
Initial Experience with Humanized Allogeneic and Autologous Exosome-Based Therapies in Androgenetic Alopecia: A case series
*Corresponding Author(s):
Suruchi GargDirector And Chief Consultant, Aura Skin Institute, Chandigarh, India
Email:gargsuruchi01@gmail.com
Introduction
Hair loss, particularly androgenetic alopecia (AGA) and female pattern hair loss (FPHL), is a common dermatologic concern with significant impact on the quality of life and psychosocial well-being of patients. Although current FDA approved therapies such as minoxidil, finasteride and low-level laser therapy can slow progression and promote modest regrowth, their use is often limited by poor compliance, adverse effects and need for long term use. This has prompted the need for regenerative, cell-free therapies that target the underlying follicular microenvironment and promote prolonged effects. Exosomes are nano-sized extracellular vesicles (30–150 nm) secreted by nearly all cell types and play a crucial role in intercellular communication by transferring proteins, lipids, messenger ribonucleic acids (mRNAs), and microRNAs to recipient cells [1]. In the context of androgenetic alopecia, exosomes have shown therapeutic benefits by modulating inflammation, enhancing angiogenesis, promoting follicular regeneration, and reversing miniaturization of hair follicles [2,3]. Although both humanized exosomes and autologous exosomes are commercially available for clinical use, clinical evidence supporting their effectiveness and safety in hair loss is still emerging. We describe our experience using humanized and autologous exosome therapies in patients with hair loss, discussing outcomes, tolerability, and practical considerations, contributing to the growing evidence base for exosome-based hair restoration. There is a paucity of literature in this field and through this case series, we hope to contribute to the existing body of literature supporting the use of exosomes.
Case Series
In this prospective interventional study, we enrolled 10 cases after obtaining informed consent and a detailed medical history. Clinical photography and trichoscopy were performed at baseline and subsequent follow-ups.
In this study we aimed to study efficacy, safety of three different variants (derivative source) of exosomes, namely Metacell Technology (MCT) blood derived exosomes, Exoxe and AdvancExo, along with 3 different methods of administration - Intradermal Injection, Microneedling and Microneedling Radiofrequency (MNRF), for hair growth in Androgenetic alopecia.
Out of 10 cases, 7 cases were thoroughly evaluated clinically and trichoscopically at follow-up along with routine blood reports and detailed dietary history. Two cases (Pt. 7 and 9) were lost to follow up.
Methodology
All 10 cases were evaluated for the grade of AGA and were treated with exosomes derived from autogenic or allogenic source by various delivery methods. Baseline demographic and clinical characteristics of the patients are summarized in (Table 1).
|
Sr. No. |
Patient Code |
AGE |
SEX |
AGA GRADE- Hamilton-Norwood Scale |
DURATION |
|
1 |
Pt. 1 |
48 |
F |
1(Sinclair’s) |
1yr |
|
2 |
Pt. 2 |
34 |
M |
IIIA |
2yrs |
|
3 |
Pt. 3 |
27 |
M |
IIIA |
6months |
|
4 |
Pt. 4 |
64 |
F |
5(Sinclair’s) |
7-8yrs |
|
5 |
Pt. 5 |
37 |
M |
V |
2yrs |
|
6 |
Pt. 6 |
70 |
F |
2(Sinclair’s) |
4-5yrs |
|
7 |
Pt. 7 |
40 |
M |
IIIA |
3yrs |
|
8 |
Pt. 8 |
32 |
M |
IV |
8months |
|
9 |
Pt. 9 |
25 |
M |
IIIA |
2yrs |
|
10 |
Pt. 10 |
49 |
F |
1(Sinclair’s) |
1yr |
Table 1: Demographic profile of all 10 cases in tabulated form.
AGA grade- Hamilton-Norwood (Male AGA) and Sinclair’s Classification (FPHL)
Patient 1(Pt. 1) was treated with intradermal injection of MCT blood derived exosomes with dermaroller on androgen sensitive area of scalp. MCT exosomes was prepared from autologous source (blood) as per set protocol. First, the PRP was prepared by harvesting 20 ml of venous blood, mixed with 3 ml of ACD-A anticoagulant, processed for 4 minutes at 3400 rpm by single spin method in two YCELL Biokits in Remi cold centrifuge. Eight ml of PRP thus harvested was preconditioned with MCT system, a novel device based on Thermo-photobiomodulation (TPBM) with blue light (467nm) at a temperature-controlled setting of 37 °C for 10 minutes and finally 8 ml of exosomes were harvested [4]. On trichoscopy, an abrupt eruption of terminal hairs from base of vellus hairs (candle hairs) was seen at 2 months and multirooted hair follicles were noticed at 4 months on the right frontal area of scalp (Figures 1A-1C).
 Figure 1A: Pt 1 A. At baseline, hair diameter variation with primary roots seen.
