
Mesenchymal Stem Cells for Treatment of Retinitis Pigmentosa: Short Review
*Corresponding Author(s):
Antonio FloridoUniversity La Sapienza Of Rome, Polo Pontino - Ospedale A. Fiorini Di Terracina, Italy
Tel:+39 3486500312,
Email:antonioflorido@hotmail.it
Abstract
The management of retinitis pigmentosa (RP) and its progression has always been a difficult issue, but promising developments have been shown by the use of mesenchymal stem cells (MSCs). It has thus been evidenced how these cells have significant paracrine and immunomodulatory properties: they secrete trophic factors that stimulate RPE or that are similar to those produced by RPE. In studies using animal models, MSCs have been found to be effective in stopping the progression of retinal degeneration and for rescuing photoreceptors in the dormant phase. Furthermore these cells are hypoimmunogenic and have been shown to suppress chronic inflammation, prevent apoptosis and stimulate progenitor cells in the retina promoting self-repair mechanisms.
Previous studies have already evidenced how grafting autologous mesenchymal cells, in a scleral pocket above the choroid, with the limoli retinal restoration technique (LRRT) could exert a beneficial effect on the residual retinal cells in patients with retinitis pigmentosa (RP).
This surgical procedure can improve the clinical and rehabilitative prognostic parameters in RP patients even though further researches and studies will be needed to evaluate its efficacy.
Keywords
Limoli retinal restoration technique; Mesenchymal stem cells; Retinitis pigmentosa; Retinal Pigment epithelium
Introduction
Talking about RP we refer to an heterogeneous group of hereditary retinal diseases known by the progression in the degeneration of photoceptors. This group of pathologies primarily affects the rods, with subsequent involvement of cone function, which consequently leads to an early night blindness and progresses to the restriction of peripheral vision, culminating with a central vision loss on the very end stage, due to delayed macular degeneration [1].
Its etiology is variable and involves the progressive photoreceptor cells death by apoptosis. Patients with autosomal recessive RP (50-60% of cases) and autosomal dominant RP (30-40% of cases) have better visual prognosis and slower progression of the pathology compared to those with X-linked RP.
The suspect of the disease, caused by visual concerns, could be confirmed by specific examinations such as visual field testing, full-field electroretinogram (ERG), and optical coherence tomography (OCT). The latter records a progressive reduction of the stromal thickness, which occurs initially at the periphery and then affects the whole retinal stroma, eventually leading to its atrophy. A further study has shown that there is a positive linear correlation between the decrease in visual field sensitivity and the thinning of the outer segments. Therefore, in RP, retinal thinning secondary to cell loss is linked with impairment of visual acuity [2].
Possible Roles Of MSCs In Treatment Of Retinitis Pigmentosa
Considering that until date, the disease has no curative treatment, the main goal is to slow down the photoreceptors apoptosis. This can be done by delivering embryonic stem cells (ESCs), Mesenchymal Stem Cells (MSCs), and induced pluripotent stem cells (iPSCs), to precise target locations in the eye. Those cells not only play a key role in tissue repair by self-renovation and multipotency, but could also have immunosuppressant function thus inhibiting the proinflammatory cytokine release [3]. Mesenchymal stem cells (MSCs) were successfully isolated from several tissue sources such as bone marrow, adipose tissue, dental pulp, umbilical cord blood, amniotic membrane and considered as promising candidates for therapy to regenerate and repair the degenerated retinal cells in several retinal degenerative disorders.
Some of the significant properties of MSCs, which contributed for the engagement of these cells as a treatment in RP, include the paracrine factors secreted by the cells, the exosomes and mitochondrial transfer into host cells.
