
Mesenchymal Stem Cell-Based Treatment of Osteoarthritis in Dogs - A Review
*Corresponding Author(s):
Domaniža MSmall Animal Clinic, Centre For Experimental And Clinical Regenerative Medicine, University Of Veterinary Medicine And Pharmacy, Komenského 73, 041 81 Košice, Slovakia
Email:domaniza.michal@gmail.com
Abstract
Osteoarthritis (OA) is Degenerative Joint Disease (DJD) associated with pain, inflammation and cartilage degradation resulting in lameness. This state is irreversible and actual conventional therapies are not able to provide regeneration of damaged tissue. Current therapies, mainly NSAIDs, have many adverse effects and are not able to stop degenerative process. Joint supplements, weight management, and rehabilitation therapy often fail to provide a long-term response in inflamed joint. Adult Mesenchymal Stem Cells (MSCs) might serve as an alternative therapy for OA, because they can differentiate into osteo-chondral lineages. In addition, they have shown ability to decrease pain and inflammation as well as promote cartilage function and healing in patients with OA. Thus, the aim of present review is to clarify the findings associated with MSCs application in the treatment of OA by means of synovial fluid analysis, gait analysis, clinical and x-ray examination often used in veterinary practice.
Keywords
Cartilage; Inflammation; Mesenchymal Stem Cells; Osteoarthritis
Introduction
Articular cartilage is hyaline cartilage and is principally composed of water (65-85 %), collagen (10-20 %, collagen type II is 90-95%), proteoglycans (10-20 %) and chondrocytes (1-5 %). Cartilage consists of four layers - superficial layer, transitional layer, deep layer and layer of calcified cartilage. Hyaline cartilage is a free of vascular, lymphatic and neural structures. Nourishment is accomplished through diffusion from synovial fluid and vessels in synovial membrane [1-4].
OA is described as a main reason of dog lameness which is also first clinical sign observed by owners [5]. Most important clinical signs are joints pain and decreased range of motion. Prescription of Non-Steroidal Anti-Inflammatory Drugs (NSAID) by veterinarian is still the number one choice in OA treatment. NSAIDs application is associated with some adverse effects, which is not required for long-term use [6,7]. There have been used alternatives of NSAIDs such as hyaluronic acid [8]. Mesenchymal Stem Cell (MSC) therapy is a new and safe therapeutic method in OA treatment in veterinary medicine [9-11]. For MSC treatment is typical high therapeutic index and long-term influence on joint microenvironment, despite the MSCs will not last long in vivo [12]. MSCs interact with the microenvironment of inflamed joint, through large cytokines variety, micro-vesicles (exosomes), growth factors which results in immunomodulatory, trophic, anti-fibrotic, and anti-apoptotic effects [13,14]. Stromal vascular fraction from visceral fat is comparable with MSCs from joint tissues. MSCs from bone marrow have high tendency for chondrocytes hypertrophy and bone tissue proliferation [15,16], which is not required. MSCs from synovial membrane are ideal for cartilage regeneration [17].
Pathophysiology of Osteoarthritis
OA is characterized by cartilage destruction, loss of cartilage matrix, bone remodeling and intermittent inflammation. The degradation of cartilage is not the only pathological process in the joint. OA involves complex changes that affect the subchondral bone and the surrounding soft tissues (synovium, joint capsule, ligaments, and muscle) [18].
Osteoarthritis development can be connected with aging changes in body constitution, such as loss of muscle mass or atrophy, increased fat tissue, a low-grade inflammatory state, less growth-promoting hormones, decreased bone mass structure, and increased microtrauma resulting from decreased proprioception and balance [19,20].
There are detectable changes at early stage of OA in subchondral bone or synovium. Increase in cartilage matrix synthesis occurs in parallel with increased degradation. Synovial and cartilage-derived proteases are key factors in cartilage matrix degradation. Matrix metalloproteinases – calcium depended and zinc containing peptidases (MMPs) and aggrecanases - proteolytic enzymes are responsible for catabolic processes in cartilage [18].
Osteoarthritis includes following changes in articular and periarticular tissue
Degeneration of the articular cartilage
Synthesis and degradation of collagen and proteoglycans is caused by release of wide spectrum of cytokines, mainly the matrix metalloproteinases and collagenases, along with growth factors [18].
