
Human Prostate Stem Cells and Their Niche - A Comprehensive Review
*Corresponding Author(s):
Rakesh HeerNorthern Institute For Cancer Research, Newcastle University, Newcastle-upon-Tyne, United Kingdom
Email:rakesh.heer@newcastle.ac.uk
Abstract
Several recent major findings in the field of adult prostate stem cells have advanced our understanding of the cell biology. Earlier seminal studies in the murine prostate demonstrated and defined the cell biology and dynamics. However, it remained unclear how these findings correlated to the human prostate stem cell until very recently. The location and dynamics of the human adult prostate stem cell niche have now been identified. This has implications for further research into the origins of benign prostatic hypertrophy and prostate cancer. This review summarises the current evidence for prostate stem cells and their niche in both the murine and human models.
Keywords
Adult stem cell; Niche; Progenitor; Prostate; Stem cell
INTRODUCTION
The past decade has seen rapid developments in our understanding of adult stem cell biology and the central role they play in tissue maintenance, ageing and disease [1-3]. These insights are now providing new approaches to tissue regeneration, disease modelling and treatments [4].
In this review we focus on the prostate, where benign and malignant disease is common. Symptomatic Benign Prostatic Hypertrophy (BPH) affects more than 40% of men over 50 [5], whilst prostate cancer is the most common male cancer and the second leading cause of cancer death worldwide [6]. A better understanding of the normal mechanism of prostate epithelium maintenance will underpin new approaches to treatment as this will lay the foundations for understanding the effects of ageing and abnormal regulation in disease. The mouse prostate field has predominantly defined stem cells and mechanisms for homeostasis in pioneering techniques involving lineage tracing [7,8] and organoid culture [9,10]. How the findings from these studies correlate to human prostate stem cell biology remained an open question until very recently.
This review will focus on recent advances in describing human prostate stem cell biology, the prostate stem cell niche and the opportunities these findings afford for a translational impact.
EVIDENCE FOR HUMAN ADULT PROSTATE STEM CELLS - BASAL, LUMINAL OR BOTH?
The existence of somatic adult stem cells has been widely illustrated within various human organs, including hair follicles [11], skin [12], bone marrow [13] and intestinal tissue [3], which allow for tissue maintenance during ageing and rapid regeneration in cases of injury.
The initial evidence for stem cells within the prostate came from studies in rat, or murine, prostatic epithelium. The prostate epithelium consists of 3 types of cell: basal, luminal and neuroendocrine. The luminal cells line the ductal lumens, whilst the basal cells lie underneath, adjacent to the basal membrane [14]. Neuroendocrine cells are rare, secretory cells expressing neuropeptides and are distinct from the basal and luminal cells [14,15].
The first studies showed that androgen deprivation led to regression of adult prostate by preferential apoptosis of luminal cells. Re-administration of androgens then led to regeneration of new, fully developed prostate [16,17]. Another piece of evidence came, again from murine models. This time from transplantation of embryonic murine p63+ Urogenital Sinus (UGS) into immunodeficient mice [18]. The UGS then differentiated into luminal and neuroendocrine cells, but not basal, suggesting basal cells are not needed for generation of prostate-like tissue in transplantation assays. This study was followed closely by murine chimaeric studies between wild type and p63 knockout mice by Signoretti et al. [19]. They demonstrated that prostate epithelium, including basal and luminal cells, were derived only from p63+ mice, suggesting that during normal murine embryonic development all prostate cells arise from p63+ progenitors. Together, these studies suggest the presence of castrate-resistant adult prostate stem cells within the prostatic epithelium.
These studies ignited a search for the location of the stem cell. As p63 null mice do not develop a prostate, and p63 is expressed in basal cells only, it was suggested that both basal and luminal cells arise from basal p63 progenitors in the embryonic model [19]. By this logic, the search for an adult prostate stem cell was focussed on the basal compartment. Leong et al. demonstrated that a single adult basal cell can regenerate prostate tissue on transplantation, supporting the existence of adult basal multipotent prostate stem cells [20].
At the same time, functional prostate regeneration assays demonstrated that a small population of basal cells in both human and murine prostate exhibit multipotency [20-24]. However, it is worth noting that these assays do not reflect the normal physiological condition.