Paracrine neuroprotective factors
The secretome of bone marrow derived mesenchymal stem cells (BMSCs) contain an array of neurotrophic factors (NTFs) such as ciliary neurotrophic factor (CNTF), BDNF, glial cell derived neurotrophic factor (GDNF), platelet derived growth factor (PDGF), nerve growth factor (NGF), neurotrophin-3, 4/5 (NT-3, 4/5), insulin-like growth factor 1 (IGF1), basic Fibroblast growth factor (FGF2), PEDF and erythropoietin (EPO). The neurotrophic factors secreted by BMSCs, bind to their cognate receptors on the recipient cells and enhance the neural cell survival, differentiation, axonal outgrowth, neural cell attachment and inhibit neural cell apoptosis. The signaling pathways activated by the NTFs, such as P13K/AKT, P13K/IAP, PLC/IP3/PKC, MAPK/ERK and JAK/STAT3 have neuroprotective effect on the neuro-retinal cells [4]. The neuroprotective role was demonstrated in an ex vivo study by Cui et al, where co-culturing BMSCs with retinal ganglion cells (RGCs) reduced hydrogen peroxide (H2O2) induced injury in RGCs through the expression of neurotrophins, BDNF, CNTF and reduced the expression of pro-inflammatory factors interleukin 1β (IL1β) and tumor necrosis factor α (TNFα) by RGCs [5]. Moreover, Osborne et al and Johnson et al found that PDGF secreted by BMSCs protected RGCs in an ex vivo and preclinical models respectively [6]. Mead et al proposed that NGF, BDNF and NT-3 secreted by BMSCs have protective effects on RGCs and this neuroprotective effect induced by BMSCs was ablated when tropomyosin related kinase (TrK) and PDGF receptor α (PDGFRα) were inhibited on RGCs [7]. Intravitreal transplantation of GDNF and BDNF secreting BMSCs resulted in higher number of RGCs compared to the control group in an experimental optic nerve crush model. Similarly, long-term neuroprotection and axon regeneration of RGCs was observed after transplantation of BMSCs, which was attributed to an increased expression of FGF2 and IL1β in the RGC layer that activated the PI3/AKT signaling cascade and rescued RGCs. Martin et al found a significant increase in neuroprotective (Dll4, Crim-1, Glupican-3, Cntn1), anti-inflammatory (Transforming Growth Factor β and IL10, 13, 11, 4) molecules as well as proteins associated with anti-oxidant (haptoglobin), anti-apoptotic (Apex1) activity and protein homeostasis (Hsp10, Hsp60, Hsp70, Hsp20, Hsp27, Kctd10, Pyk2, clusterin) in the secretome of human BMSCs co-cultured with neuroretinal explants [8].
MSCs derived extracellular vesicles (MSC-EVs)
MSC-EVs or exosomes are secreted, bilipid layered, nano dimensional micro vesicles which encapsulates functional molecules such as proteins, lipids, miRNAs and can provide important therapeutic effects. MSC-EVs were found to be endocytosed by retinal neurons, microglia and RGCs via caveolar mediated endocytic pathway, facilitated by heparin sulfate proteoglycans (HSPGs). Furthermore, the endocytosis of MSC-EVs took place in a dose, temperature dependent manner and saturable interaction of MSC-EVs with proteins of the vitreous humor was responsible for prolonged retention of EVs in the eye. Yu and co-workers showed that intravitreally injected MSC-EVs were as efficient as transplanted MSCs in reducing damage and apoptosis in addition to improving vision in an experimental model of retinal laser injury. Moreover, MSC-EVs ameliorated retinal damage by downregulating the expression of pro-inflammatory mediators, intercellular adhesion molecule 1 (ICAM1), monocyte chemoattractant protein 1 (MCP1), TNFαand VEGF-A [9].
MSCs dampen inflammatory responses
The ability of the eye to prevent intraocular inflammation in order to protect the visual elements from damage and thus, conserving visual acuity, is defined as ocular immune privilege. This highly complex phenomenon is maintained by the blood-retinal barrier (BRB) which efficiently separates the eye from the immune system along with local inhibition of both innate and adaptive immune responses by the ocular microenvironment, and ocular-specific mechanisms cause systemic activation of immunosuppressive regulatory T cells. However, pathological conditions such as RP, age-related macular degeneration (AMD), glaucoma and diabetic retinopathy (DR), arecharacterized by an abundance of proinflammatory cytokines in addition to infiltration of immune cells leading to breakage of the BRB. Studies have shown that intravitreal and periorbital administration of BMSCs resulted in significant reduction of inflammatory cytokines in the retinal microenvironment, infiltration of macrophages and CD4+ T cells [10].
