
Mesenchymal Stem Cells Derived from Adipose Tissue Ameliorate Chronic Allograft Rejection in the Long Term in a Rat Experimental Model of Kidney Transplantation
*Corresponding Author(s):
Irene L NoronhaLaboratory Of Cellular, Genetic And Molecular Nephrology, Renal Division, University Of Sao Paulo School Of Medicine, Av. Dr. Arnaldo, 455, 4o Andar, Lab 4304, Sao Paulo, CEP 01246-903, Brazil
Tel:+55 1130618403,
Fax:+55 1130618361
Email:irenenor@usp.br
Abstract
Chronic allograft nephropathy remains the leading cause of late allograft failure after renal transplantation. Current immunosuppressive regimens significantly reduce the incidence of acute rejection, but do not influence long-term graft survival. Mesenchymal Stem Cells (MSC) may represent a new strategy to prevent allograft rejection due to its anti-inflammatory and immunomodulatory properties. The aim of this study was to analyze the possible beneficial effects of Adipose-Derived Stem Cells (ASC) on chronic renal allograft nephropathy model. ASCs were isolated and expanded until the 4th passage, characterized by flow cytometry and by their ability to differentiate into mesenchymal lineages. Orthotopic kidney transplantation was established using Fisher F334 rats as donors and Lewis rats as graft recipients, without immunosuppression Animals were divided into 3 groups, and followed for 6 months: Syngeneic (SYNG), kidney transplantation from Lewis to Lewis (n = 6); Allogeneic (ALLO): Lewis rats transplanted with kidney from Fisher rats(n=10); and ALLO+ASC: allogeneic transplantation treated with ASCs. ASCs were effective in the prevention of proteinuria, hypertension and renal dysfunction. Histological analysis showed that ASCs prevented interstitial fibrosis in the chronic allograft nephropathy model, with a significant reduction in the number of lymphocytes and macrophages, pointing to an anti-inflammatory effect of ASCs. Both macrophage phenotypes coexisted in the kidney biopsies of the ALLO group, with M1 phenotype predominance. Treatment with ASCs decreased both macrophages subpopulations, maintaining M1 predominance over M2 phenotype. In addition, there was a significant increased expression of renal gene proinflammatory cytokines, such as IL-1b, TNF-a, IL-6, and IFN-g. ASCs promoted a shift from the pronounced proinflammatory Th1 profile towards an increase in regulatory Th2 cytokines. In conclusion, in the chronic allograft rejection model, administration of ASCs was effective in protecting kidney allograft function, interstitial fibrosis and tissue inflammation supporting cell therapy as possible new strategy of treatment in organ transplantation.
Keywords
Chronic allograft nephropathy; Kidney transplantation; Rat model; Mesenchymal stem cells
INTRODUCTION
Kidney transplantation is recognized as the best alternative treatment for patients with end-stage renal disease. Despite the advances achieved over the last decades, which clearly improved short-term patient and graft survival, no significant change has been observed in long-term graft survival [1]. Chronic allograft nephropathy remains the leading cause of late allograft failure after renal transplantation, leading to end stage renal disease and return to dialysis after renal allograft loss [2].
Chronic allograft rejection is clinically characterized by gradual, slow and progressive kidney allograft dysfunction with increasing serum creatinine, accompanied by proteinuria and hypertension [3]. The typical histopathological changes of chronic rejection are fibrous intimal thickening of arteries, disruption of the elastic layer of the vessel, inflammatory cells in arterial intimal fibrosis, leading to a significant narrowing of the arterial lumen with subsequent ischemia and organ failure. Interstitial fibrosis and tubular atrophy (IF/TA) as non-specific lesions are also hallmarks of chronic allograft injury. The presence of glomerular sclerosis and mononuclear cell infiltration is also observed to some degree [3,4].
There is currently no specific treatment for this clinical condition and thus, new therapeutic approaches are needed to develop alternative treatments to improve long-term outcomes following kidney transplantation. In this regard, there is great interest in using stem cells for tissue repair and regenerative medicine in organ transplantation.
Mesenchymal Stromal Cells (MSC) may represent a new strategy to prevent allograft rejection due to their anti-inflammatory and immunomodulatory properties, including the ability to suppress the innate and adaptive immune response, targeting several cell types involved in the immune response. The first studies to demonstrate the ability of MSC to induce T-cell unresponsiveness were carried out using co-culture of MSC with peripheral blood mononuclear cells. MSC strongly suppress lymphocyte proliferation in response to a variety of stimuli such as allogeneic lymphocytes, T-cell mitogens or anti-CD3 and anti-CD28 activating antibodies [3,5-9]. In vivo effects of MSC were also recognized for their ability to prolonged third party skin graft survival [6]. MSC have also been shown to inhibit cytotoxic effector cells such as CD8+ and NK cells [10,11] and to induce regulatory T cells - Tregs [12,13].
Another interesting aspect relies on fact that MSC are considered immune privileged cells, due to low expression of MHC class II and absence of costimulatory molecules such as B7-1, B7-2, and CD40, CD40L. Thus, MSC escape the immune system and have the advantage of not inducing rejection when infused into an allogeneic host [8]. In addition, MSC exert immune regulatory functions by secreting soluble factors that promote an immunosuppressive milieu [14].
The present study was designed to analyze the possible beneficial effects of Adipose-Derived Stem Cells (ASC) administration on the chronic renal allograft injury model after kidney transplantation. For this purpose, an experimental model of chronic renal allograft rejection was established in our laboratory, using Fisher (F334) rats as donors and Lewis (LEW) rats as graft recipients.