Molyneux et al. demonstrated that determination of the cell of origin in the absence of functional lineage tracing studies would lead to misleading results [25]. Previous methods i.e transplantation of UGS [26] and in vitro methods of primary prostate culture [27], although both demonstrate bipotency of basal cells, they favour basal cell growth over luminal. This left the luminal cells relatively unexplored. Wang et al. demonstrated that a murine luminal stem cell existed, and was a cell of origin for prostate cancer [28]. However, this did not definitively demonstrate the existence of a luminal stem cell in the normal prostate as this was performed in a castrate resistant model, therefore not reflective of normal physiology.
The turn of the decade saw a revolution in technology with the emergence of lineage tracing studies, which have become the gold standard in stem cell research. The initial murine lineage tracing studies by Wang et al., demonstrated that Nkx3.1 is mainly expressed in luminal cells with expression in a small minority of basal cells. However, when the mice were castrated, they found that Nkx3.1 expression in luminal cells was much reduced [28,29]; these were termed castration-resistant Nkx3.1-expressing cells (CARNs). They then demonstrated that CARNs can generate both basal and luminal cells. However, this model did not represent the normal physiology, given the castration.
Two further, seminal lineage tracing studies in the murine model by Ousset et al. and Choi et al. were able to examine both luminal and basal cells for the potential of stemness, without predominance of basal cells. Choi et al. demonstrated by labelling cytokeratins 14 (CK14; basal-specific) and 8 (CK8; luminal-specific), in vivo, that neither lineage was able to differentiate into the other, suggesting the existence of independently sustained populations of unipotent luminal and basal stem cells in the adult murine model [30]. In the work by Ousset et al., they examined the embryonic and adult model, using the same labels as Choi et al. (CK14 and CK5 for basal cells and CK8 for luminal cells) [7]. They found in early developmentthat CK14 labelled basal cells demonstrated a large expansion and differentiation into both CK5+ basal and CK8+ luminal cells. CK5 lineage tracing demonstrated a far higher proportion of basal cells than luminal cells, whilst CK8 luminal cell lineage tracing demonstrated a stable frequency of luminal labelled cells but no basal differentiation. Lastly, they demonstrated a proportion (15%) of ‘intermediate’ adult cells. These are basal cells expressing both CK5 and CK8, which are strongly associated with luminal differentiation. Here, the existence of multipotent basal progenitors during prostate postnatal development contrasts with the distinct pools of unipotent basal and luminal stem cells that mediate adult prostate regeneration.
Together, these findings suggest the presence of unipotent luminal progenitors in both the embryonic and the adult mouse, whilst basal cells lose their embryonic multipotency and become predominantly unipotent in the adult. However, there appears to be a minor population amongst the luminal and basal cells that retain some potential for multipotency (Figure 1).
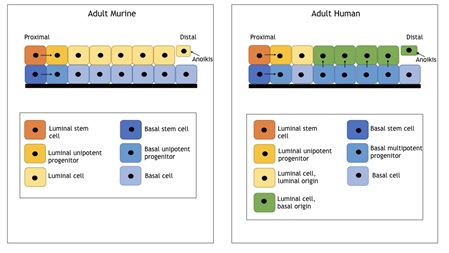 Figure 1: Diagram demonstrating adult murine and human prostatic epithelium.
Figure 1: Diagram demonstrating adult murine and human prostatic epithelium.
The study of human prostate stem cells was lagging behind that of the murine model, as lineage tracing models were limited until the discovery of the ability to use mitochondrial (mt) DNA mutations marks linked with ageing. These lead to respiratory chain deficits that can be measured by Cyclo-Oxygenase activity (CCO) [31,32]. The study by Ousset et al. in the murine model echoed the initial results in human clonal analysis in prostatectomy specimens showing the presence of bipotent clones containing both basal and luminal CCOdeficient cells, as well as unipotent basal or luminal clones of CCO deficient cells [31,32].
A land mark paper came from Karthaus et al. [10], who argued that in vitro culture systems (UGS transplantation and primary prostate cell culture) don’t generate tissues resembling the in vivo composition or contain Androgen Receptors (AR) at physiological levels. Utilising novel organoid culture methods pioneered in the investigation of the gastrointestinal tract [33,34], they produced sustainable R-spondin1 based adult prostate organoids from mouse and human prostate cells, respectively. The human model’s luminal cells expressed physiological CK8 and AR, whilst basal cells expressed p63 and CK5, both in keeping with their epithelial position. The CK5-expressing basal and CK8-expressing luminal cells were then separated and cultured as organoids. Basal (CK5)-derived organoids expressed mostly CK5, with CK8 cells surrounding a sporadic array of lumens, with patchy AR expression. Luminal derived organoids immediately formed lumens. The majority of cells expressed CK8 and AR, with a small minority being CK5+, CK8- i.e basal cells. This indicates that human luminal cells are able to generate basal cells and vice versa i.e both luminal and basal stem cells have bipotency within the organoid model [10]. Finally, Moad et al. [35], using lineage tracing methods of human prostatectomy specimens with subsequent in vitro organoid culture, demonstrated multipotency of basal stem cells and unipotency of luminal stem cells (Table 1).