MSCs modulate angiogenesis
Pathological retinal angiogenesis, unlike vasculogenesis and physiological angiogenesis, leads to disorderliness and creates physiologically deficient blood vessels that disrupt the neuronal histology. These newly formed blood vessels intrude into the outer retina and the macular pit, where absence of vascularity is essential for human vision [4]. Many retinal pathological conditions lead to aberrant angiogenesis with progressive loss of vision.
Kim et al., reported that intraperitoneal injection of human placental amniotic membrane derived MSCs (AMSCs) in a mouse model of oxygen induced retinopathy resulted in significant abrogation of neovascularization through TGFβ1 expression, which was blocked when AMSCs were transfected with TGFβ1 siRNA [11]. Ghazaryan et al., reported that sub-conjunctival injection of BMSCs encouraged corneal wound healing and significantly reduced the neovascularization by downregulating VEGF and matrix metalloproteinase-9 (MMP-9) expression [12]. When murine ADSCs were intravitreally administered in a diabetic mouse model, although the intraocular levels of VEGF and PDGF was unaffected, the expression levels of TSP1 increased significantly [13]. TSP1, primarily produced by RPE, choroid and mÜller glial cells in the healthy eye prevents VEGF receptor 2 (VEGFR2) activation by disrupting the receptor’s association with CD47 and terminates the VEGF signaling to AKT- endothelial nitric oxide synthase pathway [14,15]. TSP1 also binds to CD36 and recruits Src homology 2 domain- containing protein tyrosine phosphatase (SHP1) to the CD36-VEGFR2 complex in the microvascular endothelial cells, which in turn dephosphorylates VEGFR2 and inhibits angiogenesis [16]. Several studies have suggested that the successful reconstruction of damaged ocular tissues by MSCs was more dependent on the release of paracrine anti-inflammatory and anti-angiogenic factors than differentiation into ocular cells. Thus, when human BMSCs were intravitreally implanted in an oxygen induced retinopathy mouse model, it significantly reduced retinal neovascularization.
MSCs donate mitochondria
Several studies have reported that MSCs transfer healthy, functional mitochondria via tunneling nanotubes (TNTs) [17], gap junctions [18] and exosomes [19,20] to the damaged cells for its regeneration [21]. Numerous studies have demonstrated enhancement of mitochondrial bioenergetics by MSCs in the injured cells in spinal cord [22], bronchial epithelia [23], corneal epithelia [24], cardiomyocytes [25,26] and cells affected by neurotoxicity [27,28]. When MSCs are injected intravitreally, although they do not pass through inner limiting membrane (ILM) of the retina, the mitochondria donated efficiently permeate the ILM, limiting the RGC death.
MSCs differentiate into retinal cells
Amniotic membrane derived mesenchymal stem cells (AMSCs), Bone marrow derived mesenchymal stem cells (BMSCs), Dental pulp derived mesenchymal stem cells (DPSCs) and Umbilical cord blood derived mesenchymal stem cells (UMSCs) have been found to efficiently differentiate into various cells of retinal lineages in vitro and express genes related to retinal cells. Some studies also tested the functionality of the differentiated cells in in vitro systems. Autologous MSC transplantation could be a promising strategy for cell replacement therapy in retinal diseases, however; further preclinical studies are required to understand the safety, immunogenicity and function of the transplanted cells in vivo [4].
Treatments In RP
The degeneration of photoreceptors in RP is usually associated with gene mutations. Until date, ~4500 mutations have been discovered in 70 genes involved in the causation of RP. RP is linked with Usher Syndrome, Bardet-Biedl Syndrome, and can also exist as non-syndromic RP. Pathogenesis of autosomal recessive RP is due to mutation in genes involved in photo-transduction pathway like cGMP phosphodiesterase (PDE6), and intra-ocular delivery of recombinant Adeno-associated virus (rAAV) containing corrected PDE6 gene, led to disease remission in mouse disease models. Since, mutation in Mer receptor tyrosine kinase (MERTK) is also known to play a role in autosomal recessive RP, gene replacement through AAV vector have resulted in improvement in retinal function in RP models as well as human patients. Gene therapy tested for autosomal dominant RP include rAAV carrying ribozymes designed to specifically inhibit mRNA of defective rhodopsin gene. Positive outcomes were reported in X-linked RP (XLRP), caused by mutant retinitis pigmentosa GTPase regulator (RPGR) when treated with AAV8 vectors expressing normal RPGR gene. The first in vivo gene therapy to be approved by Food and Drug Administration (FDA) for RP is, Luxturna, a AAV2 virus carrying the complementary DNA (cDNA) of the gene RPE65, whose biallelic mutation causes recessive RP. FDA also approved transplantation of an artificial retina, resulting in recovery of vision in late-stage RP patients. RP patient-derived iPSCs corrected for mutations in Pro23His variant of rhodopsin (RHO) gene and homozygous Alu insertion in exon 9 of male germ cell-associated kinase (MAK) gene by CRISAP/Cas9 mediated gene editing was proposed for autologous retinal cell replacement. Correction of RPGR gene by CRISPR/Cas9 gene editing also resulted in repair of defective photoreceptors and ciliopathy in the patient iPSC-derived organoids [4].