Figure 1A: Pt 1 A. At baseline, hair diameter variation with primary roots seen.
 Figure 1B: After 2 months of single session of intradermal injection of MCT blood derived exosomes combined with derma-roller, fine vellus hair abruptly converted to terminal hair – ‘Candle hair sign’ (red arrow), seen at frontal area.
Figure 1B: After 2 months of single session of intradermal injection of MCT blood derived exosomes combined with derma-roller, fine vellus hair abruptly converted to terminal hair – ‘Candle hair sign’ (red arrow), seen at frontal area.
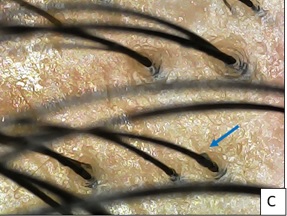 Figure 1C:At 4 months, secondary and tertiary hair follicles with increased hair shaft diameter (blue arrow) are seen in the frontal area.
Figure 1C:At 4 months, secondary and tertiary hair follicles with increased hair shaft diameter (blue arrow) are seen in the frontal area.
Pt. 2 was treated with 2 sessions of MNRF with application of exosomes (EXOXE). On trichoscopy, new secondary and tertiary follicles were observed at 4 months following the 1st session and increased density with thickened hair shaft diameter was seen at 2 months after 2nd session on the vertex and fronto-temporal region (Figures 2A-2C). On clinical examination, significant increase in hair density was visible at vertex region (Figure 3).
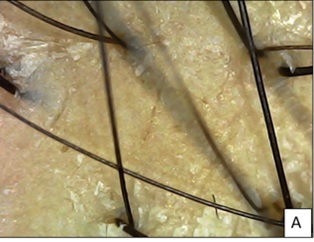 Figure 2A: Pt 2 at baseline showing miniaturization of hair with anisotrichosis and sparse density and scaling seen.
Figure 2A: Pt 2 at baseline showing miniaturization of hair with anisotrichosis and sparse density and scaling seen.
 Figure 2B: After 4 months of 1st session, multiple secondary and tertiary hair roots seen (red arrow) with uniform hair shaft diameter.
Figure 2B: After 4 months of 1st session, multiple secondary and tertiary hair roots seen (red arrow) with uniform hair shaft diameter.
 Figure 2C: After 2 months of 2nd session, new multirooted terminal hairs with increased density seen at vertex.
Figure 2C: After 2 months of 2nd session, new multirooted terminal hairs with increased density seen at vertex.
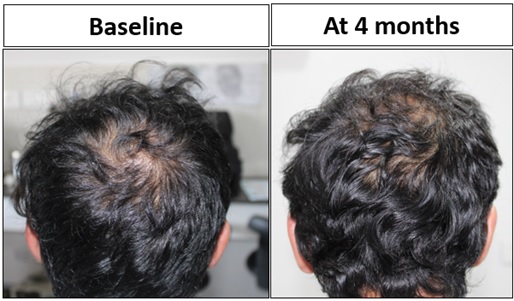 Figure 3: Pt. 2 had significant increased hair density at vertex region at 4 months from baseline.
Figure 3: Pt. 2 had significant increased hair density at vertex region at 4 months from baseline.
Six patients (Pt 3,4,5,6,7 and 9) underwent single session of MNRF with infusion of Advancexo for AGA. Pt. 4 was followed up at 5 weeks post treatment, showing significantly increased terminal: vellus hair ratio at bilateral frontotemporal angles (Figure 4).
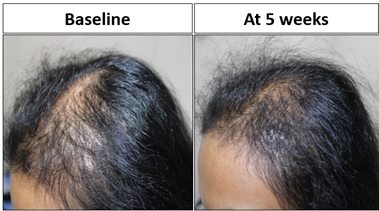 Figure 4: Pt 4 showed significant improved terminal hair growth in temporal and saggital areas at 5weeks.
Figure 4: Pt 4 showed significant improved terminal hair growth in temporal and saggital areas at 5weeks.
Pt.8 and Pt. 10 underwent a single session of MNRF followed by EXOXE infusion, with follow up at 4 weeks and 1 week, respectively. Pt. 8 had significant secondary new hair follicles at 4 weeks at the vertex region (Figures 5A-5C), with significant terminal hair growth clinically at the parietal and vertex regions of scalp (Figure 6). While Pt. 10 at 3 days and 7 days at trichoscopy showed oedema erythema and increased hair shaft diameter at base at 2 weeks respectively (Figures 7A-7C).
 Figure 5A: Pt 8 At baseline, multiple primary rooted telogen hairss seen at vertex.
Figure 5A: Pt 8 At baseline, multiple primary rooted telogen hairss seen at vertex.