|
Model |
Embryonic/ Adolescent |
Adult |
|
|
Murine |
In Vitro |
Burger et al., 2005; Goldstein et al., 2010; Lawson et al., 2007; Leong et al., 2008; Xin et al., 2003 [20-24] |
Leong et al. 2008; Karthaus et al. 2014 [10,20] |
|
In Vivo |
Ousset et al. 2012 [7] |
Wang et al. 2009; Ousset et al. 2012; Choi et al. 2012 [7,28,30] |
|
|
Human |
In Vitro |
N/A |
Karthaus et al. 2014; Moad et al. 2017 [10,35] |
|
In Vivo |
N/A |
Moad et al. 2017 [35] |
|
Table 1: Summary of evidence for prostate stem cells in differing models.
We therefore have definitive evidence in humans for multipotency in basal stem cells and conflicting evidence of multipotency (organoid model) vs. unipotency (lineage tracing and organoid culture) in luminal stem cells.
PROSTATE STEM CELL NICHE - PROXIMAL OR DISTAL?
As adult stem cells are required for the life of the tissue they are thought to reside in highly specialized, spatially confined locations, which provide unique microenvironments to maintain these essential cells; the stem cell niche. A functional niche should maintain a homeostatic balance between cellular quiescence and activity [2].
Until the work by Moad et al. [35], very little was understood about the true location, and definitive identity of human prostate stem cells and their niche. Prior to this, the stem cell niche had been described in other tissues, but not for the prostate. The relationship between other epithelial stem cells and their surrounding tissues (Extracellular Membrane [ECM], stromal cells, neighboring epithelial cells and possibly immune cells) had been described in differing tissues, notably the gut with intestinal crypts [2,36,37]. Intestinal crypts lie at the bottom of epithelial invaginations at the base of each villus within the small intestine [38]. This provides a physical space in which the cells can be contained and maintained.
The adult human prostate stem cell niche has only been described recently. As mentioned above, Moad et al. [35] demonstrated the multipotency of basal stem cells and unipotency of luminal stem cells using lineage tracing. Following from this, they performed 3D glandular reconstructions with proliferation kinetics and functional assays of differentiation. These demonstrated several key findings. Firstly, stem cells generated continuous migratory streams flowing from individual stem cells located in the basal layer of proximal ductal epithelium to peripheral distal epithelium. This finding of proximal stem cell location echoed the findings from animal studies, where Tsujimura et al. [39] demonstrated that murine prostate stem cells originate in the proximal portion of the duct and more recently a scRNA sequencing-based comprehensive cellular atlas of whole prostates revealed proximally residing putative progenitor cells (Club and Hillock cells). These proximal basal progenitors differentiate into both basal and luminal epithelial cells in all but the most proximal region [35]. Secondly, they made the observation that these basal progenitors never gave rise to proximally located luminal cells. These particular cells have their own, unipotent cells within the most proximal part of the duct, which give rise to short luminal-only clonal expansions. Thirdly, they demonstrated that the urothelium interdigitates with the prostatic epithelium into the proximal prostatic ducts. The proximal basal progenitors lie at the interface between the urothelium and prostatic epithelium within these separating interdigitations, topographicallysimilar in structure to intestinal crypts.
Together these findings show that human prostatic stem cells originate in the basal layer in the stem cell niche nested within interdigitationsof urothelium and flow, unidirectionally into the distal ducts, where they differentiate into both luminal and basal cells. A separate, limited pool of luminal stem cells maintains the luminal epithelium at the most proximal part of the duct (Figure 1).
STEM CELL DYNAMICS IN THE PROSTATE STEM CELL NICHE
Stem cell dynamics, or modes of cellular division, is a key process in maintaining the stem cell population and providing differentiated cells for replenishment of specific tissues. Stem cell division can be either symmetrical, whereby two daughters cells of equivalent fates are produced, or asymmetrical where the daughter cells have differing fates, namely a stem cell and a committed progenitor of fully differentiated tissue.