Gene therapy holds great potential, but it is currently at an experimental stage and has obtained only limited therapeutic results in vivo. Consequently, scientific interest is particularly directed at restoratory therapy based on stem cells. The latter aims to recover cell density as well as to preserve the remaining retinal cells by improving intra/extracellular conditions [2].
Recently, the Limoli retinal restoration technique (LRRT) has been developed as a potential therapy for currently untreatable retinal disorders. This surgical technique is a variant of Pelaez’s intervention, wherein only orbital autologous fat is transplanted in the subscleral space. The technique exploits the use of GFs to create an environment conducive to the neuroenhancement of still functioning retina. The source of autologous GFs in LRRT is an implant of certain cell types of mesenchymal origin, such as adipose stromal cells, adipose tissue-derived stem cells (ADSCs) contained in the stromal vascular fraction of adipose tissue, and platelets (PLTs) obtained from PLT-rich plasma (PRP) prepared from fresh whole blood by centrifugation [3] (Figure 1).
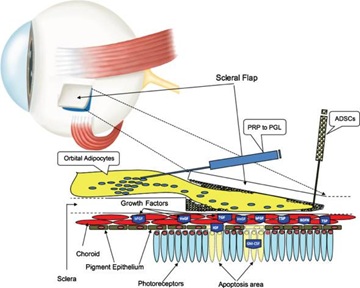
Figure 1: The suprachoroidal autograft obtained by Limoli Retinal Restoration Technique (LRRT) allows placing fat stromal cells, adipose tissue-derived stem cells (ADSCs) and platelets (PLTs) obtained from PLT-rich plasma (PRP) close to the choroid. The production of growth factors (GF), characteristic of these cells, is poured directly into the choroidal flow, helping to maintain retinal cell trophism. The GFs, through the choroidal flow, have a direct action on the choroid, on the Müller cells, on the retinal pigment epithelium (RPE) cells with improvement of physiology of the outer segments (OS), on the rods and on the cones.
Clinical Trials With MSCs For RP
The encouraging outcomes seen with injecting MSCs in animal models of retinal degeneration led to initiation of several clinical trials. Whilst most trials are ongoing (Table 1), outcomes of some of the phase I trials are discussed below.