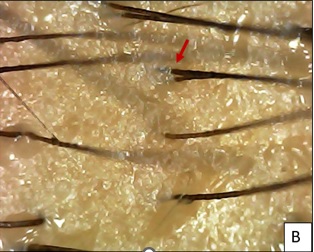 Figure 5B: After 1 month of 1st session, few secondary hair follicles (Red arrow) seen with increased hair shaft diameter.
Figure 5B: After 1 month of 1st session, few secondary hair follicles (Red arrow) seen with increased hair shaft diameter.
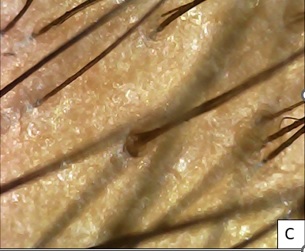 Figure 5C: After 5 months of 1st session.
Figure 5C: After 5 months of 1st session.
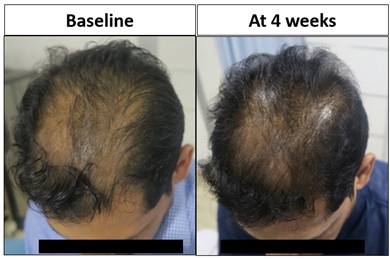 Figure 6: Pt. 8 had significantly increased hair density at vertex region on 4th week from baseline.
Figure 6: Pt. 8 had significantly increased hair density at vertex region on 4th week from baseline.
 Figure 7A: Pt 10 A. At baseline, single rooted hair noted at frontal hairline.
Figure 7A: Pt 10 A. At baseline, single rooted hair noted at frontal hairline.
 Figure 7B: After 3 days of 1st session, mild oedema and erythema seen.
Figure 7B: After 3 days of 1st session, mild oedema and erythema seen.
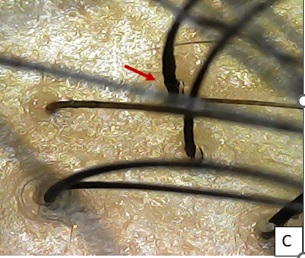 Figure 7C: After 1 week of 1st session, Increased hair shaft diameter at base (red arrow) with mild erythema seen.
Figure 7C: After 1 week of 1st session, Increased hair shaft diameter at base (red arrow) with mild erythema seen.
Pt. 5 after single session of MNRF with Advancexo was followed at 2nd month, with an interesting finding of abrupt conversion of telogen hair to anagen hair with 90o angulation named as ‘Tricho-angulation in Rescue Hair’ (Figure 8).
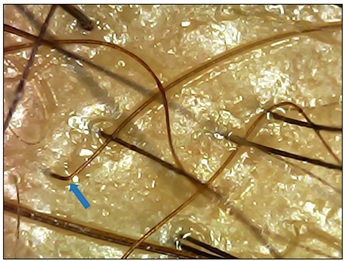 Figure 8: Pt. 5, after 2 months of single session (MNRF with Advancexo), finding of telogen hair converting to anagen with acute change in angle – ‘Trichoangulation’ - Rescue hair (blue arrow) with significant change in color of hair shaft is seen at vertex.
Figure 8: Pt. 5, after 2 months of single session (MNRF with Advancexo), finding of telogen hair converting to anagen with acute change in angle – ‘Trichoangulation’ - Rescue hair (blue arrow) with significant change in color of hair shaft is seen at vertex.
There was only one patient (Pt. 3) who reported aggravation of hair loss for 2 weeks after Advanceexo. Rest of the cases reported significant reduction or stoppage of hair loss within 7 days.
The treatment protocol and clinical outcomes along with trichoscopic findings for all the patients is summarised in (Tables 2 & 3).
|
Patient Code |
Past history of treatment taken |
Family History of AGA/Chronic Illness |
Dietary deficiency and Blood reports |
||
|
Oral |
Topical |
Procedural |
|||
|
Pt. 1 |
Cyclical Micronutrients Therapy |
Peptides |
PRP*, GFC* |
None |
All WNL* |
|
Pt. 2 |
Cyclical Micronutrient therapy |
Peptides, Minoxidil 5% Finasteride |
PRP, GFC |
Yes, Paternal |
Low Vitamin (Vit) D3 and Iron |
|
Pt. 3 |
Micronutrient, Minoxidil 1.25mg (2 months) |
Finasteride, Peptides |
PRP, GFC |
Yes, Both Maternal and Paternal |
Low iron, Vit D3, high cholesterol |
|
Pt. 4 |
Calcium |
Peptides |
PRP, GFC |
None |
Low iron and Vit D3 |
|
Pt. 5 |
Cyclical Micronutrient therapy |
Peptides |
PRP, GFC |
AGA in Father, History of hair transplant (hair line and fronto-temporal angles) 3 yrs back |
Low Iron, Vit D3 and ferritin |
|
Pt. 6 |
Cyclical Micronutrient therapy |
Peptides |
PRP, GFC |
History of breast cancer, treated with lumpectomy along with chemotherapy and radiotherapy for 9 months, 4 yrs back |
Low hemoglobin |
|
Pt. 7 |
Micronutrient, Iron, D3 |
Minoxidil, Peptides |
PRP, GFC |
Hypothyroidism |
Low Vit D3, Vit B12 |
|
Pt. 8 |
None |
None |
None |
Yes, Paternal |
WNL |
|
Pt. 9 |
None |
Minoxidil |
PRP |
None |
WNL |
|
Pt. 10 |
Cyclical Micronutrient therapy |
Peptides |
GFC |
None |
WNL |
Table 2: Detailed Past treatment history, Family history and Blood profile in tabulated form.