MATERIALS AND METHODS
Animals
Inbred male Lewis (LEW, RT1) and Fisher 344 (F344, RT1lv1) rats, weighing 200-250 g, were used in the experiments for syngeneic and allogeneic renal transplantation. The strain matrices were purchased from the Multidisciplinary Center for Biological Research (UNICAMP, Brazil). All animals used in this study were raised in our local laboratory obtained from crosses between siblings (inbreeding) of the same generation, by at least 20 consecutive generations in permanent monogamous pairs, providing genetic uniformity among the lineages. The rats were housed under standard conditions with controlled 12-hour light/dark cycles, temperature, and humidity and had unrestrained activity and free access to water and rat chow. All experimental procedures were approved by the local Animal Ethical Research Board (CEUA University of Sao Paulo 070/15).
Adipose Tissue Derived Mesenchymal Stem Cells (ASCs)
Adipose tissue from inguinal regions were isolated from adult male Lewis rats and digested with 0.075% collagenase IA (Sigma Aldrich, MO, USA) at 37ºC for 40 minutes, under constant stirring. Subsequently, the collagenase was inactivated with fetal bovine serum and the cell suspension was washed with phosphate buffer and centrifuged twice, for 5 minutes, at 260 g each. Cells were resuspended in ASCs culture medium, consisting of low-glucose Dulbecco’s modified Eagle medium (DMEM, Gibco, Invitrogen Corporation, USA) supplemented with 10% fetal bovine serum (Emcare, SP, Brazil), transferred to a 175 cm2 culture flask (Greiner Bio-one, Essen, Germany), and kept at 37°C, 5% CO2, 20% O2 and 95% humidity. Medium was changed every 3-4 days. When more than 90% confluent was reached, ASCs were detached using 0.05% trypsin-EDTA at 37°C and were directly used for experiments between passages 6 and 8.
ASCs were characterized by: 1) adherence to plastic, 2) negative for hematopoietic cell surface marker as CD45 and positive markers for CD105, CD90, CD44 and CD29 by flow cytometry using FACS Calibur (Becton Dickinson, CA, USA), 3) the ability to differentiate into adipocytes, chondrocytes and osteoblast-like cells. ASCs differentiation was carried out using a mouse MSC functional identification kit (STEMPRO® differentiation kit, Life Technologies, Nova York, USA). After 21 days, alizarin red staining was used for calcium phosphate deposits to evaluate osteogenic differentiation, oil Red O for fatty vacuoles assessed adipogenic differentiation and finally the alcian blue staining for sulfated proteoglycans to confirm chondrogenic differentiation.
Orthotopic rat kidney transplantation model
The experimental transplantation was performed under general anesthesia with Ketamin (5mg/Kg (Ketamin-S, Sao Paulo, Brazil) and Xilasin 2mg/kg (Xylazine Rompun, Bayer, Leverkusen, Germany). Briefly, the left kidney of the donor was perfused through the aorta with cold heparinized saline solution, for approximately 5 min. After perfusion, the kidney was removed by cutting the renal artery and vein in the proximal regions, and the ureter in the region proximal to the bladder. The removed kidney was cooled in 4oC saline solution for approximately 2minutes, and orthotopically placed after removal of the recipient’s kidney. The anastomoses between the donor and recipient renal artery and vein were performed through end-to-end suture, using 9-0 polypropylene sutures. The cold ischemia time varied from 25 to 30 min. The ureter was also anastomosed with an end-to-end suture. During the surgical procedure, all animals were appropriately hydrated (physiological saline solution), and their body temperature was kept at approximately 37°C using an adjustable heating pad. Recipient rats received 5 mg/kg tramadol hydrochloride during surgery and at days 1 and 2 post transplantation. No immunosuppression therapy was given.
The nephrectomy of the right kidney was performed 10 days later. During this surgical procedure, a group of allogeneic kidney transplantation rats received 1 × 106 ASCs diluted in 20 µL of saline solution, administered in the subcapsular region of the kidney graft, as previously described [15]. A small cut was made at the renal capsule in the renal hilum region. The renal capsule was carefully separated using a needle holder. The pellet of cells was allocated under the kidney capsule. The allogeneic group treated with ASCs received two additional intraperitoneal injections of 1 × 106 ASCs diluted in 3ml of saline solution at right inguinal region, 4 and 12 weeks after transplantation.
Six months after transplantation, all rats were euthanized, the renal graft was removed and blood samples were obtained through the inferior vena cava.
Experimental design
The rat model of chronic allograft nephropathy was established performing allogeneic transplants using Fisher 344 as donors and Lewis rats as graft recipients (ALLO). The isogeneic transplantation control consisted of LEW kidney to LEW animals (ISO). Three experimental groups were studied: Syngeneic (SYNG), syngeneic transplantation from Lewis to Lewis (n=8), Allogeneic (ALLO): allogeneic transplantation from Fisher 344 to Lewis (n=10); and ALLO+ASC, allogenic transplantation, treated with ASCs (n = 8). Ten days after transplantation, the animals were again submitted to a median laparotomy for nephrectomy of the remaining kidney. After removal of the kidney, the isolated ASCs were submitted to subcapsular infusion (1.106 cells). Additional ASCs infusions were performed intraperitoneally 4 and 24 weeks after transplantation. The animals were followed for 6 months and analyses of caudal pressure, renal function, histology, immunohistochemistry and real time PCR were performed. Recipient survival at week 24 was determined.
Biochemical analysis
On weeks 4, 12, and 24 after transplantation, body weight and tail cuff pressure were measured, and 24-hour urine samples were collected using metabolite cages, and stored at -80oC, until assayed. Urinary protein, serum and urinary creatinine, sodium and potassium as well as blood urea nitrogen (BUN) levels were measured by an automated method (Cobas C111 analyzer (Roche, Indianapolis, USA). Creatinine clearance was calculated as urinary creatinine (mg/dL) × diuresis volume (mL) / serum creatinine (mg/dL) × 1440 (min). Fractional excretion of sodium (FeNa) was calculated using a spot urine sample, using the equations: uFeNa (%) = urinary Na+(mEq/L) × serum Creatinine(mg/dL) /urinary creatinine(mg/dL) × serum Na+(mEq/L) × 100. Fractional excretion of sodium (FeNa) was calculated using the equation: uFeK (%) = urinary K+(mEq/L) × serum Creatinine(mg/dL) / urinary Cr (mg/dL) × serum K+(mEq/L) × 100.
