, Friedrichshafen, D). D) Bottom view of computer assisted design of the Type IV unit showing the two tanks. Each tank contains culture medium for their respective unit. Tank 1 contained medium with BrdU. The medium from Tank 2 was sent to the cell chamber on T+5d in order to stop BrdU incorporation. The cells were then removed from the facility prior to moving them to 4°C. E) View of the external cover designed to self-contain the units and their contents in case of a potential leak. F) View of the inner portion of facility where units were installed once contained in their black external cover. Cells travelled and were incubated in this facility while in space. The arrow shows the position of the units during space flight. The STaARS-1 EF was designed by STaARS, inspired on this study having in mind the use of minimal astronaut time. Therefore, this facility is activated and controlled from STaARS’ headquarters in Houston, Tx. A total of four Automated Type IV units were flown aboard SpaceX-16 on December 5, 2018. To view more details of the hardware please see please see information from Yuri in Supplement 1 or visit (https://www.yurigravity.com/our-service - right-hardware).
Figure 1: Automated Experiments. A) Shows the synopsis of the experiment and hardware used for the in-flight proliferation study. T indicates the time when cells started being in space microgravity or time 0days (d), T+2d (means time from berth plus 2days). B) To prevent cells from detaching from the surface of the hardware, during ascent and descent, cells were pre-seeded onto mesh carriers. These carriers were previously tested for this purpose, the arrow shows the mesh where cells grew freely forming tridimensional structures, mesh carriers used in these units were 2mm × 3mm. C) View of the Type IV chamber where cells were seeded (Airbus-Yuri, Friedrichshafen, D). D) Bottom view of computer assisted design of the Type IV unit showing the two tanks. Each tank contains culture medium for their respective unit. Tank 1 contained medium with BrdU. The medium from Tank 2 was sent to the cell chamber on T+5d in order to stop BrdU incorporation. The cells were then removed from the facility prior to moving them to 4°C. E) View of the external cover designed to self-contain the units and their contents in case of a potential leak. F) View of the inner portion of facility where units were installed once contained in their black external cover. Cells travelled and were incubated in this facility while in space. The arrow shows the position of the units during space flight. The STaARS-1 EF was designed by STaARS, inspired on this study having in mind the use of minimal astronaut time. Therefore, this facility is activated and controlled from STaARS’ headquarters in Houston, Tx. A total of four Automated Type IV units were flown aboard SpaceX-16 on December 5, 2018. To view more details of the hardware please see please see information from Yuri in Supplement 1 or visit (https://www.yurigravity.com/our-service - right-hardware).
Passive experiments, recovery of the hardware and harvesting of live samples
We used the 8-well Petri dishes from Airbus-Kiwi (Friedrichshafen, DE) as shown in figure 2. NSCs were seeded on mesh carrier 2mm × 2mm and they were flown onto space onboard SpaceX-16. This experiment was designed mimicking the trajectory astronauts’ brains undergo during space flight (i.e., launch, stay in space and splash down when returning to earth). Thus, these units allowed for cells to be flown back to Earth alive, four mesh pieces were placed in each well. Pre-flight seeding: 0.5 × 106 cells were used for 4 wells (one side of the passive hardware) and flown to the International Space Station (ISS), and installed in the STaARS-1 EF at 37°C. The cells remained onboard the ISS for 39.3 days and then returned to Earth. We referred to the 8 well units as “passive” because they remained unattended, and without medium change while in the ISS. During descent and after splash-down, live cells were maintained in a controlled environment at 37°C inside a conditioned double bag and transported at 37°C from Long Beach (CA, USA) airport to University of California Los Angeles (UCLA). The secretome together was collected and the cells were detached from the floor and walls of each well and were recovered separately. Secretome samples were frozen at -80°C. Next, NSCs that were attached to the mesh-carrier, were retrieved from the hardware, plated onto poly-d-lysine coated flasks in stem cell medium (STM) as previously described [6] and allowed to recover from space flight. After 20h in the incubator [5% CO2 and 36.8°C], flasks were placed in a Zeiss Axio Observer 7 (Oberkochen, Germany) fully motorized inverted research microscope with the Zeiss Axiocam 506 monochrome camera with Zeiss ZEN software. The system was equipped with the Zeiss Full Incubation XL chamber. We want to emphasize that during ascent and while in the ISS, all cells were kept at 37°C. Subsequently, waiting for unberth and descent, cells were kept at 37°C in a conditioned double bag then placed in 37°C controlled environment during transit until their delivery to UCLA. The synopsis for the passive experiments is shown (Figure 2). For details on this hardware please see information from Yuri in Supplement 2. Asynchronous ground control experiments were performed post-flight. The experiment timeline and environmental parameters from the ISS were used to simulate ground controls as close as possible from flight.