*WNL: Within normal limits, *PRP: Platelet rich plasma, *GFC: Growth factor concentrates
|
Pt. Code |
Treatment Given |
Clinical Evaluation |
Trichoscopic evaluation |
||||
|
|
Oral |
Topical |
Procedural |
Hairfall Aggravated |
Hairfall Arrested |
Hair density |
|
|
Pt. 1 |
Cyclical Micronutrient therapy and Plant based protein |
Peptides |
Intradermal injection of MCT blood derived Exosomes with dermaroller |
Not applicable (NA) |
NA |
30% improvement and mild increase in density |
Multiple terminal upright hair growth seen at 2 weeks in frontal region and abrupt change of vellus hair into terminal hair - Candle hair |
|
Pt. 2 |
Cyclical Micronutrient therapy, Multivitamin, Vit D3, Plant based protein |
Peptides, Minoxidil 5%, Finasteride once |
2 sessions of EXOXE at 4 months interval |
NA |
Abrupt stop in hair fall in 1 week |
40% increased density at vertex at 4months |
New multirooted terminal hairs with good density seen at vertex area (4months) & frontotemporal area (6months) |
|
Pt. 3 |
Minoxidil 1.25mg, Micronutrient, Iron, D3, Statin |
FinasteridePeptides |
1 session of MNRF with Advancexo |
Aggravated for 2weeks |
NA |
20% improvement, increased density in vertex after 4weeks |
Multirooted vellus hairs with improved density at vertex and temporal angles seen |
|
Pt. 4 |
Micronutrient and Plant based protein |
FinasteridePeptides |
1 session of MNRF with Advancexo |
NA |
NA |
30-35% improved density at temporal areas at 5 weeks |
Increased terminal to vellus hair ratio, significant improvement in temporal thinning |
|
Pt. 5 |
Micronutrient, Iron and D3, Plant based protein |
FinasteridePeptides |
1 session of MNRF with Advancexo |
NA |
1 week after 1 session |
30% improvement in hair density at frontal and vertex region |
At 2 months, ‘Trichoangulation/Rescue hair’ seen at vertex |
|
Pt. 6 |
Micronutrient, Antioxidant, Plant based protein |
FinasteridePeptides |
1 session of MNRF with Advancexo |
NA |
NA |
10-15% Improved hair density in sagittal area |
Few multirooted vellus hairs seen with improved hair shaft diameter |
|
Pt. 7 |
Micronutrient, Iron, D3, Plant based protein |
Minoxidil, Peptides, Finasteride |
1 session of MNRF with Advancexo |
NA |
NA |
NA |
NA |
|
Pt. 8 |
Plant based protein supplement |
None |
Single session of Derma-pen with EXOXE |
NA |
NA |
30% Improvement in Hair density at parietal and vertex region |
Few secondary hair follicles with greater hair shaft diameter seen at 1month and effect waned off in 5 months |
|
Pt. 9 |
Micronutrient, Plant based protein supplement |
Finasteride,Peptides |
1 session of MNRF with Advancexo |
NA |
Within 7 days |
NA |
NA |
|
Pt. 10 |
Micronutrient, Plant based protein supplement |
Peptides |
1 session MNRF and EXOXE |
NA |
NA |
No specific change in hair density seen at 1 week |
Increased terminal to vellus hair ratio with mild erythema at 1week |
Table 3: Describes the treatment prescribed with respective Exosome procedure along with clinical and trichoscopic evaluation.
Discussion
The growing need for regenerative therapies in the field of androgenetic alopecia and female pattern hair loss has triggered research into cell free approaches towards hair restoration. This case series highlights the potential of exosome therapy as an emerging therapy in AGA/FPHL.
Exosomes are nano-sized extracellular vesicles, secreted particularly by mesenchymal stem cells (MSCs) and various cell types such as human umbilical vein endothelial cells, T and B cells, macrophages, dendritic cells, natural killer cells [5]. They are generated from multivesicular bodies through the endosomal pathway and mediate cell-to-cell communication via membrane fusion, endocytosis (clathrin/caveolin-mediated), phagocytosis, and pinocytosis. Exosomes carry a rich cargo of diverse materials such as nucleic acids (mRNA, miRNA, circRNA), lipids, enzymes, cytokines, and surface proteins such as CD9, CD63, and CD8. Upon internalisation, this rich cargo of bioactive molecules such as miRNA, proteins and lipids modulates stem cells, fibroblasts and endothelial cells. Owing to their size, they seep easily into the hair follicles. The regenerative effects appear to be mediated by paracrine signalling mechanisms that stimulate follicular cell proliferation, prolong the anagen phase, promote angiogenesis, and mitigate local inflammation [6].