Histology
Kidney grafts were carefully harvested, washed with saline solution, immediately fixed in 4% buffered formaldehyde solution for 24h and paraffin-embedded. Paraffin sections of 4mm thick were stained using H&E as well as with periodic acid-Shiff, to evaluate glomerular, tubular and vascular changes. Sections were also stained with Masson's trichrome technique and Picrosirius Red to evaluate interstitial fibrosis. To analyze the collagen content in the interstitial space, sections were stained for 30 min with Sirius red (0.1% of Sirius red in saturated aqueous picric acid) and analyzed under polarized light microscopy. The presence of tubular atrophy was evaluated semiquantitatively using 1 to 3+ score, analyzed in 25 consecutive microscopic fields of PAS-stained sections of each experimental group, under 200× magnification. The extent of interstitial fibrosis was quantitatively evaluated in Masson Trichrome-stained sections by a point counting method in 25 consecutive microscopic fields, at high magnification of 400× under a 176-point grid or 100-point ocular grid or in a grid containing 117 sampling points (13 × 9) which was superimposed on the monitor. The number of points falling on stained structures of interstitial space extracellular matrix components was counted.
Immunohistochemistry and immunofluorescence
Immunohistochemistry for inflammatory cells was carried out as previously described [16]. Briefly, after deparaffinization, renal biopsy specimens were submitted to microwave antigen retrieval in citrate buffer, pH 6.0. Slides were incubated at 4°C overnight with the primary monoclonal antibodies anti-rat ED1 (CD68; Serotec, Oxford, UK), diluted at 1: 400, and anti-rat CD43 (Serolab, Oxford, UK), diluted at 1: 200. Sections were, then, incubated with biotinylated anti-mouse IgG (BA 2001, Vector Labs, Burlingame, USA). The streptavidin-biotin-alkaline phosphatase complex reaction was performed (Vector) using a freshly prepared substrate consisting of naphthol-AS-MX-Phosphate (Sigma Chemical Co., St. Louis, USA) and fast red dye (Sigma, St. Louis, USA). To detect the CD206+ subpopulation, renal samples were submitted to microwave antigen retrieval in tris EDTA buffer, pH 9.0. Endogenous peroxidase activity was blocked, and the slides were incubated with a polyclonal anti-mannose receptor antibody (CD206; Abcam, San Francisco, USA), followed by incubation with biotinylated anti-rabbit IgG (BA 1000, Vector Labs, Burlingame, USA). Quantitative analysis of macrophages (CD68 and CD206) and T-cells (CD43) was performed in a blind fashion under magnification (×200) and expressed as cells/mm2. For each section, 20 microscopic fields were examined, and the results were expressed as the mean number of positive cells/mm2.
Indirect immunofluorescence to analyze the M1 and M2 macrophages subpopulations was carried out in paraffin sections. After deparaffinization, renal samples were submitted to antigen retrieval, in 4°C water bath with pH 9.0 tris EDTA buffer + 0.05% Tween for 30 minutes. Nonspecific protein binding was blocked with 5% albumin bovine fraction for 30 minutes (37°C). The sections were, then, incubated with the primary antibody anti-rat ED1 CD68 Serotec, Oxford, UK), diluted at 1:50 and anti-mannose receptor antibody (CD206; Abcam, San Francisco, USA), diluted at 1:800, overnight at 4°C. After washing, the slides were incubated with Alexa Fluor-labeled secondary antibodies (Alexa 546 anti-rabbit, and Alexa 488 anti-mouse; Life Technologies, Oregon, USA), for 1 hour at room temperature. Finally, the slides were incubated for 15 minutes with DAPI for nuclear staining, diluted at 1:1000 (Life Technologies, Carlsbad, USA). After washing, the slides were mounted with glycerol 1:1 in PBS, and the sections were analyzed under a confocal microscopy (Zeiss LSM 880, Germany), at 400x magnification, using the 488 nm filter for CD68 (green), 546nm filter for CD206 (red) and 504 nm for DAPI (blue). The M1 macrophage subtype was recognized by CD68+/CD206- cells. The M2 macrophage subpopulation was recognized by CD206+/CD68+cells [17], stained as merged green and red colors.
Real-time PCR and cytokines detection
Total RNA was extracted from snap-frozen portions of kidney by the guanidinium isothiocyanate/phenol-chloroform extraction method. Gene expression of the cytokines IL-1β, TNF-α, IL-6, INF-g, IL-4 and IL-10 was analyzed by Real time PCR as previously described [18]. The following PCR cycle profile was used: 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C for denaturation, 20 sec at 60°C for combined annealing, and 10 sec at 72°C for extension. β-actin was used as housekeeping control. Primer sets used for qRT-PCR are presented in Supplementary Table 1.
Statistical analysis
Data are expressed as mean±SEM. Statistical analyses were performed with the Prism statistical program (GraphPad, San Diego, USA). One-way ANOVA with Tukey’s multiple comparisons test. P < 0.05 was considered significant.
RESULTS
Animal model and mortality
The orthotopic rat kidney transplantation model of chronic allograft nephropathy was established in our laboratory. At 6 months after transplantation, the mortality rate was 25%, 40% and 0% in SYNG, ALLO and ALLO+ASCs groups, respectively (Supplementary figure 1). As shown in figure 1A, body weight gain was lower, but not significant, in ALLO and ALLO+ASCs recipient rats compared with SYNG animals.
