
Mesenchymal Stromal Cells and Micro Fragmented Adipose Tissue: New Horizons of Effectiveness of Lipogems
*Corresponding Author(s):
Carlo TremoladaImage Regenerative Clinic, Via Pietro Mascagni 14, 20122 Milan, Italy
Email:carlo.tremolada@gmail.com
Abstract
In the last three years there has been an explosion of published in vivo and clinical data discussing micro fragmented adipose tissue and its regenerative properties. In fact, the paracrine activity of micro fragmented adipose tissue has been well described, showing the importance of the maintenance of an intact microvascular environment of fat tissue during the processing in order to guarantee cellular activation and long-term viability there by maintaining their signaling capacity in order to effectively modulate inflammation and immune response.
Lipogems®, patented in 2010 and clinically available since 2013, is a novel technology that reproducibly and simply obtains, micro fragmented adipose tissue with enhanced regenerative properties with somewhat astonishing results that are now confirmed from recent clinical trials.
In this review we have summarized all the new evidence published, focusing on possible new clinical indications from intriguing new in vivo studies.
Keywords
Lipogems®; Medicinal signaling cells; Mesenchymal stromal cells; Micro fragmented adipose tissue; Pain; Plastic surgery; Regenerative medicine
INTRODUCTION
Since its first clinical use in 1995 [1], we have seen an exponential increase of the literature pertaining to Mesenchymal Stromal Cells (MSCs) during the last twenty years. Their activity has been deeply characterized in several articles [2] both in humans and in animals [3]. It has been also proven in vitro that MSCs can differentiate into different adult cells according to the environment in which they have been injected [1].
MSCs have been harvested from several different tissues [4,5] (and using a variety of different methods), even though the most easily accessible site is from adipose tissue [6,7]. Importantly, MSCs derived from either/any site have common biological characteristics, with some biological differences in vitro but no discernable differences in activity or behavior in clinical practice [8]. The most promising source of MSCs, adipose tissue, presents a significantly higher concentration of MSCs compared to bone marrow (1% versus >0.01%), and also other sources such as umbilical cord, dental pulp or menstrual blood [9]. Furthermore harvesting from adipose tissue is less invasive than bone marrow with less risk of severe complications.
Considering the multipotent properties of MSCs derived from adipose tissue [10-16], they have been proposed as potential reparative and regenerative candidates for the treatment of diseases where an angiogenic, anti-inflammatory, anti-fibrotic or anti-apoptotic mechanism was a critical component needed to treat the pathophysiology of the disease (covers most major disease processes potentially).
A recent review [17] analyzed all of the currently available methods of isolation of MSCs from adipose tissue and there in vitro and in vivo applications. Autologous adipose tissue has been used as intact lipo-aspirate, enzymatically derived Stromal Vascular Fraction (SVF) or Micro Fragmented Fat (MFAT). Vezzani et al., [18] has demonstrated that the “regenerative properties” of these three methods are quite different; with the MFAT preparations being significantly more productive in the release of grow factor and cytokines concomitant with notably superior effects on repairing tissues, induction and modification of immunomodulatory activity and in supporting vascular angiogenesis.
These results may be attributed to the importance of maintaining the perivascular environment/niche, with MSCs being a critical component of this and of perivascular origin. The perycites and other perivascular cells have a key role in the regenerative medicine as they can be reprogrammed into “regenerative cells” that act as immunomodulatory and anti- inflammatory cells to restore and repair damaged tissue [19]. Pericytes are multi-potent structural cells localized within the vascular basement membrane and present on all parts of the vascular system or ‘stromal vascular fraction’ of adipose tissue. Fascinatingly, when activated during tissue damage or inflammation, they transform into MSCs with the capability to create a pro-regenerative environment and have the ability to differentiate into mesodermal lineage terminal cells [20].