|
Condition |
Cell Type |
Route of administration |
Dosage |
Number of patients enrolled |
Recruitment Status |
Phase of Study |
Clinical Trial
|
Start Date |
Actual/ Estimated Completion Date |
|
|
RP |
Allogenic WJMSCs |
Intravitreal |
2-6x106 cells/1,5ml |
32 |
Completed and published [1] |
III |
NCT04224207
|
April, 2019 |
January, 2020 |
|
|
RP |
UMSCs |
Peribulbar |
1x106 cells/1,8ml |
18 |
Completed and Published[29] |
I/II |
NCT04315025
|
October, 2018 |
September, 2019 |
|
|
RP |
Autologous BMSCs |
Intravitreal |
1x106 cells/0,1ml |
10 |
Enrolling by invitation |
I |
NCT01531348
|
February, 2012 |
December, 2020 |
|
|
RP, AMD, DR, VO, HRD |
Autologous BMSCs |
Intravitreal |
3,4x106 cells/0,1ml |
15 |
Enrolling by invitation[30] |
I |
NCT01736059 |
July, 2012 |
January, 2022 |
|
|
RP |
Autologous BMSCs |
Intravitreal |
- |
50 |
Active, not recruiting |
I/II |
NCT02709876 |
April, 2014 |
March, 2021 |
|
|
RP, RD, AMD, SD |
Autologous BMSCs |
Intravitreal |
- |
30 |
Enrolling by invitation |
I |
NCT03772938
|
December, 2018 |
March, 2020 |
|
|
RP, AMD, SD, ON, OA, OND, RA, VLP. VLN, Maculopathy, Glaucoma |
Autologous BMSCs |
Retrobulbar, Subtenon, Intravitrea, Subretinal, Intravenous |
- |
500 |
Recruiting |
II |
NCT03011541 |
January, 2016 |
January, 2023 |
|
Table 1: Some of the phase I trials. RP - Retinitis Pigmentosa, VO - Vein occlusions, HRD - Hereditary retinal disease, SD - Stargardt’s disease, ON - Optic Neuropathy, OND - Optic Nerve Disease, OA - Optic Atrophy, RA - Retina Atrophy, VLN - Vision Loss Night, VLP - Vision Loss Partial.
Weiss and Levy conducted a SCOTS (Stem cell ophthalmology treatment study) clinical trial in 17 patients suffering from bilateral vision loss due to progressive RP with autologous BMSCs transplantation. A 6 months follow up found an improvement in visual acuity in 11 out of 17 patients (64.7%), 8 patients (35.3%) exhibited stability in their condition and none experienced vision loss. This study also found that the ability of the eyes to respond to cell therapy was irrespective of the duration of the disease [31].
However, Satarian et al., reported that intravitreal injection of autologous BMSCs in three patients suffering from advanced RP, resulted in improvement in visual acuity in only two of the patients whereas the third patient developed severe and progressive adverse effects. The patient developed vitreal and pre-retinal fibrosis two weeks after transplantation which led to total tractional retinal detachment at the end of the three-month follow-up period [32].
Özmert and Arslan recently reported the result of an open label, phase III clinical trial (NCT04224207) with WJMSCs. In this study, WJMSCs were implanted in the sub-tenon space in 32 patients diagnosed with RP. In the 6 month follow up period, a significant improvement in mean BCVA, outer retinal thickness values, mf-ERG results and decrease in the visual field mean deviation value was observed. The authors did not observe any severe ophthalmic or systemic complication, thus, assuring its safety [1]. Mangunsong et al., tested the safety and efficacy of peribulbar infusion of UMSCs in a prospective, multi-center, randomized clinical study (NCT04315025) involving 18 individuals suffering from RP. An improvement in light perception and visus was observed one week after the treatment and no serious side effects was seen during that period [29].
Conclusion
LRRT cell therapy has been demonstrated to have a notable impact on certain functional parameters after the interaction with residual cells. The direct contact of the autograft with the choroid enhances the incretion of the bioactive factors produced by mesenchymal cells into the choroidal flow and therefore promotes a widespread dissemination through the retinal tissue, finally exuding in vitreous body [2].
To increase the safety and efficacy of stem/progenitor cell-based transplantation therapy, several unsolved issues and corresponding strategies need to be resolved:
- Currently, research on stem cell-derived exosome-based strategies for biomedical applications is still in its infancy. One of the main weaknesses of nanovesicle exosomes is their rapid clearance from tissues or organs. Clearance should be verified in ophthalmology after topical, intravitreal or subconjunctival applications to determine how to overcome this issue. On the other hand, exosomes as a sustained delivery platform greatly rely on the generation of vesicles of consistently high purity and quality on a large scale, which is another challenge. Hence, more methods and technologies for exosome-based systems should be developed to generate the next stem cell-derived exosome nanomedicine for retinal diseases (RD) management.
- The proliferation and differentiation mechanisms of stem/progenitor cells still require better understanding. Thus, besides clinical trials, more basic experiments need to be conducted for a deeper comprehension of the mechanism, which will facilitate the application of progenitor/stem cell-based transplantation therapy in more RD patients as early as possible.