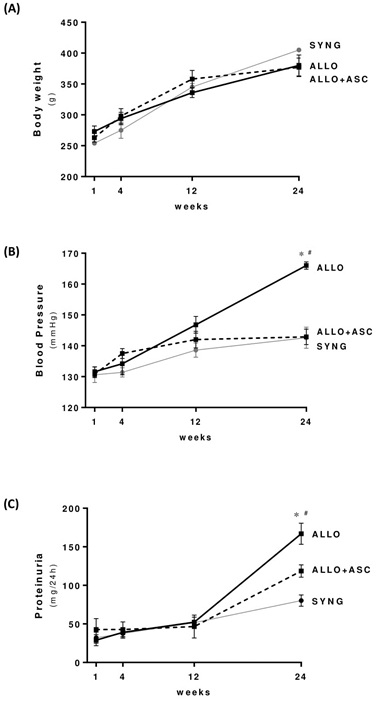
Figure 1: Body weight, blood pressure and proteinuria in the 3 different groups of chronic allograft nephropathy in rats, measured at 4, 12 and 24 weeks after transplantation. * p< 0.05 versus SYNG and #p < 0.05 versus ALLO+ASC group.
Renoprotective effects of ASCs therapy
Rats submitted to the experimental model of chronic allograft nephropathy (ALLO group) presented marked hypertension with elevated blood pressure levels observed at 12 weeks after transplantation, and significantly higher at 24 weeks compared with syngeneic recipients (SYNG). It is noteworthy that ALLO animals treated with ASCs did not develop hypertension, maintaining blood pressure levels similar to the control group (Figure 1B). In parallel, ALLO rats developed progressive proteinuria, with the highest levels detected 6 months after transplantation. ALLO animals treated with ASCs presented significantly lower levels of urinary protein excretion.
The experimental model of chronic allograft nephropathy (ALLO group) promoted a marked degree of renal dysfunction, with significantly higher creatinine and BUN levels compared with control animals, in parallel with decreased creatinine clearance. Tubular injury, characterized by elevated fractional excretion of sodium and potassium, was also observed in this model (Table 1). Administration of ASCs to ALLO rats ameliorated all parameters of kidney dysfunction including tubular function protection.
|
Parameters |
SYNG |
ALLO |
ALLO+ASC |
|
BUN (mg/dL) |
50.1±1.3 |
70.4±8.3* |
43.4±1.1# |
|
Serum Creatinine (mg/dL) |
0.5±0.2 |
1.6±0.7* |
0.5±0.1# |
|
Creatinine Clearance (ml/min) |
1.30±0.10 |
0.68±0.18* |
1.49±0.25# |
|
FeK+(%) |
42±5 |
118±61* |
33±60# |
|
FeNa+(%) |
0.3±0.1 |
1.2±0.5* |
0.3±0.1# |
Table 1: Effects of ASC on renal function and urinary biochemistry at 6 months after transplantation in the rat model of chronic allograft nephropathy.
*p < 0.05 vs SYNG; #p < 0.05vs ALLO.
ASCs attenuate morphological changes and interstitial inflammation
In the SYNG group, renal architecture was preserved, with only mild changes. In contrast, the histological analysis of rat kidneys of the ALLO group by 6 months showed that the tubulointerstitial compartment was affected by interstitial fibrosis/tubular atrophy (IFTA) changes (Figure 2).The degree of interstitial fibrosis, evaluated both by Masson’s trichrome and picrosirius red staining, was significantly higher in the ALLO compared with the SYNG group (Table 2). In addition, tubular atrophy, and different degrees of dilated tubules, many of them with protein casts within the lumen were also more prominent in the kidney of ALLO rats. In the ALLO group, vascular changes, characterized by endothelial swelling and intimal expansion, as well as some degree of glomerulosclerosis and basement membrane thickening with marked mononuclear cell infiltration in periglomerular and perivascular regions were also observed. Treatment of rats with chronic allograft nephropathy (ALLO group) with ASCs analyzed at 6 months resulted in significantly lower interstitial fibrosis, tubular atrophy and perivascular inflammation (Table 2).
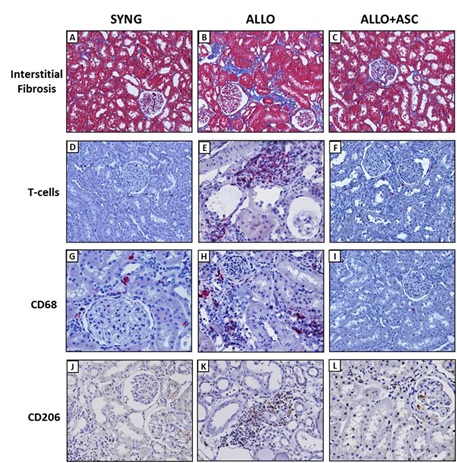 Figure 2: Effects of ASC injection in transplanted kidney. (A, B and C) Representative photomicrographs of kidney sections (×400) marked for T-cells. (A) SYNG with low number of stained cells; (B) ALLO group showing a high number of stained cells (red staining); (C) In the ALLO + ASCs group, stained cells were attenuated. (D, E and F) Representative photomicrographs of kidney sections (×400) marked for total macrophages (CD68); (D) SYNG group stained very few cells; (E) ALLO group showing an intense number of stained cells (red staining); (F) ASC treatment attenuated CD68 cells staining. (G, H and I) represents kidney sections (×400) marked for macrophages M2 (CD206); (G) SYNG group with very few stained cells; (H) ALLO group with intense staining cells for CD206; (I) ASC treatment reduced the number of CD206 stained cells. (J) Graph representing the quantification of T-cells in all groups. (L) Graphs representing the total macrophages number CD68 and CD206 (M2) in all groups. Results are expressed as mean ± standard error of mean. * p< 0.05 versus SYNG and #p < 0.05
Figure 2: Effects of ASC injection in transplanted kidney. (A, B and C) Representative photomicrographs of kidney sections (×400) marked for T-cells. (A) SYNG with low number of stained cells; (B) ALLO group showing a high number of stained cells (red staining); (C) In the ALLO + ASCs group, stained cells were attenuated. (D, E and F) Representative photomicrographs of kidney sections (×400) marked for total macrophages (CD68); (D) SYNG group stained very few cells; (E) ALLO group showing an intense number of stained cells (red staining); (F) ASC treatment attenuated CD68 cells staining. (G, H and I) represents kidney sections (×400) marked for macrophages M2 (CD206); (G) SYNG group with very few stained cells; (H) ALLO group with intense staining cells for CD206; (I) ASC treatment reduced the number of CD206 stained cells. (J) Graph representing the quantification of T-cells in all groups. (L) Graphs representing the total macrophages number CD68 and CD206 (M2) in all groups. Results are expressed as mean ± standard error of mean. * p< 0.05 versus SYNG and #p < 0.05
|
Parameters |
SYNG |
ALLO |
ALLO+ASC |
|
Interstitial fibrosis index (Masson's trichrome) |
7.4±0.6 |
24.5±2.0* |
17.3±1.0# |
|
Picrosirius Red (semiquantitative index) |
0.6±0.4 |
2.3±0.3* |
1.4±0.4 |
|
Tubular atrophy (semiquantitative index) |
1.6±0.4 |
2.6±0.2 |
1.6±0.4 |
|
Perivascular inflammation (semiquantitative index) |
1.4±0.4 |
2.3±0.3 |
1.3±0.4 |
|
T-cells (cells/mm2) |
8.0±2 |
26.0±1.0* |
10.0±1.0# |
|
Macrophages CD68 (cells/mm2) |
13.0±2.0 |
95.0±3.4* |
53.0±3.8*,# |
|
Macrophages CD206 (cells/mm2) |
6.2±1.8 |
41.5±3.9 |
19.4±.2.5*,# |
Table 2: Effects of ASC on kidney allograft histology and cellular infiltration at 6 months after transplantation in the rat model of chronic allograft nephropathy.