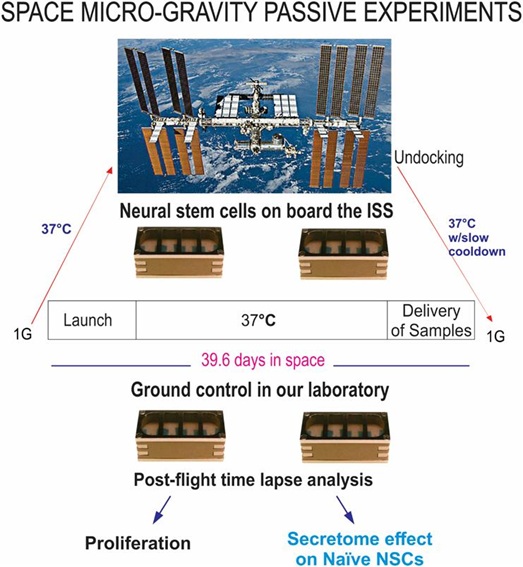 Figure 2: Synopsis of passive experiments. NSCs were seeded on floating mesh carriers to which cells adhere strongly; this feature was necessary to ensure that cells would not detach and die during launching or while returning to Earth. Upon splash-down they were transferred to the Long Beach (CA, USA) Airport and brought to the laboratory (UCLA) at 37°C. Upon arrival, cells were seeded onto flasks or flaskettes and allowed to recover during six hours in the incubator prior to placing them in the imaging system. Like for the Type IV units, these units also had been placed in the self-contained black cover. For details on the functioning of this hardware please see information from Yuri in Supplement 2.
Figure 2: Synopsis of passive experiments. NSCs were seeded on floating mesh carriers to which cells adhere strongly; this feature was necessary to ensure that cells would not detach and die during launching or while returning to Earth. Upon splash-down they were transferred to the Long Beach (CA, USA) Airport and brought to the laboratory (UCLA) at 37°C. Upon arrival, cells were seeded onto flasks or flaskettes and allowed to recover during six hours in the incubator prior to placing them in the imaging system. Like for the Type IV units, these units also had been placed in the self-contained black cover. For details on the functioning of this hardware please see information from Yuri in Supplement 2.
Secretome collection
The culture medium that fed the cells during space flight was recovered from the hardware separately, medium from the cell chamber, and each tank were placed in numbered tubes and saved frozen at -80°C. This medium is commonly known as conditioned medium. For the purpose of this manuscript, we named this conditioned medium “secretome”. In comparative figures we use the term “space CM” to differentiate from conditioned medium derived from ground control or naïve cells. For treatments with secretome from space-flown NSCs the volumes were 2:1, meaning there were 2 parts culture medium per 1 part secretome.
Examination of NSCs cultures that were stopped during space flight
For the “in space proliferation study”, the mesh pieces containing the cells, were preserved in RNA Later. Prior to unberth and upon return to UCLA, the cells were post-fixed with 2% paraformaldehyde for 30 min and transferred to tubes containing phosphate buffer saline (PBS) to be examined by triple immunofluorescence as previously described [8]. Cells rescued from the passive hardware were recovered one well at a time and were plated onto coated plastic 8-well chambers (Nunc 177445), or on poly-d-lysine coated glass cover slips as previously described [9].
Immunofluorescence
Cell lineages were confirmed using double immunofluorescence with established markers for stem cells, PSA-NCAM supernatant, a sialylated form of neural cell adhesion molecule, at 1:10 (Developmental Studies Hybridoma Bank, University of Iowa). Proliferation was ascertained with anti-BrdU, 1:200, and nestin antibody at 1:100 (BD Pharmingen, Franklin Lakes, NJ). Three secondary antibodies were used to visualize the markers mentioned above: goat anti-mouse IgM AMCA 1:100 (Jackson Immunology Research (JIR) Laboratories, West Grove, PA); goat anti-rabbit IgG Texas Red 1:800 (Jackson laboratories, west Grove, PA); and goat anti-mouse IgG FITC 1:300 (Sigma, St. Louis, MO). The immunofluorescence procedures were carried out as previously described [8,10]. Briefly, the cells were fixed with 4% paraformaldehyde. Samples were blocked for 1 h in 1% BSA (Sigma-Aldrich), 0.3%, Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA), and 10% normal goat or donkey serum in PBS. Primary antibodies were diluted in carrier solution (1% BSA and 0.3%, Triton X-100 in PBS) and incubated overnight at 4°C. After washing with PBS, secondary antibodies (anti-rabbit Texas red, 1:800 dilution; anti-mouse IgG Alexa Fluor 488, 1:1000 dilution; and anti-mouse IgM Alexa Fluor 633 ABCAM, 1:1000 dilution) were incubated for 1 hour at room temperature, washed with Tris-buffered saline (TBS) and mounted. The samples were imaged with the LSM 800 confocal microscope (Zeiss, Jena, Germany) and analyzed with the Zen software (Zeiss).
Statistics
Data are presented as mean ± SD and statistical analyses were performed using One-Way ANOVA, followed by Tukey post hoc test in which p
For cell proliferation, we used Student’s T-Test, in which p< 0.05 was defined as statistically significant. For the analysis of naïve cells, a two-way ANOVA was performed, followed by Sidak’s multiple comparisons [11] test, in which *p< 0.05 was defined as statistically significant. For the cell diameter study a three-way ANOVA was performed, followed by Sidak’s multiple comparisons test [12].