Cell division within a closed niche, such as that demonstrated in the prostate [35] poses a problem, namely that there is spatial limitation, allowing only a finite number of stem cells to reside there. This phenomenon has been extensively investigated in the intestinal crypts. A seminal paper by Ritsma et al. [36], demonstrated that, contrary to previous studies, intestinal stem cell division appears symmetric. However, increasing loss of contact with the niche seems to prevent the maintenance of stemness and they begin to differentiate [36].
There is no such definite work on the prostatic niche, however it has been demonstrated in the murine prostate, by labelling centrosomes with g-tubulin, that mitotic spindles among dividing basal cells occurred in two predominant orientations to the basement membrane: horizontal and vertical [40]. They observed that formation of the luminal layer was marked with an increased number of vertical divisions, with vertical division increasing from around 36% at postnatal day 5 to around 65% at postnatal day 15. Examination of horizontally and vertically oriented daughter cells revealed two distinct fates for the dividing basal cells (asymmetrical and symmetrical). Horizontally-divided basal cells always gave rise to two daughter basal cells (as detected by p63 staining), whilst vertically divided cells gave rise to a basal cell (p63 positive) and an apical, luminal cell (p63 negative, but CK8 positive). Luminal cells only gave rise to other luminal cells, by dividing horizontally. These findings fitted with previous observations of a multipotent basal progenitor and a unipotent luminal progenitor in the embryonic murine model [7].
REGULATORY PATHWAYS IN THE PROSTATE STEM CELL NICHE
As described earlier, a stem cell niche not only provides an anatomical location, in this case in the prostatic epithelium surrounded by interdigitating urothelium at the proximal portion of the prostatic duct, but also a microenvironment, which supports and sustains the stem cell. Loss of contact to which, appears to induce loss of stemness [36], at least in the intestinal stem cell niche. A seminal study in mammalian skin first identified the importance of a Notch-dependent pathway in apparent asymmetric division in epidermal stem cells [12].
In a series of embryonic murine studies, Notch1 was initially identified in basal progenitor cells [41]. Notch1 was then shown to be indispensable for prostatic morphogenesis and regrowth following androgen deprivation and replacement [42]. Finally, [43] demonstrated that, by stimulating the Notch pathway directly, there was a stimulatory effect on cell differentiation and proliferation. These series of experiments demonstrated that the Notch pathway was a major regulator of embryonic murine prostatic basal stem cells. A further study in adult murine prostates, then demonstrated that Notch ligands were present in both the basal and luminal layers [44], consistent with contemporaneous studies showing separate pools of unipotent progenitors within the separate layers [7]. Disruption of the pathway increased differentiation and proliferation of the basal layer, but not the luminal. Whilst application of ectopic Notch resulted in a decrease in basal cell number and luminal cell hyper proliferation [44].
As before, human research took time to catch up with that of the murine. Ceder et al. [45], demonstrated that DLK1, a ‘dead’ ligand [46] to Notch and known regulator of prostatic epithelial homeostasis [43,44], was highly expressed in adult human prostate basal stem cells. They found that these basal stem cells displayed little to no Notch reactivity [5]. A further study demonstrated that, like the mouse, Notch is an established regulator of basal progenitor differentiation [47].
It was not until the study by Moad et al. [35], that the evidence for the effect of Notch and DLK1 in adult human prostatic stem cells was clarified. With immunohistochemistry of DLK1 and Notch in vivo they demonstrated a distinct spatial expression profile, with DLK1 only expressed in basal cells within, the now identified, prostatic stem cell niche. This was consistent with DLK1 providing an inhibitory signal, opposing Notch-stimulated basal progenitor differentiation. DLK1 was also identified in peripheral, or distal, luminal cells, where the greatest apoptotic activity was identified, suggesting a gradient in Notch expression. This was consistent with previous findings showing DLK1 inhibits Notch-regulated resistance to cell death through anoikis (detachment from the extracellular membrane) [48]. Moad et al. [35] further tested this observation with experimental organoid culture of DLK1+ and DLK1- basal cells. DLK1+ basal-cell derived organoids developed into long-lasting mature prostatic architecture expressing basal (CK5) and luminal (CK8, PSA and AR) markers. Whilst, DLK1- organoids did not mature and there was visible apoptosis.
Another signalling pathway associated with prostatic stem cells is the Wnt pathway. Hu et al. [49] demonstrated, in vitro, that prostatic stem cells expressed high levels of Wnt10B, known to be a regulator of stem cell homeostasis [50]. As yet, this has not been demonstrated in vivo, but remains a tantalising research target.