*p < 0.05 vs SYNG; #p < 0.05 vs ALLO.
ASCs attenuate macrophages and T cells infiltration in kidney allograft
No significant interstitial T cells infiltration was observed in the kidneys of control rats (SYNG group). However, a significantly inflammatory infiltration of T-cells was observed in the kidney of the ALLO group, specifically at the perivascular region graft (Table2; Figure 2). Importantly, treatment with ASCs promoted significant reduction in the T cells infiltration. Macrophage infiltration was also analyzed through CD68 and CD206 expression. Both CD68 and CD206 macrophages were present in a very small number of the SYNG group, whereas cells expressing both markers increased significantly in the ALLO group. It is noteworthy that ASC treatment reduced the staining for all macrophages (Table2; Figure 2).
In order to verify whether ASCs treatment induced a shift in the M1/M2 macrophage subtypes profile in terms of their anti-inflammatory/modulatory effects, the expression of M1 macrophages (CD68+/CD206- cells) and M2 (CD68+/CD206+) was analyzed (Figure 3). As shown in figure 3, there was a preponderance of the M1 subpopulation in kidney biopsies. Treatment with ASCs decreased both M1 and M2 macrophages subtypes.
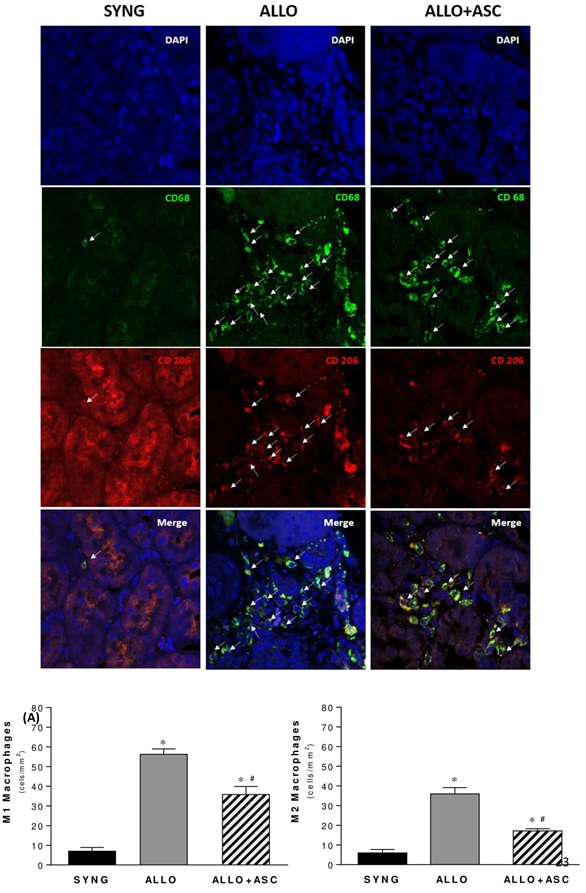 Figure 3: Double immunostaining of CD68 (green) and CD206 (red) were used to identify macrophages in the kidney of rats submitted to the chronic allograft nephropathy, using immunofluorescence analyzed in a confocal microscope. The counterstaining of nuclei (DAPI) is shown in blue. Kidney specimens from controls (SYNG), chronic allograft rejection (ALLO) and chronic allograft rejection treated with ASC(ALLO+ ASC) were stained with DAPI, CD68, CD206, and CD206+/CD68+ cells, stained as merged green and red colors. Arrows indicate cell positivity for CD68 (green), CD206 (red) and both (merged color), in the illustrative microphotographs. Quantification of M1 and M2 macrophages in kidney in each group (cells/mm2) is shown in the graphs below. The M1 macrophage subtype was recognized by CD68+/CD206-cells. The M2 macrophage subpopulation was recognized by CD206+/CD68+ cells.
Figure 3: Double immunostaining of CD68 (green) and CD206 (red) were used to identify macrophages in the kidney of rats submitted to the chronic allograft nephropathy, using immunofluorescence analyzed in a confocal microscope. The counterstaining of nuclei (DAPI) is shown in blue. Kidney specimens from controls (SYNG), chronic allograft rejection (ALLO) and chronic allograft rejection treated with ASC(ALLO+ ASC) were stained with DAPI, CD68, CD206, and CD206+/CD68+ cells, stained as merged green and red colors. Arrows indicate cell positivity for CD68 (green), CD206 (red) and both (merged color), in the illustrative microphotographs. Quantification of M1 and M2 macrophages in kidney in each group (cells/mm2) is shown in the graphs below. The M1 macrophage subtype was recognized by CD68+/CD206-cells. The M2 macrophage subpopulation was recognized by CD206+/CD68+ cells.