Exosomes can be isolated and modified to deliver proteins, DNA, RNA, or drugs, and the surface receptors can be tailored to direct exosomes to the desired tissues and cells [7]. Therefore, they can deliver tailored contents to target cells, thereby influencing gene expression and cell differentiation. This targeted form of therapy allows for minimal side effects, with a simultaneous increased therapeutic concentration dose [8]. The use of exosomes spans across various domains of aesthetic dermatology, including anti-aging and anti-inflammatory therapies, wound healing, scar reduction, and hair regeneration [9].
In our case series both humanised and commercial preparations were explored in the treatment of AGA. We utilised three types of exosomes; MCT blood derived exosomes (Autologous blood derived), EXOXE exosomes (Umblical fluid derived) and Advancexo Exosomes (Umblical cord-derived, Hair Rejuve Kit) for regenerative therapy.
MCT Blood-Derived Exosomes
MCT (MetaCell Technology) blood-derived exosomes are produced from autologous peripheral blood mononuclear cells (PBMCs). These exosomes are generated by cultivating PBMCs under temperature controlled regenerative conditions, often in hypoxia [10] or TPBM [4]and harvesting the exosomal fraction via filtration and centrifugation.
Mechanism of Action:
- Immunomodulatory and anti-inflammatory properties: Exosomes derived from PBMCs contain anti-inflammatory cytokines like interleukin-10 (IL-10) and transforming growth factor- beta (TGF-β) that reduce perifollicular inflammation [11].
- Stimulation of angiogenesis: They are rich in vascular endothelial growth factor (VEGF) and angiopoietin, promoting dermal capillary growth and improving follicular nutrition and oxygenation [12].
- Activation of Wnt/β-catenin signaling: These exosomes transfer miRNAs (e.g., miR-100, miR-21, miR-218) to dermal papilla cells (DPCs), activating the Wnt pathway and inhibiting androgen receptor (AR)-mediated apoptosis [13].
- Enhanced cell proliferation: PBMC-derived exosomes upregulate Ki67 expression, promoting keratinocyte and follicular cell proliferation [14].
Delivery is typically performed via intradermal injections to enhance dermal absorption and follicular uptake or microneedling [microneedling radiofrequency / Dermaroller/Dermapen] [10].
EXOXE Exosomes (REGENBOGEN)
EXOXE exosomes, with primary source as amniotic fluid; developed by REGENBOGEN Biotech, are derived from mesenchymal stem cells (MSCs) and manufactured under good manufacturing practice (GMP) conditions. (11) These exosomes are known for their high purity and are rich in hair growth-promoting factors.
Mechanism of Action:
- Growth factor delivery: EXOXE exosomes are enriched in keratinocyte growth factor (KGF), VEGF, platelet-derived growth factor (PDGF), and insulin- like growth factor-1 (IGF-1), all of which are known to stimulate dermal papilla cell (DPC) activity and prolong the anagen phase [12,15].
- Anti-apoptotic signaling: They activate the AKT and ERK pathways in DPCs, helping to counteract dihydrotestosterone (DHT)-induced follicular miniaturization [14]
- Immunomodulation: The exosomes suppress inflammatory mediators like tumour necrosis factor-alpha (TNF-α) and IL-6, improving scalp homeostasis [16].
- Extracellular matrix remodeling: Matrix metalloproteinases (MMPs) and tissue inhibitors (TIMPs) in the exosomes enhance follicular anchoring and scalp structure.
The delivery method involves microneedling, nano-infusion, or dermal stamping, typically in sessions spaced 2–4 weeks apart [17].
Advancexo Exosomes (Hair Rejuve Kit)
Advancexo, part of the Hair Rejuve Kit, uses exosomes derived from adipose or umbilical cord-derived MSCs. It combines exosomal therapy with peptides, growth factors, and supportive agents.
Mechanism of Action:
- Hair follicle cycling: Exosomes promote the transition from telogen to anagen by upregulating key regenerative signals [14,18]
- Androgen modulation: Certain miRNAs in Advancexo exosomes reduce androgen receptor expression and inhibit 5α-reductase, lowering local DHT activity.
- Nutrient enrichment: They contain matrix proteins, adenosine triphosphate (ATP) precursors, and amino acids that enhance keratinocyte and DPC activity [16].
- Scalp repair: By reducing inflammation and supporting ECM remodelling, Advancexo restores a healthy follicular microenvironment [10].