These studies provide the latest evidence for how the adult human prostate epithelium is organized. Proximal basal stem cells propagate, differentiating distally into both basal and luminal cells, replacing distal luminal cells lost through apoptosis. It appears reasonable to hypothesize that the interaction between Notch and DLK1 induces a quiescent state in basal prostate stem cells, whilst loss of DLK1 distally and therefore loss of this interaction then drives luminal differentiation as shown in the murine studies [44]. DLK1 expression at the periphery, associated with apoptosis, suggests a Notch gradient, maximal at the niche, tapering to negligible in the periphery, although this, along with a role for the Wnt pathway have yet to be demonstrated.
SUMMARY
In this review, we define the current understanding of the biology of the human prostate stem cell niche. Recent findings open new lines of investigation and we outline potential cell signaling regulators and cell-to-cell interactions of interest, which could be explored as potentially aberrant in diseased states. Furthermore, there is unique topography to the prostate niche which may provide increasing gradients to stem cell niche factors toward the apexes of the niche between the interdigitating urothelial tongues and will be the focus of future research investigating the role of the niche in prostate cancer.
REFERENCES
- Clevers H (2006) Wnt/β-Catenin Signaling in Development and Disease. Cell 127: 469-480.
- Moore KA, Lemischka IR (2006) Stem cells and their niches. Science 311: 1880-1885.
- Stange DE, Clevers H (2013) Concise review: The Yin and Yang of intestinal (cancer) stem cells and their progenitors. Stem Cells 31: 2287-2295.
- Clevers H (2011) The cancer stem cell: premises, promises and challenges. Nat Med 17: 313-319.
- Naslund MJ, Gilsenan AW, Midkiff KD, Bown A, Wolford ET, et al. (2007) Prevalence of lower urinary tract symptoms and prostate enlargement in the primary care setting. Int J Clin Pract 61: 1437-1445.
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. (2015) The Global Burden of Cancer 2013. JAMA Oncol 1: 505-527.
- Ousset M, Van Keymeulen A, Bouvencourt G, Sharma N, Achouri Y, et al. (2012). Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat. Cell Biol 14: 1131-1138.
- Shen MM (2015) Illuminating the Properties of Prostate Luminal Progenitors. Cell Stem Cell 17: 644-646.
- Drost J, Karthaus WR, Gao D, Driehuis E, Sawyers CL, et al. (2016) Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc 11: 347-358.
- Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, et al. (2014).Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159: 163-175.
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, et al. (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411-417.
- Lechler T, Fuchs E (2005) Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437: 275-280.
- LaBarge MA, Blau HM (2002) Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111: 589-601.
- McNeal JE (1968) Regional morphology and pathology of the prostate. Am J Clin Pathol 49: 347-357.
- Kasper S (2008) Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev 4: 193-201.
- English HF, Santen RJ, and Isaacs JT (1987) Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate 11: 229-242.
- Evans GS, Chandler JA (1987) Cell proliferation studies in the rat prostate: II. The effects of castration and androgen-induced regeneration upon basal and secretory cell proliferation. Prostate 11: 339-351.
- Kurita T, Medina RT, Mills AA, Cunha GR (2004) Role of p63 and basal cells in the prostate. Development 131: 4955-4964.
- Signoretti S, Pires MM, Lindauer M, Horner JW, Grisanzio C, et al. (2005) p63 regulates commitment to the prostate cell lineage. Proc Natl Acad Sci USA 102: 11355-11360.
- Leong KG, Wang BE, Johnson L, Gao WQ (2008) Generation of a prostate from a single adult stem cell. Nature 456: 804-808.
- Burger PE, Xiong X, Coetzee S, Salm SN, Moscatelli D, et al. (2005) Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci USA 102: 7180-7185.
- Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON (2010) Identification of a cell of origin for human prostate cancer. Science 329: 568-571.
- Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON (2007) Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA 104: 181-186.
- Xin L, Ide H, Kim Y, Dubey P, Witte ON (2003) In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci USA 100: 11896-11903.
- Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, et al. (2010) BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell 7: 403-417.
- Cunha GR (1973) The role of androgens in the epithelio-mesenchymal interactions involved in prostatic morphogenesis in embryonic mice. Anat Rec 175: 87-96.
- Garraway IP, Sun W, Tran CP, Perner S, Zhang B, et al. (2010) Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate 70: 491-501.
- Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, et al. (2009) A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461: 495-500.