- Potential tumorigenicity of stem cells and immune rejection caused by exogenous transplantation strategies does not meet the clinical safety threshold. Fortunately, fundamental experiments have shown that pre-induction of ESCs into neural progenitors before transplantation may reduce tumorigenicity, providing a feasible solution to this problem. Meanwhile, immunosuppressive therapies also evolve, and new techniques such as the xeno-free techniques are being developed to reduce the immune response. Thus, the issue of immune rejection is expected to be addressed in the future.
- Ethical issues of stem cell transplantation need to be resolved. On one hand, iPSC generation does not present the same ethical concerns as ESC harvesting. On the other hand, iPSC-based strategy is a completely new field and remains in its infancy; experts will reach an ethical consensus over time.
- Most of the current clinical trials are in the early I/IIa phases. Thus, there is still a long way to go before their findings can be applied to clinical practice.
All in all, with deepening research, stem/progenitor cell-based transplantation will be an essential treatment used in the clinic that will bring new hope to RD patients through the joint efforts of doctors and researchers [33].
References
- Özmert E, Arslan U (2020) Management of retinitis pigmentosa by Wharton’s jelly derived mesenchymal stem cells: preliminary clinical results. Stem Cell Res Ther 11: 25.
- Limoli PG, Limoli CSS, Morales MU, Vingolo EM (2020) Mesenchymal stem cell surgery, rescue and regeneration in retinitis pigmentosa: clinical and rehabilitative prognostic aspects. Restor Neurol Neurosci 38: 223-237.
- Limoli PG, Vingolo EM, Limoli C, Nebbioso M (2019) Stem Cell Surgery and Growth Factors in Retinitis Pigmentosa Patients: Pilot Study after Literature Review. Biomedicines 7: 94.
- Adak A, Magdalene D, Deshmukh S, Das D, Jaganathan BG (2021) A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem Cell Reviews and Reports 2021.
- Cui Y, Xu N, Xu W, Xu G (2017) Mesenchymal stem cells attenuate hydrogen peroxide-induced oxidative stress and enhance neuroprotective effects in retinal ganglion cells. In Vitro Cell Dev Biol Anim 53: 328-335.
- Osborne A, Sanderson J, Martin KR (2018) Neuroprotective Effects of Human Mesenchymal Stem Cells and Platelet-Derived Growth Factor on Human Retinal Ganglion Cells: Stem Cell Protection and the Human Retina. Stem Cells 36: 65-78.
- Mead B, Berry M, Logan A, Scott RAH, Leadbeater W, et al. (2015) Stem cell treatment of degenerative eye disease. Stem Cell Res 14: 243-257.
- Usategui-Martín R, Puertas-Neyra H, García-Gutiérrez M-T, Fuentes M, Pastor JC, et al. (2020) Human Mesenchymal Stem Cell Secretome Exhibits a Neuroprotective Effect over In Vitro Retinal Photoreceptor Degeneration. Molecular Therapy - Methods & Clinical Development 17: 1155-1166.
- Yu B, Shao H, Su C, Jiang Y, Chen X, et al. (2016) Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Scientific Reports 6: 34562.
- Hermankova B, Kossl J, Bohacova P, Javorkova E, Hajkova M, et al. (2019) The Immunomodulatory Potential of Mesenchymal Stem Cells in a Retinal Inflammatory Environment. Stem Cell Reviews and Reports 15: 880-891.
- Kim K-S, Park JM, Kong TH, Kim C, Bae S-H, et al. (2016) Retinal Angiogenesis Effects of TGF-β1 and Paracrine Factors Secreted from Human Placental Stem Cells in Response to a Pathological Environment. Cell Transplant 25: 1145-1157.
- Ghazaryan E, Zhang Y, He Y, Liu X, Li Y et al. (2016) Mesenchymal stem cells in corneal neovascularization: Comparison of different application routes. Mol Med Rep 14: 3104-3112.
- Ezquer M, Urzua CA, Montecino S, Leal K, Conget P, et al. (2016) Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res Ther 7: 42.
- Bazzazi H, Zhang Y, Jafarnejad M, Isenberg JS, Annex BH, et al. (2018) Computer Simulation of TSP1 Inhibition of VEGF–Akt–eNOS: An Angiogenesis Triple Threat. Front Physiol 9: 644.
- Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, et al. Thrombospondin-1 Inhibits VEGF Receptor-2 Signaling by Disrupting Its Association with CD47. J Biol Chem 285: 38923-38932.
- Chu LY, Ramakrishnan DP, Silverstein RL (2013) Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood 122: 1822-1832.
- Feng Y, Zhu R, Shen J, Wu JM, Lu W, et al. (2019) Human Bone Marrow Mesenchymal Stem Cells Rescue Endothelial Cells Experiencing Chemotherapy Stress by Mitochondrial Transfer Via Tunneling Nanotubes. Stem Cells Dev 28: 674-682.
- Sinclair KA, Yerkovich ST, Hopkins PMA, Chambers DC (2016) Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res Ther 7: 91.
- Monsel A, Zhu Y, Gennai S, Hao Q, Hu S, et al. (2015) Therapeutic Effects of Human Mesenchymal Stem Cell–derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med 192: 324-336.
- Phinney DJ, Giuseppe MD, Njah J, Sala E, Shiva S, et al. (2015) Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 6: 8472.
- Li C, Cheung MKH, Han S, Zhang Z, Chen L, et al. (2019) Mesenchymal stem cells and their mitochondrial transfer: a double-edged sword. Biosci Rep 39: BSR20182417.
- Li H, Wang C, He T, Zhao T, Chen Y-Y, et al. (2019) Mitochondrial Transfer from Bone Marrow Mesenchymal Stem Cells to Motor Neurons in Spinal Cord Injury Rats via Gap Junction. Theranostics 9: 2017-2035.
- Ahmad T, Mukherjee S, Pattnaik BR, Kumar M, Singh S, et al. (2013) Miro 1 Knockdown in Stem Cells Inhibits Mitochondrial Donation Mediated Rescue of Bronchial Epithelial Injury. Biophysical Journal 104: 659.
- Jiang D, Gao F, Zhang Y, Wong DSH, Li Q, et al. (2016) Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis 7: e2467-e2467.
- Pacak CA, Preble JM, Kondo H, Seibel P, Levitsky S, et al. (2015) Actin-dependent mitochondrial internalization in cardiomyocytes: Evidence for rescue of mitochondrial function. Biol Open 4: 622-626.
- Plotnikov EY, Khryapenkova TG, Vasileva AK, Marey MV, Galkina SI, et al. (2008) Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J Cell Mol Med 12: 1622-1631.
- Babenko VA, Silachev DN, Popkov VA, Zorova LD, Pevzner IB, et al. (2018) Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery. Molecules 23: 687.
- Boukelmoune N, Chiu GS, Kavelaars A, Heijnen CJ (2018) Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathologica Communications 6: 139.
- Mangunsong C, Putera B, Haifa R, Suwandjaja M, Shirana A, et al. (2019) Safety issues of peribulbar injection of umbilical cord mesenchymal stem cell (UC-MSC) in patients with retinitis pigmentosa. Cytotherapy 21: 83.
- Park SS, Bauer G, Abedi M, Pontow S, Panorgias A, et al. (2014) Intravitreal Autologous Bone Marrow CD34+ Cell Therapy for Ischemic and Degenerative Retinal Disorders: Preliminary Phase 1 Clinical Trial Findings. Invest Ophthalmol Vis Sci 56: 81-89.
- Weiss JN, Levy S (2018) Stem Cell Ophthalmology Treatment Study: bone marrow derived stem cells in the treatment of Retinitis Pigmentosa. Stem Cell Investig 5: 18.
- Satarian L, Nourinia R, Safi S, Kanavi MR, Jarughi N, et al. (2017) Intravitreal injection of bone marrow mesenchymal stem cells in patients with advanced retinitis pigmentosa; a safety study. J Ophthalmic Vis Res 12: 58-64.
- Wang Y, Tang Z, Gu P (2020) Stem/progenitor cell-based transplantation for retinal degeneration: a review of clinical trials. Cell Death Dis 11: 793.
Citation: Florido A, Vingolo EM, Limoli P, Contento L (2021) Mesenchymal Stem Cells for Treatment of Retinitis Pigmentosa: Short Review. J Stem Cell Res Dev Ther 7: 066.
Copyright: © 2021 Antonio Florido, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