These are delivered through microneedling-assisted transdermal infusion, with standard protocols involving 6–8 sessions, sometimes followed by booster applications [10].
The comparative summary for these therapies is listed in (Table 4).
|
Parameter |
MCT Blood-Derived Exosomes [4,19] |
EXOXE (REGENBOGEN) [15] |
Advancexo (Hair Rejuve Kit) [10] |
|
Source |
Autologous PBMCs |
Allogenic amniotic fluid MSCs |
Allogenic Adipose/Umbilical MSCs |
|
Key Action |
Immunomodulation, angiogenesis |
Follicle stimulation, anti-apoptotic |
DHT modulation, follicle cycling |
|
Wnt/β-catenin Activation |
Moderate |
Strong |
Moderate to strong |
|
Delivery Method |
Microneedling, injections |
Microneedling, nano-infusion |
Microneedling + topical application |
|
Inflammation Control |
Strong |
Moderate to strong |
Strong |
|
Ideal for AGA Stage |
Early to moderate |
Moderate-to-severe |
All stages |
Table 4. Comparative parameters for the three types of Exosomes used in study.
Numerous pre-clinical and clinical studies have demonstrated the potential of exosome-based therapy in regeneration of hair. Most of the pre-clinical and clinical studies have explored mesenchymal stem cells, dermal papilla cells and adipose derived stem cells (ADSC’s) as the source of exosomes. Li et al. demonstrated increased dermal papilla cell proliferation and migration, reduced apoptosis and activation of Wnt/β-catenin pathway in vitro and in murine models using DPC derived exosomes [20]. Similarly, Kwack et al. reported upregulation of growth factors such as IGF-1, KGF, and hepatocyte growth factor (HGF) by three-dimensional cultured DPCs and human hair follicles, which promoted both anagen induction and prolongation in mice [21]. In addition, the enhancement of VEGF and PDGF expression, suppression of TGF-β, and acceleration of the telogen-to-anagen transition has been demonstrated in various murine studies [22-24]. In an interesting study by Zhou et al. [23] DPC derived exosomes were injected into the hair follicles at different stages of the hair follicle and evaluated by histological and IHC analysis. They noted accelerated onset of anagen, delayed catagen and upregulation of beta catenin and sonic hedgehog levels in mice. In vitro, DPC-Exo treatment enhanced proliferation and migration of outer root sheath cells, stimulated the expression of β-catenin and sonic hedgehog levels. Apart from these sources, bovine derived colostrum has been show to promote dorsal hair regrowth in mice at levels comparable to minoxidil, without associated adverse effects [25]. In another study, hyaluronic acid–preconditioned MSCs significantly improved DPC function and promoted hair regrowth, suggesting that preconditioning stem cells can significantly enhance the clinical outcome [26].
Although there is a paucity of clinical studies on exosomes in hair growth, the existing literature supports the findings of the pre-clinical studies. In a study by Gupta et al, statistically significant increase in hair thickness and density in 39 patients of androgenetic alopecia (AGA) using ADSC-derived exosomes [27]. An open-label study conducted in 2023 on 16 AGA patients combined microneedling with ADSC-exosomes, resulting in an average increase of approximately 35 hairs per cm² at 12 months. Similar results were noted in a retrospective analysis in South Korea, where hair density and thickness were notably enhanced among 39 patients with alopecia after 12 weeks of ADSC-Exos treatment, without adverse effects [28]. Interestingly, Sasaki et al. investigated intradermal injection of human bone marrow MSC-derived extracellular vesicles (EVs) in 31 early AGA patients (22 females, 9 males), and observed frequent positive responses, particularly in older females, younger males, and patients at earlier stages of hair loss, with no significant adverse effects [29].
The method of delivering exosomes to the scalp is critical for ensuring their effective penetration and interaction with hair follicle cells. Currently, for autologous exosomes, intradermal injection, similar to the technique used in platelet rich plasms (PRP) is the most commonly employed technique, where exosomes suspended in fluid are injected directly into the scalp, providing localized delivery near the follicular unit. Clinical and preclinical studies evaluating exosomes for androgenetic alopecia have reported concentrations ranging from 2 × 109 to 1 × 1010 particles/mL, as determined by nanoparticle tracking analysis (NTA), with a total injection volume of approximately 3 mL per treatment session [30-32]. However, this method can be painful, operator-dependent, and may result in uneven distribution. Microneedling combined with topical application is another widely used approach, where the microchannels created by the process of microneedling facilitates exosome penetration while also stimulating follicular regeneration through mechanical injury. Authors have utilized Impact ultrasonic infusion through Alma Hybrid laser after creating microchannel through micro needling radiofrequency. An emerging, innovative method involves dissolvable microneedle patches, which embed exosomes in biodegradable microneedles that painlessly penetrate and dissolve in the skin, ensuring uniform delivery and improved patient comfort; although this remains largely experimental [33-35]. Although topical application is the least invasive method, poor penetration through intact skin limits its efficacy unless combined with adjunctive techniques. In a review of various exosome delivery methods across multiple indications, including hair loss, four studies specifically utilized microneedle-based delivery systems for exosomes in hair growth [36]. These studies demonstrated improved targeted and uniform deposition, as well as longer retention of exosomes within the dermal layer and around hair follicles, compared to intradermal injections.