ASCs diminished the expression of inflammatory cytokines in kidney allografts
In order to investigate the mechanisms involved in the anti-inflammatory effects of ASCs administration in the rat model of chronic allograft nephropathy, cytokine gene expression was analyzed in graft samples of the different groups (Figure 4). The ALLO group exhibited significantly higher mRNA levels of the pro-inflammatory cytokines TNF-α, IFN-g, IL-1β and IL-6 compared with SYNG group. However, animals treated with ASCs showed a significantly decreased expression of these cytokines, with mRNA levels similar to the SYNG group, corroborating the role of ASCs on inflammation control. On the other hand, the Th2 cytokines IL-4 and IL10, which were depressed in the ALLO group, were strikingly elevated in ALLO animals treated with ASCs, pointing to an immunoregulatory role upon inflammation that could be beneficial to protect the kidney injury.
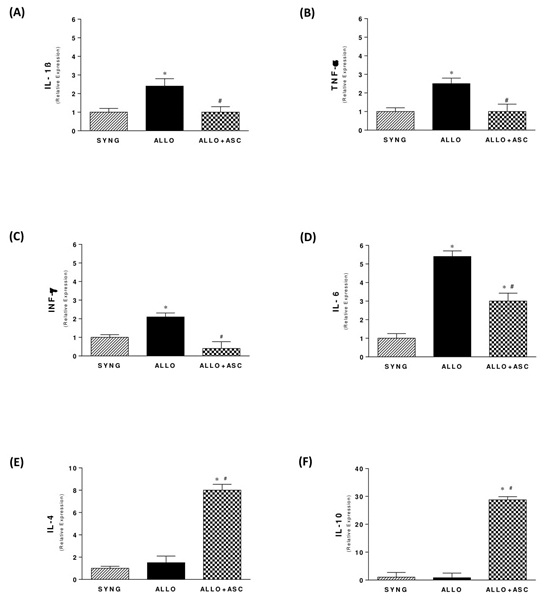
Figure 4: Effects of ASC on pro-inflammatory and anti-inflammatory gene expression in the kidney graft of all groups, 6 months after transplantation (A-F).
*p< 0.05 versus SYNG and #p < 0.05 versus ALLO group.
DISCUSSION
In the present study, the effect of ASCs was analyzed in the rat kidney model of chronic allograft nephropathy, established in our laboratory. In this Fisher 344 to Lewis rat renal transplant model, the immune response is a result of partial MHC or RT1 disparity between the strains, and the clinical and histological findings observed in these animals resemble the characteristics of chronic renal allograft injury observed in patients, including functional and histological changes. In this setting, the stem cells effects were analyzed as a new possible therapeutic approach for this clinical condition.
Among different sources of MSCs, adipose tissue has emerged as an attractive alternative source to bone marrow, considered the main source of MSC for clinical application. Human adipose tissue is distributed in the body, accumulated in the subcutaneous layer, easily obtained under anesthesia with less discomfort for the patient compared with bone marrow tissue. Large amounts of ASCs can be isolated from adipose tissues exhibiting higher proliferation potential compared do Bone Marrow-derived MSC (BM-MSC) [19]. Although ASCs present similar features to BM-MSC, differences in immunophenotype, differentiation potential and immunomodulatory activity have been described [20]. It is still not clear whether ASCs can be as effective as BM-MSC for clinical application. Higher concentration of angiogenic paracrine factors are produced by ASCs compared to BM-MSC, suggesting that ASCs may be better suited concerning angiogenic capacity than BM-MSC [14].
In this study, the delivery route for ASCs administration was the renal subcapsular region, in order to provide adequate number of MSC near the site of injury, aiming to facilitate their homing. Although the number of stem cells in the kidney necessary to play a role in the renoprotective effects has not yet been defined, the approach of inoculating ASCs underneath the kidney capsule likely avoids systemic dispersion of stem cells to other organs, mainly to the lungs and to the liver, as described with intravenous or intraperitoneal administration [21-23]. ALLO rats receiving ASCs had a higher survival rate than ALLO animals. Although the survival rate of ALLO+ASCs was higher than SYNG, this difference was not statistically significant, and may be due to other issues related to the transplantation procedure.
Administration of ASCs in the rat chronic nephropathy transplant model was effective in preventing proteinuria, hypertension and renal dysfunction. Proteinuria post-transplantation represents a hallmark of chronic allograft injury and is associated with worse long-term prognosis [24]. The positive effects of ASCs administration in reducing proteinuria levels are possibly due to direct effects on the glomerular filtration barrier, particularly on the structural and functional integrity of podocytes. We have recently reported renoprotective effects of MSC in a podocytopathy model induced by puromycin aminonucleoside recovering WT1 expression, ameliorating podocyte slit diaphragm and cytoskeletal proteins, such as nephrin and podocin synaptopodin and podocalyxin [25]. Similar results using BM-MSC in diabetic mice were reported by Wang and coworkers [26].
In the present study, beneficial effects of ASCs on kidney dysfunction of ALLO rats were also observed, with improvement in creatinine clearance, and decreased serum levels of BUN and creatinine. In addition, tubular function was also improved in animals treated with ASCs, as measured by the fractional excretion of sodium and fractional excretion of potassium. We have previously described renoprotective effects of BM-MSCs treatment in the rat remnant kidney model and in the puromycin model [15,25]. These effects were also described in models of experimental glomerulonephritis [27] and renal transplantation [28] treated with BM-MSC and sepsis-induced kidney injury, treated with human wharton's jelly-derived MSC [29]. Indeed, in vitro cultured ASCs release large amounts of the proangiogenic factor VEGF, which contributes to glomerular and tubular [30,31], and insulin-like growth factor (IGF-1), which is crucial for nephrogenesis.