The literature also provides evidence that tissue or cellular treatments beyond a ‘minimal’ mechanical manipulation of adipose tissue (for example adipose fat or other derived MSC containing cell mixture), such as enzymatic dissociation, leads to a different gene expression pattern [21] and exosome content of the MSCs [22]. Currently, researchers are studying the cytokine retention and delivery capacity and safety profile of exosomes derived from MSCs (MEX) [23,24]. Even though the MEX regulatory properties are well demonstrated, the most important being that their activity is highly variable depending upon the tissue they are isolated from-making tissue sourcing an important consideration before extraction, banking or use.
A more detailed characterization of MEX will be of high value for further research in the field of regenerative medicine and in the future development of MSC-based drug delivery protocols [24].
One example where MSCs derived from adipose tissues have been shown to be effective is in the treatment of complex-non-healing wounds. In vivo studies in mice demonstrated a significantly better healing response and final outcome compared with controls treated using a standard protocol [25]. As with all the experimental data, large differences in the experimental findings are seen when comparing the capability of MSCs derived from different sources. Hence, it is important to adopt, both in experimental models and in a clinical settings, a fully standardized Standard Operating Procedures (SOPs) to harvest and to manipulate adipose tissue that will minimize the damage to the perivascular environment and maximize the regenerative effect concomitantly reducing the risk of contamination [26]. Lipogems® technology is a closed sterile system that allows the collection of MSCs from MFAT without contamination.
In this review we present an update of previously published data demonstrating a clear rationale, based on in vivo results and clinical studies produced using this technology and justifying it as an important tool for regenerative medicine [27].
LIPOGEMS®: TECHNOLOGY AND NEW INSIGHTS IN ITS ACTIVITY
Lipogems® is a new device that efficiently harvests, processes and transfers adipose tissue with unique characteristics including long-term expression and secretion of physiologically active concentrations of angiogenic, immunomodulatory, anti-inflammatory, anti-apoptotic and anti-fibrotic secretome [25].
The resulting Lipogems product is composed of small specifically sized ‘adipose’ clusters of adipocytes maintaining an intact perivascular environment and a pericytes’ activation through ball-bearing-induced mechanical shock. Lipogems®, creates a minimally manipulated fat-derived product according to and in line with the regulations set forth by US Food and Drug Administration (FDA).
It has received FDA clearance as a class II medical device for processing of autologous adipose tissue. According FDA we could defined Lipogems® as (1) autologous; (2) minimally manipulated; (3) intended for homologous use; (4) enzyme-free; (5) not dependent on the metabolic activity of the cells for its primary function; (6) used in the same surgical procedure and (7) not combined with anything other than saline.
The harvest technique has been already described in several other articles [27,28], and here it will be described briefly.
The procedure is divided into three phases:
- The first phase is the harvest and process of a small quantity of fat tissue after local anesthesia and Klein solution subcutaneous fat tissue injection (mini-liposuction). Klein solution is a tumescent anesthesia that uses large volumes of saline with local anesthetic (usually lidocaine at 0.05-0.1% concentration) and epinephrine (1:500.000-1:1.000.000)
- The second phase is carried out (Figure 1) in a closed full immersion low pressure cylindrical system in order to obtain two parts: a micro fragmented fat tissue (fat tissue clusters of 0.3-0.7 mm) and a fluid containing oil, all the excreted proinflammatory cytokines and dead/dying or apoptotic cells, that are subsequently eliminated. The system obtains the micro-fragmented fat only through mechanical forces without any disruption of the integrity of the fat tissue and/or stromal vascular fraction and with “activation” of pericytes and within the microvascular environment of MSCs [29].
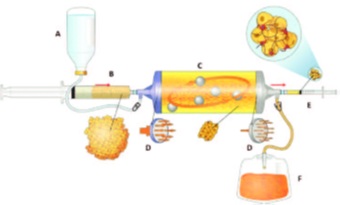 Figure 1: Lipogems device is a complete closed system filled with physiologic solution. Figure shows how the lipoaspirate is reduce in micro clusters after washing of oil, blood and cellular debris. A, Sac with physiologic solution; B, Syringe with lipoaspirate clusters; C, Washing chamber containing marbles for the emulsion of fluid and elimination of oil and blood against gravity; D, Mechanical filters; E, Syringe with clusters of reduced size; F, Sac with waste oil and blood [30].