In our case series, the intradermal injections of humanized (commercial) and autologous (blood-derived) exosomes were well tolerated and associated with visible improvements in hair density, scalp coverage, and patient satisfaction over the observation period.
The growing body of evidence for role of exosome therapy in hair loss is compelling. Our observations align with preclinical and clinical studies which demonstrate arrest of active hair fall, conversion of telogen hair into anagen (trichoangulation in rescue hair), conversion of vellus hair into terminal hair (candle hair), the acceleration of onset of anagen, prolongation of duration of anagen, enhancement of hair follicle diameter and density. The comparative use of both humanized and autologous exosomes in our series demonstrates safety in both the sources, with minimal transient side effects, suggesting that exosome therapy is an innovative adjunct or alternative to existing hair loss treatments.
Limitations
Limitation of this series include the small sample size, lack of a control group, and assessment measures. Nonetheless, our case series contributes to the emerging literature supporting use of exosome therapy in hair loss. Large-scale, randomised control trials studies need to be conducted to standardise the production of exosomes, optimise the protocols, and investigate the potential of combining exosomes with modalities such as microneedling or energy-based devices. As the process of hair loss is chronic & progressive, the longevity of exosome induced improvement and maintenance schedule remain interesting areas of future research.
Conclusion
Exosome therapy represents a novel and promising approach in the treatment of androgenetic alopecia. Only a few small-scale clinical studies or case series exist, and standardized protocols are lacking. Our case series is among the few reported real-world experience with both humanized and autologous exosomes.
While the humanized exosomes are standardized, convenient for use, the autologous exosomes are safer in terms of lack of immunogenicity.
Each formulation—MCT, EXOXE, and Advancexo—offers a unique combination of regenerative properties. The selection of exosome type may be guided by AGA stage, inflammation severity, DHT sensitivity, and patient-specific response. As research evolves, standardized protocols and head-to-head clinical trials will further validate and refine the role of exosomes in hair regeneration.
References
- Pegtel DM, Gould SJ (2019) Exosomes. Annu Rev Biochem 88: 487-514.
- Kim YJ, mi Yoo S, Park HH, Lim HJ, Kim YL, et al. (2017) Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochemical and Biophysical Research Communications 493: 1102-1108.
- Zhang B, Wang M, Gong A, Zhang X, Wu X, et al. (2015) HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem cells 33: 2158-2168.
- Cordero L, Domingo JC, Mengual ES, Pinto H (2025) Autologous Platelet-Rich Plasma Exosome Quantification after Two Thermo-photobiomodulation Protocols with Different Fluences. Journal of Photochemistry and Photobiology 100267.
- Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. science 367: eaau6977.
- Cheng M, Ma C, Chen HD, Wu Y, Xu XG (2024) The Roles of Exosomes in Regulating Hair Follicle Growth. Clin Cosmet Investig Dermatol 17: 1603–1612.
- Familtseva A, Jeremic N, Tyagi SC (2019) Exosomes: cell-created drug delivery systems. Molecular and cellular biochemistry 459: 1-6.
- Liang Y, Duan L, Lu J, Xia J (2021) Engineering exosomes for targeted drug delivery. Theranostics 11: 3183.
- Bai G, Truong TM, Pathak GN, Benoit L, Rao B (2024) Clinical applications of exosomes in cosmetic dermatology. Skin Health and Disease 4: ski2-348.
- Al Abadie M, Abed N, Mahfoudh M (2025) Exosome Therapy for Hair Loss. International Journal of Clinical & Experimental Dermatology (IJCED) 10: 01-7.
- Bae SH, Kim MH, Lee SJ (2020) The effects of MSC-derived exosomes on alopecia: comparative study with PRP. Stem Cell Res Ther 11: 150.
- Andriopoulos D, Kolilekas L, Kostikas K (2021) Vascular endothelial growth factor in hair follicle biology. J Cosmet Dermatol Sci Appl 11: 29-36.
- Hu S, Li Z, Lutz H, Huang K, Su T, et al. (2020) Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Science advances 6: eaba1685.
- Shin H, Yoo B, Lee S (2022) Clinical efficacy and safety of topically applied exosomes for hair growth: a randomized placebo-controlled trial. J Dermatolog Treat 33: 1883–1890.
- Nahm WJ, Nikas C, Goldust M, Horneck N, Cervantes JA, et al. (2025) Exosomes in Dermatology: A Comprehensive Review of Current Applications, Clinical Evidence, and Future Directions. International Journal of Dermatology.