Changes in bone
Marginal osteophytes and subchondral sclerosis are two visible signs of OA by X-ray, CT or arthroscopy examination [18].
Changes in synovial membrane
Inflammatory mediators are responsible for an increase in cells in the synovial lining layer and subsynovial layer. Permeability in capillaries increase fluid in the subsynovial layer, leads to thickening of the synovium and increase fluid in the joint (joint effusion). Flexibility in the thickened synovium is decreased in comparison with normal synovium, resulting in decreased range of motion [18].
Changes in articular cartilage
Early changes in articular cartilage are characterized by changes in colour and structure. Cartilage is not more white and smooth, but became yellow with soft or velvety areas [18].
Clinical signs
Most common clinical sign of OA is pain. Joint pain occurs after the hard joint loading, or during palpation and manipulation with the OA joints. Stiffness is another clinical sign, is associated with inflammation, and can be result of oedema of periarticular tissues or joint effusion. Joint crepitus is defined as sound or vibration detected with movement of the joint. It is most commonly associated with irregular joint surfaces that result from the loss of cartilage and osteophytes formation [9,18,21].
Regenerative Medicine for OA Treatment
In areas where tissue is not responding by healing in traditional circumstances, innovated strategies of regenerative medicine are suggested. Every tissue is able to heal or perform scarring after traumatic damage. Some tissues are able to heal to their previous structure and resilience. Cartilage does not heal itself as well as most other tissues because chondrocytes rarely replicate or repair, thus their self-repair capacity is very limited. The objective of regenerative medicine is to promote cartilage healing, decrease inflammation and pain and secure return the functionality of the damaged cartilage.
Autologous/Allogeneic Conditioned Serum (ACS) and Platelet Rich Plasma (PRP) are most often used in regenerative cell based therapies. However, Interleukin-1 Receptor Antagonist Protein (IRAP) enjoys great popularity in last years, especially in horse medicine. Other products such as bone marrow aspirate concentrate, adipose derived MSCs, cultured bone marrow derived stem cells, synovium derived mesenchymal stem cells, allantois and placenta can be also a source of stem cells for various cell-based therapies. These products contain growth factors, chemokines, cytokines and cells, which can secure anti-inflammatory or immune-mediated response Furthermore they can heal and regenerate damaged tissue, stimulate neovascularization, activate adult stem (resident) cells, produce a scaffold for new tissue and its protection against scar formation [22-24].
Isolation And Cultivation Of Bone Marrow From Dogs - Derived MSC In Vitro
The bone marrow can be easily obtained from epiphysis of the proximal humerus, proximal femur (great trochanter, trochanteric fossa), pelvis (iliac crest), sternum, rib under general anaesthesia. For bone marrow, sample collection is suitable Jamshidi™ Bone Marrow Biopsy Needles. The surgical site is aseptically prepared and surgical site is draped. Incision of the skin and subcutis can be made with scalpel blade. Needle for bone marrow biopsy is applied to bone with firm pressure and slightly twisting motion [25]. Post procedural analgesia is maintained with NSAIDs or opioids. Donors must be healthy, young dogs without any neoplastic process or hemopoetic problems (Figure 1). 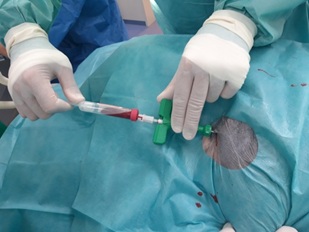
Figure 1: Bone marrow biopsy with Jamshidi needle.
Because of high risk of contamination stem cells must be handled carefully. The samples are collected and send to the laboratory, centrifugation of samples is at 500 x g for 10 minutes. The mononuclear fraction can be applied directly to the patient or used for cultivation of MSCs. Isolated bone marrow is diluted in phosphate buffered saline, which contains antibiotics. Cells are counted using trypan-blue method and then laid on T75 cell culture flask in 5 x 107 cells/cm2 density in commercial culture medium Dulbecco's Modified Eagle Medium (DMEM) or in Minimum Essential Medium Eagle - alpha modification (alpfa MEM) containing 100 units/ ml of penicilin, 100mg/ml of streptomycin and 2.5 μg/ml of amphothericin B. Temperature and humidity (usually at 37°C and 5% CO2) are strictly controlled. The cells remain under these conditions in special tissue culture flasks until they will reach 80% confluence. Thus, the MSCs are characterized by forming cellular monolayer and displaying fibroblast-like morphology in phase-contrast microscope [26].