It is noteworthy the beneficial effects of ASCs in preventing interstitial fibrosis, as observed by significant decrease of the interstitial compartment by Masson's trichrome and picrosirius red staining. Interstitial fibrosis represents the final pathway of progressive renal diseases, both in native and allograft kidneys. The extent of interstitial fibrosis strongly predicts the degree and progression to renal failure. Treatment with ASCs prevented fibrogenesis and maintained the integrity of kidney architecture. Other studies have already demonstrated the potential of ASCs in experimental models to prevent fibrogenesis in liver [27] and kidney [32].The mechanisms for the fibrosis protection are not clear but may rely on the modulation of the local inflammatory response induced by ASCs.
The results observed in this study demonstrated that ALLO rats presented a marked increase in the number of inflammatory cells in the renal interstitium. In ALLO rats treated with ASCs, there was a significant reduction in the number of lymphocytes and macrophages, pointing to an anti-inflammatory effect of ASCs. Macrophages are the main cell type infiltrating chronically rejected allografts, frequently localized to areas of interstitial fibrosis. Previous studies have shown that the number of macrophages correlate with the degree of interstitial fibrosis at 5 and 10 years post- transplantation [33].
Macrophages can adopt an M1, proinflammatory, or an M2, anti-inflammatory phenotype [34]. Both macrophage phenotypes coexisted in the kidney biopsies of the chronic allograft nephropathy model, with M1 phenotype predominance. The proinflammatory milieu in the kidney of rats with chronic allograft nephropathy might have induced the polarization of macrophages into M1 phenotype. Treatment with ASCs decreased both macrophages subpopulations, maintaining M1 predominance over M2 phenotype [35]. These results paralleled the findings of renal proinflammatory gene expression, which demonstrated increased expression of pro-inflammatory cytokines, such as IL-1b, TNF-a, IL-6, and IFN-g in ALLO rats. In the ALLO group treated with ASCs, there was a significant decreased expression of these pro-inflammatory mediators. The anti-inflammatory effect of ASCs decreasing proinflammatory mediators such as interferon-g likely promoted the reduction of the number of M1 macrophages in the tubulointerstitial compartment. In parallel, ASCs induced a Th2 immune response, characterized by a significant increase in IL-4 and IL-10 gene expression in the renal tissue in the ALLO+ASC group. Taken together, the present data showed that ASCs promoted a shift from the pronounced proinflammatory Th1 profile towards an increase in regulatory Th2 cytokines.
In conclusion, treatment with ASCs in the rat chronic allograft nephropathy model induced renoprotective effects ameliorating the progression renal disease. This outcome is probably due to the modulation of inflammatory response, decreasing the pro-inflammatory infiltrate and pro-inflammatory cytokines and increasing the anti-inflammatory cytokines. These results reinforce the importance of new studies in both, basic and preclinical research areas not only to understand the MSC action mechanisms, but also the strategies of treatment in cell therapy.
ACKNOWLEDGEMENT
We are grateful to Giselle L Tavora, Cleonice da Silva and Marcia Ribalta for their excellent technical support.
REFERENCES
- Meier-Kriesche H, Schold JD, Kaplan B (2004) Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 4: 1289-1295.
- Nankivell BJ, Chapman JR (2006) The significance of subclinical rejection and the value of protocol biopsies. Am J Transplant 6: 2006-2012.
- Joosten SA, Sijpkens YWJ, van Kooten C, Paul LC (2005) Chronic renal allograft rejection: pathophysiologic considerations. Kidney Int 68: 1-13.
- RB Colvin (2003) Transplant Mac attack: Humor the macrophages. Kidney Int 63: 1953-1954.
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, et al. (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838-3843.
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, et al. (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30: 42-48.
- Le Blanc K, Pittenger Mf (2005) Mesenchymal stem cells: progress toward promise. Cytotherapy 7: 36-45.
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC (2003) Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 75: 389-397.
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, et al. (2003) Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101: 3722-3729.
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P (2003) Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol 171: 3426-3434.
- Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, et al. (2005) Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 90: 516–525.
- Aggarwal S, Pittenger M F (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815-1822.
- Engela AU, Hoogduijn MJ, Boer K, Litjens NHR, Betjes MGH, et al. (2013) Human adipose-tissue derived mesenchymal stem cells induce functional de-novo regulatory T cells with methylated FOXP3 gene DNA. Clin Exp Immunol 173: 343-354.
- Hsiao STF, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, et al. (2012) Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev 21: 2189-2203.
- Cavaglieri RC, Martini D, Sogayar MC, Noronha IL (2009) Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model. Transplant Proc 41: 947-951.
- Dellê H, Rocha JRC, Cavaglieri RC, Vieira Jr JM, Malheiros DMAC, et al. (2012) Antifibrotic effect of tamoxifen in a model of progressive renal disease. J Am Soc Nephrol 23: 37-48.
- Jensen KY, Jacobsen M, Schrøder HD, Aagaard P, Nielsen JL, et al. (2019) The immune system in sporadic inclusion body myositis patients is not compromised by blood-flow restricted exercise training. Arthritis Res Ther 21: 293.
- Silva FM, Costalonga EC, Silva C, Carreira ACO, Gomes SA, et al. (2019) Tamoxifen and bone morphogenic protein-7 modulate fibrosis and inflammation in the peritoneal fibrosis model developed in uremic rats. Mol Med 25: 41.
- Peng L, Jia Z, Yin X, Zhang X, Liu Y, et al. (2008) Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev 17: 761-774.
- Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J (2012) Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 21: 2724-52.
- Kinomura, M, Kitamura S, Tanabe K, Ichinose K, Hirokoshi K, et al. (2008) Amelioration of cisplatin-induced acute renal injury by renal progenitor-like cells derived from the adult rat kidney. Cell Transplant 17: 143-158.