- Kim YJ, Lim HJ, Park HH (2021) Exosomes and hair follicle homeostasis: regulation by growth factors and matrix proteins. Biochem Res Int 2021: 1-10.
- Gupta AK, Talukder M (2021) Therapies for androgenetic alopecia: a systematic review and meta-analysis. J Eur Acad Dermatol Venereo 35: 1245-1253.
- Kang JI, Lee WJ, Kim BJ (2020) The effect of exosomes derived from human dermal papilla cells on hair regeneration. Korean J Dermatol 58: 153-158.
- Internal Report (2022): MCT Blood-Derived Exosomes in Aesthetic & Hair Therapy. MicroCell Technologies.
- Li J, Zhao B, Dai Y, Zhang X, Chen Y, et al. (2022) Exosomes derived from dermal papilla cells mediate hair follicle stem cell proliferation through the Wnt3a/β-catenin signaling pathway. Oxidative medicine and cellular longevity 2022: 9042345.
- Kwack MH, Seo CH, Gangadaran P, Ahn BC, Kim MK, et al. (2019) Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Experimental dermatology 28: 854-857.
- Rajendran RL, Ahn BC, Gangadaran P (2025) Potential of mesenchymal stem cell-derived exosomes in the treatment of androgenetic alopecia. World Journal of Stem Cells 17: 107078.
- Zhou L, Wang H, Jing J, Yu L, Wu X, et al. (2018) Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochemical and biophysical research communications 500: 325-332.
- Fu Y, Han YT, Xie JL, Liu RQ, Zhao B, et al. (2025) Mesenchymal stem cell exosomes enhance the development of hair follicle to ameliorate androgenetic alopecia. World Journal of Stem Cells 17: 102088.
- Kim H, Jang Y, Kim EH, Jang H, Cho H, et al. (2022) Potential of colostrum-derived exosomes for promoting hair regeneration through the transition from telogen to anagen phase. Frontiers in Cell and Developmental Biology 10: 815205.
- Oh HG, Jung M, Jeong SY, Kim J, Han SD, et al. (2024) Improvement of androgenic alopecia by extracellular vesicles secreted from hyaluronic acid-stimulated induced mesenchymal stem cells. Stem cell research & therapy 15: 287.
- Gupta AK, Renaud HJ, Halaas Y, Rapaport JA (2020) Exosomes: a new effective non-surgical therapy for androgenetic alopecia? Skinmed 18: 96-100.
- Park BS, Choi HI, Huh G, Kim WS (2022) Effects of exosome from adipose-derived stem cell on hair loss: A retrospective analysis of 39 patients. Journal of Cosmetic Dermatology 21.
- Sasaki GH (2022) Clinical use of extracellular vesicles in the management of male and female pattern hair loss: a preliminary retrospective institutional review board safety and efficacy study. InAesthetic Surgery Journal Open Forum 4: ojac045.
- Ersan M, Ozer E, Akin O, Tasli PN, Sahin F (2024) Effectiveness of exosome treatment in androgenetic alopecia: outcomes of a prospective study. Aesthetic plastic surgery 48: 4262-71.
- Poddar N, Aratikatla A, Gupta A (2025) Therapeutic potential of stem cell-derived exosomes in hair regeneration: A systematic review. World Journal of Stem Cells 17: 108519.
- Yu A, Zhang Y, Zhong S, Yang Z, Xie M (2025) Human umbilical cord mesenchymal stem cell-derived exosomes enhance follicular regeneration in androgenetic alopecia via activation of Wnt/β-catenin pathway. Stem Cell Research & Therapy 16: 418.
- Bui VD, Son S, Xavier W, Nguyen VQ, Jung JM, et al. (2022) Dissolving microneedles for long-term storage and transdermal delivery of extracellular vesicles. Biomaterials 287: 121644.
- Nainggolan AD, Anjani QK, Hartrianti P, Donnelly RF, Kurniawan A, et al. (2023) Microneedle-mediated transdermal delivery of genetic materials, stem cells, and secretome: An update and progression. Pharmaceutics 15: 2767.
- Fathi-Karkan S, Heidarzadeh M, Narmi MT, Mardi N, Amini H, et al. (2023) Exosome-loaded microneedle patches: promising factor delivery route. International Journal of Biological Macromolecules 243: 125232.
- Schaffer S, Tehrani L, Koechle B, Chandramohan P, Hilburn B, et al. (2025) A Scoping Review of Exosome Delivery Applications in Hair Loss. Cureus 17.
Citation: Garg S, Jain N, Lobo C (2025) Initial Experience with Humanized Allogeneic and Autologous Exosome-Based Therapies in Androgenetic Alopecia: A case series. HSOA J Stem Cell Res Dev Ther 11: 117.
Copyright: © 2025 Suruchi Garg, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