Figure 1: Lipogems device is a complete closed system filled with physiologic solution. Figure shows how the lipoaspirate is reduce in micro clusters after washing of oil, blood and cellular debris. A, Sac with physiologic solution; B, Syringe with lipoaspirate clusters; C, Washing chamber containing marbles for the emulsion of fluid and elimination of oil and blood against gravity; D, Mechanical filters; E, Syringe with clusters of reduced size; F, Sac with waste oil and blood [30].
- In the third phase milliliter quantities of Lipogems® are injected into the area to be treated, resulting in a transplant of MFAT containing a complex spectrum of bioactive molecules. As previously described, Lipogems® has a significantly higher content and concentration of MSCs and exosomes compared to other fat-derived products including those derived from enzymatic methods [as there is no any digestions of extracellular matrix, the exosomes are not altered and the adipose structural niche is not damaged, but reduced in its size increasing the surface area of the active tissue] [22,31-35].
The whole process is performed in less than twenty minutes in a sterile environment and with minimal manipulation.
Through this autologous adipose tissue graft we have created a fast, reproducible and regulatory compliant technology that effectively supports the natural healing of joints or tissues, through its capacity to cushion, lubricate and 'stick' or remain viable in the area of interest for significant length of time via a self-regenerative mechanism.
In addition as already described in our previous review [27], MFAT has been demonstrated to be effective as a “regenerative drug” due to its content of mesenchymal stromal cells and high paracrine activity.
Interestingly, very recently, Nava et al., demonstrated that micro-fragmented fat tissue displayed not only a higher, but also a longer antinflammatory activity compared to normal fat tissue graft [36]. In fact, its anti-inflammatory activity persists for more than 1 month as defined by measurement of paracrine activity and its ability to inhibit monocyte and macrophage migration.
Probably, this phenomenon relates to the long term survival of MSCs within the in vivo environment where they are injected, due to the optimized Lipogems® adipo-clusters functioning to stabilize their survival and allowing targeted therapeutic responses following mechanical activation of bound pericytes following Lipogems extraction [37].
These results underline that micro-fragmented fat could represent not only a regenerative technique, but an autologous long-lasting drug able to modulate chronic inflammation in clinical conditions in which inflammation can be difficult to control, such as chronic joint inflammation or chronic systemic inflammation (see last chapter).
CLINICAL APPLICATIONS OF MICRO FRAGMENTED ADIPOSE TISSUE
In this chapter we will summarize the diseases where micro-fragmented fat tissue has demonstrated its effectiveness in clinical practice. In fact, in several clinical applications there a growing interest has been shown in the literature regarding clinical effectiveness and safety over the last decade [27].
Plastic surgery
Initially, MFAT has been used for plastic surgery not only for lipofilling but also associated with traditional aesthetic surgery where a fat tissue graft is needed, such as blepharoplasty, facelift or breast augmentation. In this setting Lipogems® demonstrated not only an “augmentation” activity, but also a real anti-inflammatory activity with better short- (reduced postoperative pain and swelling) and long-term results [27].
In a randomized controlled trial [38] the effectiveness of micro-fragmented fat tissue versus conventional treatment has been investigated in a series of patients destined for diabetic foot amputation. At 6 months, 80%, of the group treated with Lipogems MFAT healed compared to 46% of those in the group of conventional treatment. This randomized controlled trial underlined the potential wound healing and tissue regenerative properties of MFAT injection.
Lipogems is also frequently used also for treatment of ulcers and systemic sclerosis, -as have other types of fat tissue graft [39,40], but with significantly better outcomes and possible indications also for the treatment of chronic non-healing wounds as recently demonstrated [41]. These authors demonstrated, using an in vitro model, the pathophysiological basis that defines how micro-fragmented fat tissue, such as Lipogems®, is effective in treating chronic non-healing wounds, identifying a vascular stabilization activity (with inhibition of endothelial expression) and negative modulation of macrophage migration.