- Bethel CR, Faith D, Li X, Guan B, Hicks JL, et al. (2006) Decreased NKX3.1 protein expression in focal prostatic atrophy, prostatic intraepithelial neoplasia, and adenocarcinoma: association with gleason score and chromosome 8p deletion. Cancer Res 66: 10683-10690.
- Choi N, Zhang B, Zhang L, Ittmann M, Xin L (2012) Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 21: 253-265.
- Blackwood JK, Williamson SC, Greaves LC, Wilson L, Rigas AC, et al. (2011) In situ lineage tracking of human prostatic epithelial stem cell fate reveals a common clonal origin for basal and luminal cells. J Pathol 225: 181-188.
- Gaisa NT, Graham TA, McDonald SA, Poulsom R, Heidenreich A, et al. (2011) Clonal architecture of human prostatic epithelium in benign and malignant conditions. J Pathol 225: 172-180.
- Sato K, Nagai J, Mitsui N, Ryoko Yumoto, Takano M (2009) Effects of endocytosis inhibitors on internalization of human IgG by Caco-2 human intestinal epithelial cells. Life Sci 85: 800-807.
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, et al. (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141: 1762-1772.
- Moad M, Hannezo E, Buczacki SJ, Wilson L, El-Sherif A, et al. (2017) Multipotent Basal Stem Cells, Maintained in Localized Proximal Niches, Support Directed Long-Ranging Epithelial Flows in Human Prostates. Cell Rep 20: 1609-1622.
- Ritsma L, Ellenbroek SIJ, Zomer A, Snippert HJ, de Sauvage FJ, et al. (2014) Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507: 362-365.
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, et al. (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134-144.
- Cheng H, Leblond CP (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat 141: 537-561.
- Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, et al. (2002) Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol 157: 1257-1265.
- Wang J, Zhu HH, Chu M, Liu Y, Zhang C, et al. (2014) Symmetrical and asymmetrical division analysis provides evidence for a hierarchy of prostate epithelial cell lineages. Nat Commun 5: 4758.
- Shou J, Ross S, Koeppen H, de Sauvage FJ, and Gao WQ (2001) Dynamics of notch expression during murine prostate development and tumorigenesis. Cancer Res 61: 7291–7297.
- Wang XD, Shou J, Wong P, French DM, Gao WQ (2004) Notch1-expressing cells are indispensable for prostatic branching morphogenesis during development and re-growth following castration and androgen replacement. J Biol Chem 279: 24733–24744.
- Wang XD, Leow CC, Zha J, Tang Z, Modrusan Z, et al. (2006) Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev Biol 290: 66-80.
- Valdez JM, Zhang L, Su Q, Dakhova O, Zhang Y, et al. (2012) Notch and TGFβ form a reciprocal positive regulatory loop that suppresses murine prostate basal stem/progenitor cell activity. Cell Stem Cell 11: 676-688.
- Ceder JA, Jansson L, Helczynski L, Abrahamsson PA (2008) Delta-like 1 (Dlk-1), a novel marker of prostate basal and candidate epithelial stem cells, is downregulated by notch signalling in intermediate/transit amplifying cells of the human prostate. Eur Urol 54: 1344-1353.
- Baladrón V, Ruiz-Hidalgo MJ, Nueda ML, Díaz-Guerra MJ, García-Ramírez JJ, et al. (2005) dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp Cell Res. 303: 343-359.
- Zhang D, Park D, Zhong Y, Lu Y, Rycaj K, et al. (2016). Stem cell and neurogenic gene-expression profiles link prostate basal cells to aggressive prostate cancer. Nat Commun 7: 10798.
- Kwon OJ, Valdez JM, Zhang L, Zhang B, Wei X, et al. (2014) Increased Notch signalling inhibits anoikis and stimulates proliferation of prostate luminal epithelial cells. Nat Commun 5: 4416.
- Hu WY, Hu DP, Xie L, Li Y, Majumdar S, et al. (2017) Isolation and functional interrogation of adult human prostate epithelial stem cells at single cell resolution. Stem cell res 23: 1-12.
- Clevers H, Loh K, Nusse R (2014) Stem cell signalling. An integral program for tissue renewal and regeneration: Wnt signalling and stem cell control. Science 346: 1248012.
Citation: Subramanian S, Geraghty R, Hepburn AC, Wilson L, Heer R (2019) Human Prostate Stem Cells and Their Niche - A Comprehensive Review. J Stem Cell Res Dev Ther 5: 020.
Copyright: © 2019 Seshadhri Subramanian, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