- Feng S-W, Lu X-L, Liu Z-S, Zhang Y-N, Liu T-Y, et al. (2008) Dynamic distribution of bone marrow-derived mesenchymal stromal cells and change of pathology after infusing into mdx mice. Cytotherapy 10: 254-264.
- Packthongsuk K, Rathbun T, Troyer D, Davis DL (2018) Porcine wharton’s jelly cells distribute throughout the body after intraperitoneal injection. Stem Cell Res Ther 9: 38.
- Oliveira CMC, Pereira IS, Souza LCL, Cruz TA, Pinheiro Jr FML, et al. (2015) Proteinúria pós-transplante renal - prevalência e fatores de risco. J Bras Nefrol 37: 481-489.
- Ornellas FM, Ramalho RJ, Fanelli C, Garnica MR, Malheiros DMAC, et al. (2019) Mesenchymal Stromal Cells Induce Podocyte Protection in the Puromycin Injury Model. Scientific Reports 9: 19604.
- Wang S, Li Y, Zhao J, Zhang J, Huang Y (2013) Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biology of Blood and Marrow Transplantation 19: 538-546.
- Kunter U, Rong S, Boor P, Eitner F, Müller- Newen G, et al. (2007) Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol 18: 1754-1764.
- Franquesa M, Herrero E, Torras J, Ripoll E, Flaquer M, et al. (2012) Mesenchymal stem cell therapy prevents interstitial fibrosis and tubular atrophy in a rat kidney allograft model. Stem Cells Dev 21: 3125-335.
- Cóndor JM, Rodrigues CE, Sousa Moreira R, Canale D, Volpini RA, et al. (2016) Treatment with human wharton’s jelly-derived mesenchymal stem cells attenuates sepsis-induced kidney injury, liver injury, and endothelial dysfunction. Stem Cells Transl Med 5: 1048-1057.
- Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, et al. (2012) Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 30: 1030-1041.
- Schrijvers BF, Flyvbjerg A, De Vriese AS (2004) The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int 65: 2003-2017.
- Ebrahimi B, Eirin A, Li Z, Zhu XY, Lerman A, et al. (2013) Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS ONE 8: 1-12.
- Ikezumi, Y, Suzuki T, Yamada T, Hasegawa H, Kaneko U, et al. (2015) Alternatively activated macrophages in the pathogenesis of chronic kidney allograft injury. Pediatr Nephrol 30: 1007-1017.
- Tang PMK, Nikolic-Paterson DJ, Lan HY (2019) Macrophages: versatile players in renal inflammation and fibrosis. Nature Reviews Nephrology 15: 144-158.
- Chen BT, Cao Q, Wang Y, Harris DCH (2019) M2 macrophages in kidney disease: biology, therapies, and perspectives. Kidney Int 95: 760-773.
SUPPLEMENTARY MATERIAL
|
|
Primer sequence 5’→3’ |
|
IL-1β |
forward 5’-CCTTGTGCAAGTGTCTGAAGCAGC-3’ |
|
TNF-α |
forward 5’-ATCTGAGGGCTCGCCCGGT-3’ |
|
IL-6 |
forward 5’-CCGGAGAGGAGACTTCACAGAGGA-3’ |
|
INF-g |
forward 5’-TCTGGAGGAACTGGCAAAAGG-3’ |
|
IL-4 |
forward 5’-TGGCCCAGACCCTCACACTCA-3’ |
|
IL-10 |
forward 5’-TGGGTCTCAGCCCCCACCTT-3’ |
|
β-actin |
forward 5-AGGAGTACGATGAGTCCGGCCC reverse GTAGTGCGGAGCTCTCCTTCA |
Supplementary Table 1: Primer sets used for qRT-PCR
|
Parameters |
SYNG |
ALLO |
ALLO+ASC |
|
Body Weight (g) |
405±2 |
380±17 |
377±15 |
|
Blood Pressure (mmHg) |
143±3.4 |
166±0.7* |
143±2.5# |
|
Proteinuria (mg/24h) |
80.2±7.3 |
166.9±13.6* |
118.6±8.0# |
Supplementary table 2: Body weight, blood pressure and proteinuria in the different groups, at 6 months after transplantation in the rat model of chronic allograft nephropathy.
*p < 0.05 vs SYNG; #p < 0.05 vs ALLO.
|
Parameters |
SYNG |
ALLO |
ALLO+ASC |
|
CD68+CD206- (M1) |
7.0±1.9 |
56.2±2.8 |
35.8±4.1# |
|
CD68+CD206+ (M2) |
6±1.7 |
36±3.1 |
17.2±1.1 |
|
CD68+ (CD68+CD206-) +(CD68+CD206+) |
13±2 |
41.5±3.9 |
53±3.8 |
|
CD206+ |
6.2±1.8 |
31.2±1.5* |
19.4±2.5 |
|
CD68–CD206+ |
0.2±0.2 |
3.6±1.5 |
2.2±.1.7 |
Supplementary Table 3: Expression of CD68 and CD206 macrophage markers.
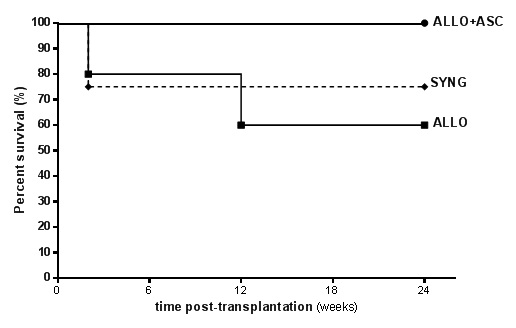
Supplementary Figure 1: Survival rate of the different groups followed for 24 weeks (6 months) after renal transplantation.
Citation: Pepineli R, Gouveia PQ, Garnica MR, Fanelli C, Martini D, et al. (2020) Mesenchymal Stem Cells Derived from Adipose Tissue Ameliorate Chronic Allograft Rejection in the Long Term in a Rat Experimental Model of Kidney Transplantation. J Stem Cell Res Dev Ther 6: 051.
Copyright: © 2020 Rafael Pepineli, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