Results
Proliferation of NSCs during space flight
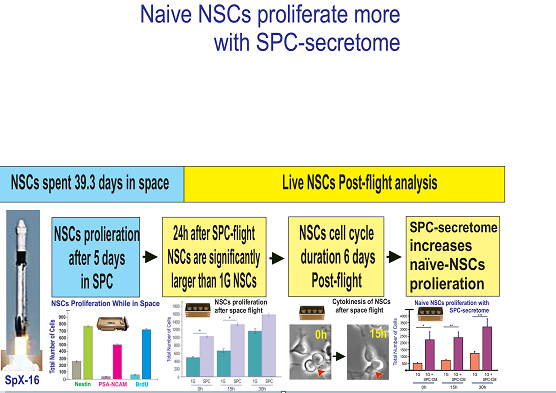
Cell proliferation was measured while NSCs were onboard the international space station (ISS) by using BrdU after 2 days from their arrival and stopping the cultures 3d later by transferring the cells to 4°C. Upon return from space, we examined BrdU incorporation with a specific antibody, in conjunction with antibodies to detect two NSC markers, polysialylated-neural cell adhesion molecule (PSA-NCAM) that is a marker of neural precursors and developing migratory neuroblasts [13], and nestin, an intermediate filament protein known as stem/progenitor marker for neural stem cells and expressed in uncommitted CNS cells [12] in combination with BrdU. Control cells were grown in the same type of hardware and pre-seeded onto mesh carriers just like those cells that travelled to space. For the proliferation study shown in figure 1, Automatic type IV units were used and tank 1 contained medium with BrdU. There were considerably fewer cells in cultures grown solely in 1G when compared to cells that had travelled to space. Most cells having flown into space expressed BrdU and colocalized with nestin and PSA-NCAM. In fact, after three days in space, 80% of NSCs were labeled for BrdU indicating that they had proliferated extensively with respect to 1G control cells (Figure 3). Comparison of the average total number of cells per field was 290 +/- 65 for cells grown in 1G and 910 +/- 132 for cells grown during the in-space flight.
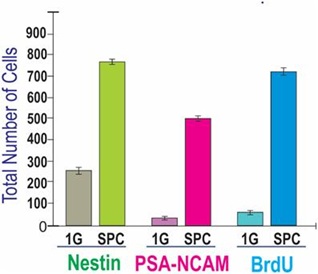 Figure 3: Comparison of proliferation of NSCs for 3 days while in space or ground control cultures. Both groups of cells were cultured in the Type IV hardware in the same conditions the only difference being space microgravity (SPC) vs. Earth gravity (1G). The figure depicts the triple immunofluorescence for NSCs markers, Nestin in green and polysialylated-neural cell adhesion molecule (PSA-NCAM) in red, and for BrdU (in blue) as a marker of cell proliferation. Data were plotted as the mean of six fields. Statistical significance was assessed by the Student’s t-test, in which p< 0.05 was defined as statistically significant.
Figure 3: Comparison of proliferation of NSCs for 3 days while in space or ground control cultures. Both groups of cells were cultured in the Type IV hardware in the same conditions the only difference being space microgravity (SPC) vs. Earth gravity (1G). The figure depicts the triple immunofluorescence for NSCs markers, Nestin in green and polysialylated-neural cell adhesion molecule (PSA-NCAM) in red, and for BrdU (in blue) as a marker of cell proliferation. Data were plotted as the mean of six fields. Statistical significance was assessed by the Student’s t-test, in which p< 0.05 was defined as statistically significant.
Continuous live imaging of SPC-flown NSCs
Mitotic activity of NSCs after space flight
After harvesting and replating, NSCs were allowed to recover for 6h following the space flight and transit. Subsequently, NSCs were placed in a time-lapse microscope system (Zeiss, Oberkochen, Germany). Images were acquired every 15 min for a longitudinal study. We observed that cell numbers increased with time in culture for both 1G controls and NSCs-flown onto space. The ratio between 1G and post-SPC total number of cells did not decrease with time, and these differences were significant between groups for the first two time points. Nonetheless, these differences became non-significant by 30h. Therefore, NSCs flown onto space and their progenies preserved their proliferative capacity for up to 15h. This is a feature proper to NSCs, indicating that they were in good health. Nonetheless, as time on Earth increased, the rate of proliferation decreased with time tending to normalize starting at 30h where differences were not significant (Figure 4).
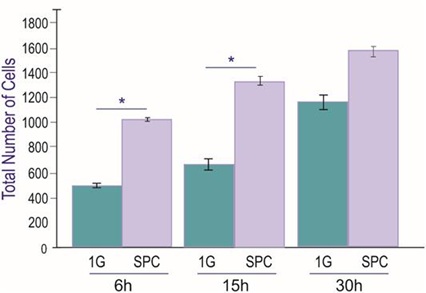 Figure 4: Proliferation of neural stem cells after spaceflight. Cultures were imaged every 15 min. uninterrupted for 30hr. Six fields were selected and imaged with a 10X objective. The total number of cells per frame was counted. For SPC cells although their numbers slowly increased in function of time, by 30h the differences between control and SPC cell numbers was not as significant as for 6 and 15h, respectively. n = 4. Data Analysis was performed with One-way ANOVA followed by Bonferroni and Holmes multiple comparisons test in which *p< 0.05 was defined as statistically significant. *p < 0.05. Data are presented as the mean of three separate cultures ± SD.
Figure 4: Proliferation of neural stem cells after spaceflight. Cultures were imaged every 15 min. uninterrupted for 30hr. Six fields were selected and imaged with a 10X objective. The total number of cells per frame was counted. For SPC cells although their numbers slowly increased in function of time, by 30h the differences between control and SPC cell numbers was not as significant as for 6 and 15h, respectively. n = 4. Data Analysis was performed with One-way ANOVA followed by Bonferroni and Holmes multiple comparisons test in which *p< 0.05 was defined as statistically significant. *p < 0.05. Data are presented as the mean of three separate cultures ± SD.