During the stem cell cultivation is crucial to remove other cell types from culture, this process is called cell passage. After repeated cell passage, reaching required confluence are MSCs characterized. Cultivated MSCs must meet criteria for MSCs according to The International Society for Cellular Therapy.
MSCs Secretome in OA Joints
MSCs are able to secrete wide range of cytokines and chemokines after exposure to the inflammatory environment [27].
MSC stem cells injected to the synovial joints migrate to synovial membrane and to articular cartilage. After migration to the synovial membrane, MSCs produce trophic factors such as PRG-4, BMP-6 a TSG-6 for chondroprotection and immunosuppression [28]. In early state of cartilage damage intraarticulary injected MSCs migrate to cartilage defect, while residual MSCs migrate to synovial membrane to produce trophic factors. Good results have been obtained with MSCs and hyaluronic acid combination [29] or combination with platelet rich plasma [30].
Apoptosis
Apoptosis of chondrocytes is associated with degenerative OA [31,32]. There is no evidence of direct MSC- cell based antiapoptotic effect in OA joints, but exosomes released from human MSCs inhibit IL-1 induced apoptosis in ex-vivo cultivated OA- chondrocytes [33].
Anti-inflammatory effect
IFNγ, TNFα, IL-1β, and IL-17 are responsible for induction of MSCs into anti-inflammatory state. High level of inflammation in environment will cause higher production of anti-inflammatary and immunomodulatory factors by MSCs. These factors are mainly TSG-6, IL-6, PGE2. This means that the immunomodulatory effect of MSCs depends on the severity of joint inflammation [11,12,14,34-37].
Tissue metabolism
MMPs reduce extracellular matrix and are regulated by TIMPs (tissue inhibitors of metalloproteinases). In OA joint is unbalance between catabolic and anabolic factors. In OA cartilage are MMP-2,-9 and -13 detectable in higher concentrations [23]. MMP-2 and MMP-9 are inhibited by TIMP-2 and TIMP-1, which are secreted from MSCs. Secretion is increased in environment with higher concentrations of IL-1β, TNF-α and hypoxia, to counterattack catabolic activity. TIMP-1 can inhibit most MMPs [38], this means MSCs are capable to keep metabolic balance in OA cartilage. In pathologic circumstances are concentrations of TIMP-1 increased.
Antifibrotic effect
Antifibrotic effect is based on decreasing fibrotic markers such as MMP-13, alkaline phosphatise, collagens type I,III,IV and vimentin. Some studies discovered that antifibrotic effect is maintained through secretion of bFGF and adrenomedullin [10,24].
Chondrogenesis
Chondrogenesis and bone proliferation in damaged cartilage is provided by thrombospondin (TSP-2), which is secreted by MSCs via autocrine mechanism ([39,40]. TSP-2 is responsible for cartilage tissue differentiation and to avoid cartilage hypertrophy [40].
Immunosuppression
Immunosuppression can be described as a potential of allogeneic MSC to suppress T-cells in recipient organism. [41,42]. PGE2 is one of the most important effectors of MSC mediated immunosupresion and is produced by MSCs. (IFN)-γ, TNF-α [43], or IL-1β [44] stimulate production of PGE2. Indoleamine 2,3-dioxygenase (IDO) is responsible for breakdown of tryptophan, causing suppression of T-cells [45]. MSCs secrete IDO after IFN-γ stimulation [42].
MSCs Application
General health condition of the recipient must be evaluated, which include blood examination (PCV, TP, CREA, BUN), capillary refill time, heart and lungs auscultation, measurement of body temperature. To diagnose the stage of osteoarthritis is necessary X-ray or CT examination. MSCs are administered intraarticulary in volume of 0.5 ml. Patient is sedated or conscious during application. After MSCs administrations is important to keep dog in restricted motion for 1-2 days. Every complications including redness, swelling of the joint or inability to use limb must be recorded. Four applications every 8-10 days are usually required to reach therapeutic effects [28,29,46].
Cartilage Regeneration after MSC Therapy
MSC stem cells injected to the synovial joints migrate to synovial membrane and to articular cartilage. After migration to the synovial membrane, MSCs produce trophic factors such as PRG-4, BMP-6 a TSG-6 for chondroprotection and immunosuppression [28]. In early state of cartilage damage intraarticularly injected MSCs migrates to cartilage defect, while residual MSCs migrate to synovial membrane to produce trophic factors. Good results have been obtained with MSCs and hyaluronic acid combination [46,47] or combination with platelet rich plasma [30].
Recommendations Associated With MSC Therapy
Since MSCs have anti-inflammatory effect it is not recommended to use them with NSAIDs because of false results or conclusions [8,48]. NSAIDs and corticosteroids can inhibit differentiation, proliferation and migration of stem cells, so during MSC treatment it is proposed to avoid their use [49-51]. Cooling joints after application is not recommended, because cold can slow the differentiation of stem cells in vitro [52,53]. Low-level laser therapy (LLLT) IIIb class and rehabilitation exercises are recommended every week in first 12 weeks after stem cells therapy, because it stimulates stem cells proliferation and viability [54-56]. Other methods of rehabilitation are not recommended in first 8 weeks after therapy because their effect on stem cells is still not well discovered.
Methods For Measuring Efficacy Of MSCs Treatment
OA can be diagnosed with clinical and radiographic examination. For describing grades of OA is recommended to use OA clinical scoring systems [57,58]. Subjective methods for treatment efficacy include owner questionnaire (Liverpool Osteoarthritis in Dogs – LOAD, Helsinki chronic pain index) about pain and lameness. Gait analysis using a force platform is objective method to evaluate the efficacy of MSCs treatment [59,60]. Force platform is modern equipment for measuring ground reaction forces from feet of the dog during the motion [61]. Joints range of motion can be detected by goniometry.
Kinetic and kinematic gait analysis in dogs
Gait analysis can be performed with special devices, which are able to record numeric comparisons between normal and abnormal gait [62,63].
Science of animal motion is kinesiology. Kinetics (the forces that affect motion) and kinematics (the temporal and geometric characteristics of motion) [63].
Kinetic gait analysis can be performed using a force plate to obtain objective notice of the forces occurring between the foot and the surface of the plate during the stance phase of the walk (ground reaction forces) [63]. Data relative to the swing phase of the walk are not measured. A single force plate cannot measure successive strides during locomotion; however, a series of plates or a force plate built into a treadmill can be used to evaluate consecutive strides [47,64].
Kinematic gait analysis performed using several cameras situated in measuring path, so they are able capturing reflection of the light from reflective targets placed on the dog's skin over required anatomic points [65,66].
Computer systems are able to measure the flexion, extension, angles, velocity during movements of joints in two or three dimension. In ideal scenario, camera analysis is combined with force plate measuring ground forces so that dynamic 3-D characteristics of limb motion are evaluated with ground-reaction force measurements [66].
Synovial fluid analysis
Biomarkers of the synovial fluid are objective indicators for the efficacy of MSCs therapy. Synovial fluid from the OA joints should be examined before and after the MSCs treatment in order to have comparable results. Proteins or enzymes that are directly or indirectly responsible for joint inflammation and pain are present in synovial fluid and may act as biomarkers for OA and provide information about treatment efficacy [67].
There is wide spectrum of markers associated with OA inflammation in joints such as interleukin-1 (IL-1), tumour necrosis factor (TNF), IL-6, MMPs, GM-CSF, PGE2. Synovial fluid from osteoarthritic joints reveal increased content of cytokines (IL-1, IL-6, and TNF), MMPs and other inflammatory mediators [68].
Synovial fluid is usually collected via arthrocentesis procedure, performed to aspirate fluid from a joint. Synovial fluid after collection is examined for cell types and numbers, protein, viscosity, and glucose content. Before synovial fluid collection, there must be hair clipping and surgical scrubbing to reduce possibility of joint infection. Surgeon must perform arthrocentesis carefully to avoid damage of cartilage. Visible synovial fluid in the needle confirms proper needle placement. Joint disease is presenting with synovial fluid effusion, what makes synovial fluid sampling easier [18]. Synovial fluid is aspirated using a 3 ml syringe [69]. The sample of synovial fluid must be stored at -80 °C until assayed. Several diagnostic methods are able to provide synovial fluid markers detection, such as ELISA, flow cytometry or Luminex Assays system.