Cytokinesis of NSCs
We also ascertained the length of the cell cycle, based on cytokinesis phenomena, that consists of the time between “birth cytokinesis” and “division cytokinesis” in different NSCs. Tracking of cells was performed by visual observation of image sequences forward and backward in time to maximize the number of linearly related cytokinesis as previously described [13]. Cytokinesis analysis one week after space flight is shown in figure 5. We determined that the average doubling time of space-flown NSCs cells ranged between 15h and 22h.
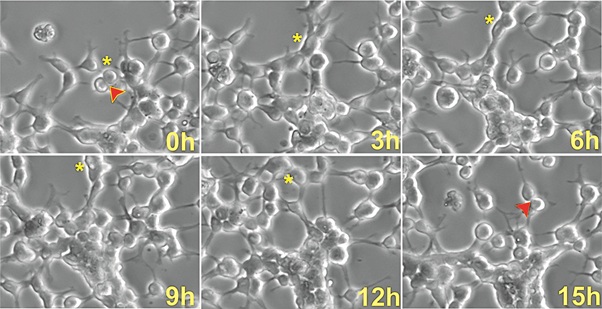 Figure 5: Cytokinesis of NSCs flown into space. Upon arrival and removal from the flight hardware, cells were allowed to acclimate to 1G for 6h. Cells were then placed in the incubation chamber for time-lapse microscopy and imaged every 15 min to examine cytokinesis. Asterisks indicate a cell that had just divided in order to follow its fate through time. Arrows represent the division of the cell and when that cell divides again. For views of ground control images, please refer to Figure S1).
Figure 5: Cytokinesis of NSCs flown into space. Upon arrival and removal from the flight hardware, cells were allowed to acclimate to 1G for 6h. Cells were then placed in the incubation chamber for time-lapse microscopy and imaged every 15 min to examine cytokinesis. Asterisks indicate a cell that had just divided in order to follow its fate through time. Arrows represent the division of the cell and when that cell divides again. For views of ground control images, please refer to Figure S1).
Naïve NSCs in the presence of NSCs-SPC medium display enhanced proliferation
In a longitudinal study, we next examined the effect of NSCs space-flown secretome on the proliferation of 1G naïve NSCs. Cells were seeded on poly-d-lysine coated flaskettes in STM medium. During the first 42 hours, NSCs grew in their regular culture medium. Starting at 43h, the same cultures were treated with the secretome from space-flown NSCs in a ratio 2:1 v/v stem medium: SPC-secretome. Cell counts were performed at 0, 15 and 30h prior to adding the secretome. Then cells were counted at 43h, 58h, and 73h after the addition of the NSCs-SPC secretome, respectively. Two-way ANOVA with repeated measures on both 3 time points and 2 treatments (1G/1G NSC+SPC). The results are shown in Figure 6.After the addition of the secretome from space-flown NSCs, the total number of cells increased by 4.6 times, 3 times, and 2.7 times for 43, 58, and 73 h, respectively.
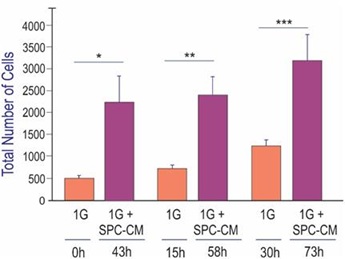 Figure 6: Naïve neural stem cells with secretome produced by SPC-flown NSCs. Naïve NSCs maintained in 1G were seeded and placed in the time-lapse system. Initially, imaging proceeded without adding the secretome from SPC-flown NSCs. Subsequently, starting at 43h cells were treated with the supernatant (secretome) of SPC-flown NSCs. We observed that as expected, NSCs proliferated as a function of time (orange bars). After adding the secretome from SPC-flown NSCs, they proliferated even more and these differences were significant (magenta bars). Data Analysis was performed with two-way ANOVA followed by Sidak’s multiple comparisons test in which *p< 0.05 was defined as statistically significant. *p< 0.0026; **p< 0.0030; ***p< 0.0017. Data represent three separate experiments reported as ± SD.
Figure 6: Naïve neural stem cells with secretome produced by SPC-flown NSCs. Naïve NSCs maintained in 1G were seeded and placed in the time-lapse system. Initially, imaging proceeded without adding the secretome from SPC-flown NSCs. Subsequently, starting at 43h cells were treated with the supernatant (secretome) of SPC-flown NSCs. We observed that as expected, NSCs proliferated as a function of time (orange bars). After adding the secretome from SPC-flown NSCs, they proliferated even more and these differences were significant (magenta bars). Data Analysis was performed with two-way ANOVA followed by Sidak’s multiple comparisons test in which *p< 0.05 was defined as statistically significant. *p< 0.0026; **p< 0.0030; ***p< 0.0017. Data represent three separate experiments reported as ± SD.
Discussion
There is significant similarity between embryonic and adult neurogenesis, indicating that some intrinsic signaling pathways are conserved. Intrinsic and extrinsic mechanisms regulate adult neurogenesis. Molecules and signaling pathways have been identified, among them, niche components such as extracellular molecules, receptors, transcriptional factors, and epigenetic regulators [14-17]. Moreover, physiological stimuli such as physical exercise or seizures increase NSCs proliferation [18-20]. Strikingly, even a transient seizure (a few hours) [21] or electro-convulsion (a few minutes) [22] leads to sustained increases in precursor proliferation for days and weeks, indicating the existence of a form of memory in the regulation of neurogenesis by neuronal activity. Therefore, experiencing the environment modifies both, functional and structural neuro-plasticity (for ref: https://www.readcube.com/articles/10.3389/fncel.2019.00066). Moreover, neuroplasticity involves not only neurons but also glial cells and both committed and uncommitted progenitors. A wide range of plasticity is observed during the development of the central nervous system (CNS), which decreases with age but is still present in adulthood and to some extent in aging and disease, depending on enriched environment [23]. Thus, the present study is relevant to humankind in space because space flight offers a completely different environment, and we sought to determine the effects of spaceflight on NSCs and their progenies in the absence of their niche and accompanying signals. This information has the potential to elucidate the effects of microgravity on cognitive and structural changes observed in astronauts.