Conclusion
Many studies described positive therapeutic effect after in vivo MSCs application in dogs. Study of MSC in dogs is perspective and needs to be discovered much more. Despite difficulties associated with cell processing and unknown mechanisms underlying MSC-mediated cartilage repair, it can be considered as an alternative treatment for OA and cartilage repair in veterinary orthopedics.
Acknowledgment
Study was supported by: APVV 19-0193, VEGA 1/0376/20.
References
- Bhosale AM, Richardson JB (2008) Articular cartilage: structure, injuries and review of management. Br Med Bull 87: 77-95.
- Bullough P, Goodfellow J (1968) The significance of the fine structure of articular cartilage. J Bone Joint Surg Br 50: 852-857.
- Imhof H, Sulzbacher I, Grampp S, Czerny C, Youssefzade, et al. (2000) Subchondral bone and cartilage disease: a rediscovered functional unit. Invest Radiol 35: 581-588.
- Zhang L, Hu J, Athanasiou KA (2009) The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng 37: 1-57.
- Johnston SA (1997) Osteoarthritis. Joint anatomy, physiology, and pathobiology. Vet Clin North Am Small Anim Pract 27: 699-723.
- Luna SPL, Basílio AC, Steagall PV, Machado LP, Moutinho FQ, et al. (2007) Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am J Vet Res 68: 258-264.
- Walton MB, Cowderoy EC, Wustefeld-Janssens B, Lascelles BDX, Innes JF (2014) Mavacoxib and meloxicam for canine osteoarthritis: a randomised clinical comparator trial. Vet Rec 175: 280.
- van der Weegen W, Wullems JA, Bos E, Noten H, van Drumpt RAM (2015) No difference between intra-articular injection of hyaluronic acid and placebo for mild to moderate knee osteoarthritis: a randomized, controlled, double-blind trial. J Arthroplasty 30: 754-757.
- Brand PW (1995) Mechanical factors in joint stiffness and tissue growth. J Hand Ther 8: 91-96.
- Suga H, Eto H, Shigeura T, Inoue K, Aoi N, et al. (2009) IFATS collection: fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through ac-Jun N-terminal kinase-dependent mechanism. Stem Cells 27: 238-249.
- Ylöstalo JH, Bartosh TJ, Coble K, Prockop DJ (2012) Human mesenchymal stem/ stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 30: 2283-2296.
- van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, et al. (2012) Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage 20: 1186-1196.
- Saulnier N, Viguier E, Perrier-Groult E, Chenu C, Pillet E, et al. (2015) Intra-articular administration of xenogeneic neonatal mesenchymal stromal cells early after meniscal injury down-regulates metalloproteinase gene expression in synovium and prevents cartilage degradation in a rabbit model of osteoarthritis. Osteoarthritis Cartilage 23: 122-133.
- Wang Y, Chen X, Cao W, Shi Y (2014) Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 15:1009-1016.
- Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, et al. (2010) Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA 107: 7251-7256.
- Vinardell T, Sheehy EJ, Buckley CT, Kelly DJ (2012) A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A 18: 1161-1170.
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T (2005) Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 52: 2521-2529.
- Decamp CE, Johnston SA, Dejardin LM (2016) Handbook of small animal orthopedics and fracture repair. Missouri: Elsevier,. 24 - 33 s. ISBN 978-1-4377-2364-9.
- Loeser RF (2012) The effects of aging on the development of osteoarthritis. HSS J 8: 18-19.
- Loeser RF (2013) Aging processes and the development of osteoarthritis. Curr Opin Rheumatol 25: 108-113.
- Shin AY, Amadio PC (2011) The stiff finger. In Green DP, Wolfe SW, Editors: Green’s operative hand surgery (6th Edn), Philadelphia, Elsevier/Churchill Livingston, pp: 355-388.
- Bogers SH (2018) Cell-based therapies for joint disease in veterinary medicine: what we have learned and what we need to know. Front Vet Sci 5: 70.
- Jackson MT, Moradi B, Smith MM, Jackson CJ, Little CB (2014) Activation of matrix metalloproteinases 2, 9, and 13 by activated protein C in human osteoarthritic cartilage chondrocytes. Arthritis Rheumatol 66: 1525-1536.
- Li T, Yan Y, Wang B, Qian H, Zhang X, et al. (2013) Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 22: 845-854.
- Humenik F, Cizkova D, Cikos S, Luptakova L, Madari A, et al. (2019) Canine Bone Marrow-derived Mesenchymal Stem Cells: Genomics, Proteomics and Functional Analyses of Paracrine Factors. Mol Cell Proteomics 18: 1824-
- Lennon DP, Caplan AI (2006) Isolation of human marrow-derived mesenchymal stem cells. Exp Hematol 34: 1604-1605.
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, et al. (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5: 54-63.
- Ozeki N, Muneta T, Koga H, Nakagawa Y, Mizuno M, et al. (2016) Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage 24: 1061-1070.
- Li L, Fan Z, Chen L, Xing F, Xu Z, et al. (2018) Mesenchymal stem cells in combination with hyaluronic acid for articular cartilage defects. Sci Rep 8: 9900.
- Yun S, Ku SK, Kwon YS (2016) Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. J Orthop Surg Res 11: 9.
- Aigner T, Kim HA, Roach HI (2004) Apoptosis in osteoarthritis. Rheum Dis Clin North Am 30: 639-653.
- Del Carlo M Jr, Loeser RF (2008) Cell death in osteoarthritis. Curr Rheumatol Rep 10: 37-42.
- Liu H, Liu S, Li Y, Wang X, Xue W, et al. (2005) The role of SDF1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One 7: 34608.
- Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815-1822.
- Carrade DD, Lame MW, Kent MS, Clark KC, Walker NJ, et al. (2012) Comparative analysis of the immunomodulatory properties of equine adult-derived mesenchymal stem cells. Cell Med 4: 1-11.
- Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, et al. (2007) Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25: 2025-2032.
- Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, et al. (2010) Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One 5: 9252.
- Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827-839.
- Hankenson KD, Bornstein P (2002) The secreted protein thrombospondin 2 is an autocrine inhibitor of marrow stromal cell proliferation. J Bone Miner Res 17: 415-425.
- Jeong SY, Ha J, Lee M, Jin HJ, Kim DH, et al. (2015) Autocrine Action of Thrombospondin-2 Determines the Chondrogenic Differentiation Potential and Suppresses Hypertrophic Maturation of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Stem Cells 33: 3291-3303.
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, et al. (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838-3843.
- Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, et al. (2006) Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24: 386-398.
- English K, Barry FP, Field-Corbett CP, Mahon BP (2007) IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett 110: 91-100.
- Chen K, Wang D, Du WT, Han Z-B, Ren H, et al. (2010) Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol 135: 448-458.
- Mellor AL, Munn DH (2004) IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4: 762-774.
- Miki S, Takao M, Miyamoto W, Matsushita T, Kawano H (2015) Intra-articular injection of synovium-derived mesenchymal stem cells with hyaluronic acid can repair articular cartilage defects in a canine model. J Stem Cell Res Ther 5: 1000314.
- Kram R, Powell AJ (1989) A treadmill-mounted force platform. J Appl Physiol 67: 1692-1698.
- Harman R, Carlson K, Gaynor J, Gustafson S, Dhupa S, et al. (2016) A prospective, randomized, masked, and placebo-controlled efficacy study of intraarticular allogeneic adipose stem cells for the treatment of osteoarthritis in dogs. Front Vet Sci 3: 81.
- Almaawi A, Wang HT, Cinobanu O, Rowas SAL, Rampersad S, et al. (2013) Effect of acetaminophen and nonsteroidal anti-inflammatory drugs on gene expression of mesenchymal stem cells. Tissue Eng Part A 19: 1039-1046.
- Muller M, Raabe O, Addicks K, Wenisch S, Arnhold S (2011) Effects of non-steroidal anti-inflammatory drugs on proliferation, differentiation, and migration in equine mesenchymal stem cells. Cell Biol Int 35: 235-248.