NSC proliferation and cell cycle duration
We have previously reported that during space flight NSCs proliferate and, in adequate conditions, they give rise to neurons [8]. Here we further quantified in-space-proliferation using automated hardware that allowed for BrdU incorporation that reflects de novo DNA synthesis. In space (SPC), NSCs preserved their self-renewal and pluripotency properties. We found that our human NSCs in SPC proliferated approximately eight times more than ground control NSCs as shown in figure 3. After space flight, NSCs continued proliferating significantly more than ground control NSCs for more than 15h. Nonetheless, with time on Earth, the differences persisted yet were not significant by 30h suggesting a tendency of NSCs and their Earth-born progenies to adopt their properties as they adapted to Earth gravity, as shown in figure 4.
The literature on human NSC proliferation offers vast information on the duration of their cell cycle depending on their origin. For example, NSC-lines derived from the embryonic human brain range from seven to 10 days in the normal brain. Nonetheless, it varies by brain regions [24]. Another example is the human neural stem cell line (HNSC.100) perpetuated/immortalized with growth factors reported to divide every 40h [25]. With time, methods and composition of culture media pertaining to NSCs proliferation and maintenance have evolved giving rise to a wealth of information on the doubling time of human normal NSCs. For example, non-induced human NSCs during normal expansion from two conditionally immortalized cell lines proliferated with a doubling time of 72 hours (70%-80% confluence) [26]. A report comparing mouse NSCs to human NSC cultures harboring an increased proportion of CD133 negative cells has shown longer doubling time of 2-3 days, thus, much longer than that of mouse NSCs (∼24 hours) [27,28]. Recent information on induced pluripotent NSCs generated from human newborn foreskin fibroblasts has shown that their doubling time was 48 hours [29]. Here, we found that the average cell cycle duration of SPC-flown NSCs when back to 1G ranged between 15h (Figure 4) and 22h (data not shown). We have previously reported shortening of the cell cycle for mouse and human neural cells while grown in simulated microgravity as well as after placed back in 1G [7] and in particular oligodendrocytes (OLs) [11]. Therefore, neural cells appear to “remember” having been in microgravity (either simulated or space µG). Nonetheless, overall SPC-NSCs tended to normalize their proliferation pace after 30h once they were back to 1G. It is possible that our cells back from space and their progenies might have built a methylation-dependent memory to increase proliferation. Reports have shown that stem cell function is determined by epigenetic regulation that supports their establishment and maintenance. Moreover, epigenetic dysregulation leads to the altered potential of stem cells during aging or disease [30]. It is possible that epigenetic phenomena are regulated by gravitational changes. Here, prior to space flight, NSCs were growing adhered to the substratum and they were seeded on the mesh carrier and sent to space as low-density cultures. After the space flight, we found an increased number of cell clusters or colonies in cultures derived from SPC-NSCs and growing in 1G revealing a role for microgravity, perhaps via memory marker(s) resulting in increased proliferation and a concomitant increase of NSCs in a colony-like formation, a phenomenon reminiscent of more primitive stem cells. Similar findings have been reported [31] where human thyroid cancer cells displayed spheroid-formation during space flight with a concomitant alteration of gene expression in these cells. It has been shown that miRNAs regulate cell cycle progression in embryonic stem cells (ES) by targeting cell cycle-associated genes (e.g., Cyclins, Cyclin-dependent kinases (CDKs) and their inhibitors (CDKIs) in ES cells [32]). Cyclins are regulated by miRNAs and expressed periodically throughout the cell cycle of totipotent stem cells, i.e., ES and induced pluripotent stem cells (iPS). Events and pathways in NSCs (that are pluripotent rather than totipotent) should not be compared directly, as one could infer that perhaps miRNAs are involved in the unique cell cycle, self-renewal, and pluripotency of NSCs and that this regulation is influenced by weightlessness. Almeida and collaborators [33] have shown that after a 15-day space flight, mouse ES cells display both reduced differentiation and poor regenerative potential [34]. The authors showed that expressions of growth factors associated with stem cell differentiation were significantly decreased in embryoid bodies that were differentiated in space. In contrast, expression of self-renewal and pluripotency markers (such as SOX1 and SOX2) was higher in space-flown ES cells when compared with ground control indicating partial maintenance of “stemness” while in space [33]. We have reported similar results for committed neural progenitors such as oligodendrocyte-progenitors, which expressed higher levels of early markers with a concomitant decrease of more mature markers expression [11].
It has been shown that ablation of cyclin A resulted in cell proliferation but prevented colony formation in both ES cells and induced pluripotent stem (iPS). Although a detailed molecular study of the players of cell cycle was not in the scope of our NASA grant proposal, based on our data and data from others, one could hypothesize that microgravity may up-regulate Cyclin A or Cyclin D1 [32,34]. Confirmation of this point would make cyclins “gravity sensing markers” for NSCs. More studies are needed to prove this hypothesis.
Numerous studies using different animal species have reported a strong correlation between shorter cell cycle and a higher proliferative potential of neural progenitors on Earth [34] suggesting that cell cycle length is functionally relevant during CNS development, because shorter cycles result in an increased cellular output in a given time slot. Nonetheless, expansion of the NSCs pool may be beneficial if it is controlled, while it may become deleterious if NSCs expansion perpetuates and leads to depletion of these uncommitted progenitors or even increased brain size. The literature shows that on Earth, cell cycle control is highly conserved across eukaryotic organisms and it is preserved in dissociated cells [34]. Other studies have identified cell fate determinants that are signaling molecules and transcription factors that control stem cell commitment. Based on our data two main questions come to mind: (i) how does microgravity influence all these events and molecules to increase NSCs proliferation? and (ii) is microgravity overdriving the cues inherent to the adult human brain leading to the production of new NSCs in the brain of astronauts? More studies are needed to elucidate these points. Microgravity can also inhibit the growth of cells such as mesenchymal stem cells, by arresting the cells in the G0/G1 phase of the cell cycle and diminishes the cellular response to growth factor stimulation [34]. Thus, understanding which key players are involved in the gravitational regulation of proliferation will allow us to design potential preventive measures for intracranial hypertension.
Is microgravity the fountain of youth for the CNS?
Our previous and present data concur with Almeida’s group, where they documented a reduction of differentiation markers and a tendency of ES cells to remain in the “stemness state” in SPC-µG [33]. Therefore, it appears that for ES cells and for cells of the neural lineage both, sim-µG and SPC-µG exert the same effect on the maturation and differentiation potential of cells. In particular, this infers that simulated microgravity device used by our group and others are suitable systems to mimic microgravity as experienced during spaceflight for NSCs. This is in sharp contrast to other biological systems such as bacteria where recent studies indicate the contrary [36].
These cells bore younger-like features typical of younger cells when compared to 1G controls. This effect was observed regardless of the species, as seen for example in the SPC-flown mouse ES [33]. Human SPC-flown NSCs (present data) and mouse and human OLPs grown in sim-µG [31] display the same younger-like features. These findings are in agreement with other reports and in particular with the famous “twins’ study”, where both Scott and Mark Kelly’s telomeres were similar in length at baseline before Scott’s flight. Nonetheless, after Scott’s arrival at the ISS, his telomeres were significantly longer [37,38]. Since telomere length is often used to determine aging (when the telomere is short) data from the twins’ study seem to confirm that cells can indeed become younger-like despite space flight stress and radiation exposure [39]. In the present study, we showed that our NSCs-flown to space preserved their self-renewal and pluripotency properties during space flight and after space flight.
There is no doubt that a cautious interpretation is appropriate, nonetheless, more spaceflight studies like the present study are of utmost importance in order to further understand the impact of SPC-µG on cells from the central nervous system. In addition, because there are different types of microgravity simulators such as bioreactors and clino-rotating robots, one needs to be prudent when comparing data generated using sim-µG vs. space flight [40]. Moreover, the source of cells including species and cell type may also influence the results generated and therefore, direct comparisons may not always be possible or advisable [41].
Since longer space flights are imminent, understanding how NSCs responded to space, as well as how they behave upon returning to Earth, is of the essence in order to ensure that astronauts’ health is protected during and after long-term space travel. Moreover, since the Moon and Mars have partial gravity, 16.1% and 62% respectively, when compared to Earth, the countermeasures must also be designed accordingly [42].
Finally, space travel implies exposure to both microgravity and radiation. Both represent health hazards, from which radiation is the main health risk for long space missions beyond low Earth orbit (LEO) [43]. Models from the NASA Space Cancer Risk (NSCR) indicate that galactic cosmic rays will lead to significant number of fatalities in long-term space missions (i.e., trip to Mars). In addition, models representing the uncertainties in the radiation quality factor (QF) parameters and the dose and dose-rate reduction effectiveness factor (DDREF) have been generated [44]. Experimental studies have shown that high atomic number energy (HZE) nuclei produce both qualitative and quantitative differences in biological effects compared to terrestrial radiation [45], predicting exposure health outcomes to humans. At the cellular level, the effects of long-term space radiation exposure to the brain are not yet fully understood. Therefore, studies on how space radiation affects NSCs are essential in order to learn how to preserve the astronauts’ CNS function in long-term space missions.
Cell replacement therapies are the most promising approach for developmental disabilities and neurodegeneration. HiPS-derived NSCs are undoubtedly a great source of uncommitted progenies and neurons [8], for powerful therapeutic interventions using cell transplants to restore CNS function. Therefore, these types of therapies might be of consideration to minimize risks associated with long-duration space flight.
Author’s Contribution
S.S, N.C and V.T, performed extensive image analysis of time-lapse files, consolidated the data and helped to prepare some figure composites. As the NASA mission scientist, F.K. was in charge of the mission and supported flight implementation. Additionally, he contributed with enticing discussion of the data and participated in the manuscript writing. A.E-J. designed the entire study, chose the appropriate hardware, performed the work at Kennedy Space Center, recovered the cells from the space hardware upon their return to UCLA, performed image acquisition of live cells and thereafter performed immunocytochemistry, global analysis of the data and prepared the manuscript.