- Salem O, Wang HT, Alaseem AM, Ciobanu O, Hadjab I, et al. (2014) Naproxen affects osteogenesis of human mesenchymal stem cells via regulation of Indian hedgehog signaling molecules. Arthritis Res Ther 16: 152.
- Belinski GS, Antic SD (2013) Mild hypothermia inhibits differentiation of human embryonic and induced pluripotent stem cells. BioTechniques 55: 79-82.
- Heng BC, Cowan CM, Basu S (2008) Temperature and calcium ions affect aggregation of mesenchymal stem cells in phosphate buffered saline. Cytotechnology 58: 69-75.
- Ginani F, Soares DM, Barboza CAG (2015) Effect of low-level laser therapy on mesenchymal stem cell proliferation: a systemic review. Lasers Med Sci 30: 2189-2194.
- Valiati R, Paes JV, De Moraes AN, Gava A, Agoatini M, et al. (2012) Effect of low-level laser therapy on incorporation of block allografts. Int J Med Sci 9: 853-861.
- Zaccara IM, Ginani F, Moto-Filho HG, Henriques ACG, Barboza CAG (2015) Effect of low-level laser irradiation on proliferation and viability of human dental pulp stem cells. Lasers Med Sci 30: 2259-2264.
- Cachon T, Frykman O, Innes JF, Lascelles B. DX, Okumura M, et al. (2018) Face validity of a proposed tool for staging canine osteoarthritis: canine osteoarthritis staging tool (COAST). Vet J 235: 1-8.
- Gordon WJ, Conzemius MG, Riedesel E, Besancon MF, Evans R, et al. (2003) The relationship between limb function and radiographic osteoarthrosis in dogs with stifle osteoarthrosis. Vet Surg 32: 451-454.
- Vilar JM, Morales M, Santana A, Spinella G, Rubio M, et al. (2013) Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Vet Res 9: 131.
- Vilar JM, Batista M, Morales M, Santana A, Cuervo B, et al. (2014) Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Vet Res 10: 143.
- Sharkey M (2013) The Challenges Of Assessing Osteoarthritis And Postoperative Pain In Dogs. Aaps J 15: 598-607.
- Anderson MA, Mann FA (1994) Force plate analysis: A noninvasive tool for gait analysis. Compend Contin Educ Pract Vet 7: 857-867.
- Jacobs NA, Skoreki J, Charnley J (1972) Analysis of the vertical component of force in normal and pathological gait. J Biomech 5: 11-34.
- Bertram JEA, Lee DV, Todhunter RJ, Foels WS, Williams AJ, et al. (1997) Multiple force plateform analysis of the canine trot: A new approach to assessing basic characteristics of locomotion. Vet Comp Orthop Traumatol 10: 160-169.
- Allen K, Decamp CE, Braden TD, Balms M (1994) Kinematic gait analysis of the trot in healthy mixed breed dogs. Vet. Comp. Orthop Traumatol 7: 148-153.
- Decamp CE, Soutas-Little RW, Hauptman J, Olivier B, Braden T, et al. (1993) Kinematic gait analysis of the trot in healthy Greyhounds. Am J Vet Res 54: 627-634.
- De Gruttola VG, Clax P, Demets DL, Downing GJ, Ellenberg SS, et al. (2001) Considerations in the evaluation of surrogate endpoints in clinical trials. Summary of a National Institutes of Health workshop. Control Clin Trials 22: 485-502.
- Fujita Y, Hara Y, Nezu Y, Schultz KS, Tagawa M (2006) Proinflammatory cytokine activities, matrix metalloproteinase-3 activity, and sulfated glycosaminoglycan content in synovial fluid of dogs with naturally acquired cranial cruciate ligament rupture. Vet Surg 35: 369-3
- Anirudh A, Ranganath L (2015) Synovial fluid analysis in dogs with elbow, hip and stifle joint disorders. Int J Appl Pure Sci Agric 7: 53-57.
Citation: Domaniža M, Trbolová A, Hluchý M, Cížková D (2021) Mesenchymal Stem Cell-Based Treatment of Osteoarthritis in Dogs - A Review. J Stem Cell Res Dev Ther 7: 083.
Copyright: © 2021 Domaniža M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