Funding
We thank NASA Space Biology for Grant: NNX15AB43G; The IDDRC Cell Culture Core is supported by NIH/ NICHD grant number U54HD087101-05; and the Cell, Circuits, and Systems Analysis Core (NIH U54HD087101).
Acknowledgments
We are grateful to Mark Mobilia and the entire Zeiss team for their support with the microscopy needs for this study; without their support, this study would not have been possible. We thank Dr. Amy Rowat for support with her microscope system as well. Dr. Amy Gresser, Elizabeth Pane, and Medaya Torres for their help with the implementation of the study. The NASA Space Biology Project team at Ames Research Center Dr. D. Tomko, K. Sato, E. Taylor, ARC Space Biology Project Manager and the support personnel at the Space Station Processing Facility at Kennedy Space Center. Thanks to Karin Perkins, Diana Ly, and Space Biology support staff. We also thank Dr. Carlos Cepeda for fruitful discussions. Special thanks to Maria Birlem and Chriss Bruderrek from Yuri, and STaARS team members Tom Kyler, Craig Walton and BreAnne MacKenzie for supporting the flight implementation. Thanks to Uli Kuebler from Airbus Defense and Space, who introduced me to the flying hardware used for this study. We also thank Elida Escalante, for preparation of the mesh micro-carriers. Dorwin Birt for help with computer-related matters, and Aurora Espinosa de los Monteros B. for help with the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goll MG, Bestor TM (2005) Eukaryotic Cytosine Methyl-transferases. Annu Rev Biochem 74: 481-514.
- Obernier K, Alvarez-Buylla A (2019) Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development 146: dev156059.
- Morales AV, Mira H (2019) Adult Neural Stem Cells: Born to Last. Front Cell Dev Biol 7: 96.
- Aleci C (2020) From international ophthalmology to space ophthalmology: The threats to vision on the way to Moon and Mars colonization. Int Ophthalmol 40: 775-786.
- Lee AG, Mader TH, Gibson CR, Tarver W, Rabiei P, et al. (2020) Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: A review and an update. NPJ Microgravity 6: 7.
- Barratt MR, Baker ES, Pool SL (2019) Principles of Clinical Medicine for Space Flight 2nd. ed., Springer, Switzerland.
- Espinosa-Jeffrey A, Nguyen K, Taniguchi A, Vergnes L, and de Vellis J (2016) Changes in energetics associated molecules, enhanced proliferation and oxygen metabolism in OLs grown in simulated microgravity, by the International Astronautical Congress (IAC); IAC-16,A1,7,6,X34299, Guadalajara, Mexico (September 26-30, 2016).
- Cepeda C, Vergnes L, Carpo N, Schibler MJ, Bentolila LA, et al. (2019) Human Neural Stem Cells Flown into Space Proliferate and Generate Young Neurons. Appl Sci 9: 4042.
- Espinosa-Jeffrey A, Becker-Catania S, Zhao PM, Cole R, de Vellis J (2002) Selective specification of CNS stem cells into oligodendroglial or neuronal cell lineage: Cell culture and transplant studies. J Neurosci Res 69: 810-825.
- Cavanagh BL, Walker T, Norazit A, Meedeniya ACB (2011) Thymidine analogues for tracking DNA synthesis. Molecules 16: 7980-7993.
- Espinosa de los Monteros A, Espejo D, de Vellis J (1997) Transplantation of Oligodendrocyte Progenitors and CG4 cells into the Developing Rat Brain: Differences and similarities. Molecular Signaling and Regulation in Glial Cells. Pg no: 329-341.
- Espinosa-Jeffrey A, Paez PM, Cheli VT, Spreuer V, Wanner I, et al. (2013) Impact of simulated microgravity on oligodendrocyte development: implications for central nervous system repair. PloS one 8: e76963.
- Abdi H (2007) The Bonferonni and Šidák Corrections for Multiple Comparisons. In Salkind NJ (Ed.) Encyclopedia of Measurement and Statistics. Thousand Oaks (CA): Sage, United Kingdom, Pg no: 103-107.
- Quartu M, Serra MP, Boi M, Ibba V, Melis T, et al. (2008) Polysialylated-neural cell adhesion molecule (PSA-NCAM) in the human trigeminal ganglion and brainstem at prenatal and adult ages. BMC Neurosci 9: 108.
- Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H (2010) The Neural Stem/Progenitor Cell Marker Nestin Is Expressed in Proliferative Endothelial Cells, but Not in Mature Vasculature. J Histochem Cytochem 58: 721-730.
- Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, et al. (2010) Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci 13: 1338-1344.
- Mu Y, Lee SW, Gage FH (2010) Signaling in adult neurogenesis. Curr Opin Neurobiol 20: 416-423.
- Ninkovic J, Götz M (2007) Signaling in adult neurogenesis: From stem cell niche to neuronal networks. Curr Opin Neurobiol 17: 338-344.
- Sun Y, Hu J, Zhou L, Pollard SM, Smith A (2011) Interplay between FGF2 and BMP controls the self-renewal, dormancy and differentiation of rat neural stem cells. J Cell Sci 124: 1867-1877.
- van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266-270.
- Krityakiarana W, Zhao PM, Nguyen K, Gomez-Pinilla F, Kotchabhakdi N, et al. (2016) Proof-of Concept that an Acute Trophic Factors Intervention After Spinal Cord Injury Provides an Adequate Niche for Neuroprotection, Recruitment of Nestin-Expressing Progenitors and Regeneration. Neurochem Res 41: 431-449.
- Jessberger S, Parent JM (2015) Epilepsy and Adult Neurogenesis. Cold Spring Harb Perspect Biol 7: a020677.
- Mora F (2013) Successful brain aging: Plasticity, environmental enrichment, and lifestyle. Dialogues Clin Neurosci 15: 45-52.
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, et al. (1997) Dentate Granule Cell Neurogenesis Is Increased by Seizures and Contributes to Aberrant Network Reorganization in the Adult Rat Hippocampus. J Neurosci 17: 3727-3738.
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, et al. (2009) Neuronal Activity-Induced Gadd45b Promotes Epigenetic DNA Demethylation and Adult Neurogenesis. Science 323: 1074-1077.
- Vescovi AL, Parati EA, Gritti A, Poulinb P, Ferrariob M, et al. (1999) Isolation and Cloning of Multipotential Stem Cells from the Embryonic Human CNS and Establishment of Transplantable Human Neural Stem Cell Lines by Epigenetic Stimulation. Exp Neurol 156: 71-83.
- Villa A, Snyder EY, Vescovi A, Martinez-Serrano A (2000) Establishment and Properties of a Growth Factor-Dependent, Perpetual Neural Stem Cell Line from the Human CNS. Exp Neurol 161: 67-84.
- Johansson S, Price J, Modo M (2008) Effect of Inflammatory Cytokines on Major Histocompatibility Complex Expression and Differentiation of Human Neural Stem/Progenitor Cells. Stem Cells 26: 2444-2454.
- Sun Y, Kong W, Falk A, Hu J, Zhou L, et al. (2009) CD133 (Prominin) Negative Human Neural Stem Cells Are Clonogenic and Tripotent. PLoS One 4: e5498.
- Wanner IB (2011) An in vitro Trauma Model to Study Rodent and Human Astrocyte Reactivity. Methods Mol Biol 814: 189-219.
- Biswas S, Chung SH, Jiang P, Dehghan S, Deng W (2019) Development of glial restricted human neural stem cells for oligodendrocyte differentiation in vitro and in vivo. Sci Rep 9: 9013.
- Beerman I, Rossi DJ (2015) Epigenetic Control of Stem Cell Potential during Homeostasis, Aging, and Disease. Cell Stem Cell 16: 613-625.
- Ma X, Pietsch J, Wehland M, Schulz H, Saar K, et al. (2013) Differential gene expression profile and altered cytokine secretion of thyroid cancer cells in space. FASEB J 28: 813-835.
- Blaber EA, Parker GC (2018) Stem Cells and Microgravity. Stem Cells Dev 27: 783-786.
- Sutherland RL, Musgrove EA (2004) Cyclins and Breast Cancer. J Mammary Gland Biol Neoplasia 9: 95-104.
- Morrison MD, Nicholson WL (2020) Comparisons of Transcriptome Profiles from Bacillus subtilis. Cells Grown in Space versus High Aspect Ratio Vessel (HARV) Clinostats Reveal a Low Degree of Concordance. Astrobiology 20: 1498-1509.
- Welsh J, Bevelacqua J, Keshavarz M, Mortazavi SAR (2019) Is Telomere Length a Biomarker of Adaptive Response? Controversial Findings of NASA and Residents of High Background Radiation Areas. J Biomed Phys Eng 9: 381-388.
- Garrett-Bakelman FE, Darshi M, Green SJ, Gur RC, Lin L, et al. (2019) The NASA Twins Study: A multidimentional analysis of a year-long human spaceflight. Science 364: 8650.
- Dai ZQ, Wang R, Ling SK, Wan YM, Li YH (2007) Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Prolif 40: 671-684.
- Espinosa-Jeffrey A, Oregel K, Ikeda M, Green J, Alana T, et al. (2016) The Implications of Simulated-Microgravity on Cell Morphology, Lineage Commitment and Proliferation of Neural Stem Cells towards Astronaut Health. 67th International Astronautical Congress (IAC), Guadalajara, Mexico, 23-30 September 2016.
- Silvano M, Miele E, Valerio M, Casadei L, Begalli F, et al. (2015) Consequences of Simulated Microgravity in Neural Stem Cells: Biological Effects and Metabolic Response. J. Stem Cell Res Ther 5: 289.
- Karouia F, Peyvan K, Pohorille A (2017) Toward biotechnology in space: High-throughput instruments for in situ biological research beyond Earth. Biotechnol Adv 35: 905-932.
- Jones JA, Kerouia F, Pinsky L, Cristea O (2019) Radiation and Radiation Disorders, in Principles of Clinical Medicine for Space Flight. Editors: Barratt MR, Baker ES, Pool SL, Springer, Pg No’s. 39-108.
- Cucinotta FA, Cacao E (2017) Non-Targeted Effects Models Predict Significantly Higher Mars Mission Cancer Risk than Targeted Effects Models. Sci Rep 7: 1832.
- Liker MA, Petzinger GM, Nixon K, McNeill T, Jakowec MW (2003) Human neuralstem cell transplantation in the MPTP-lesioned mouse. Brain Res 971: 168-177.
Citation: Shaka S, Carpo N, Tran V, Ma YY, Karouia F, et al. (2021) Human Neural Stem Cells in Space Proliferate more than Ground Control Cells: Implications for Long-Term Space Travel. J Stem Cell Res Dev Ther 7: 069.
Copyright: © 2021 Sophia Shaka, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