General and urological gynecological surgery
MFAT was demonstrated to be effective after a single injection in a case series of 15 patients with perianal fistulas with Crohn’s disease [42]. Patients were treated with a single local injection of MFAT. After 6 weeks 10 patients showed a complete clinical and instrumental remission, 4 patients slightly improved and only 1 patient did not have any improvement.
In addition, effective relief of symptoms has also been reported in case series of treatment for both anal [42,43] and urinary [44] incontinence.
Lipogems® has also been successfully demonstrated to be effective in the treatment of postmenopausal genitourinary atrophy [45] in three patients who remain symptom free and showing both clinical and histopathological complete recovery for three years. Biopsy of the area demonstrated a completely normal looking epithelial mucosa consistent with a premenopausal condition. The authors are currently confirming this data in a larger cohort of women (submitted paper).
Maxillofacial surgery
Also in maxilla surgery MFAT has been found effective in regenerative bone atrophy [46] and in 120 patients with double-jaw procedures where soft tissue had to be restored [47]. In these patients micro fragmented fat tissue demonstrated to produce less local inflammatory reaction compared to usual fat tissue graft.
Orthopedic surgery
In 2019 a group of researchers [48] used rabbit model osteoarthritis, to investigate the pattern of cell migration of three different injected fat-derived materials: expanded-Adipose Stromal Cells (ASCs) and adipose niches after enzymatic (Stromal Vascular Fraction - SVF) and mechanical processes (Micro Fragmented Fat Tissue - MFAT). They found a significantly improved long-term (30 days) migration of MFAT toward cartilage while the cells prepared using the other two methods migrated toward the synovia. Furthermore, Paolella et al., [49] demonstrated that freshly prepared MFAT had a greater effect in reducing inflammation also in synovial tissue compared to the adipose-derived mesenchymal stromal cells obtained from the same samples of MFAT.
As already underlined in our previous review, Lipogems® is currently used within the clinic in several orthopedic contraindications, where intra-articular injections are used to treat knee, hip and shoulder osteoarthritic degeneration (more than 35000 patients over the world). Initiated more than three years ago, these results have now been confirmed in several case series [50,51], and prospective clinical studies with follow up [52,53], and the “regenerative” pathophysiological mechanism of Lipogems®, when injected into the joint, have now been more carefully elucidated [54,55]. MFAT has also been demonstrated effective also in four patients with osteochondral lesions of the talus [56] who underwent to arthroscopic injection of MFAT of the osteochondral lesion after a first phase of microperforation of the cartilage. After six months all patients have a recovery of the lesion.
Jannelli E et al., [57], in an interesting position paper, proposed micro-fragmented fat tissue transplantation to treat delamination and first and second-degree chondral lesions also in knee arthroscopic surgery in order to regenerate cartilage and prevent severe grade of osteoarthritis. Schiavone Panni et al., [50] underlined in a recent case series in fifty-two patients also that MFAT treatment as an adjunct to arthroscopic debridement could be very effective also in patient with early knee osteoarthritis. They found a significative improvement after one year both of the pain scores and of the functional activity.
In a retrospective study on 76 patients (and 106 knees) no statistical difference in functional outcome or quality of life was seen between intra articular MFAT and bone marrow aspirate concentrate knee injection in osteoarthritis patients [58]. MFAT was easier and less invasive to harvest compared to bone marrow and with no any side effects.
Finally, there are some ongoing randomized clinical trials [59] that are currently investigating effectiveness of micro-fragmented fat tissue injection compared to other techniques (clinicaltrials.gov NCT03117608, NCT03379168 and NCT03922490).
Recently, MFAT was demonstrated effective also in severe osteoarthritis in dogs. After 6 months 88% of dogs treated have an improvement of osteoarthritis signs and 92% of owners declared a significative improvement of pain and functional activity of their dogs [60]
NEW POSSIBLE INDICATIONS FOR THE FUTURE
In this chapter we will review findings from investigations in animal together with other clinical data from further regenerative techniques that could suggest additional and interesting potential uses for micro-fragmented fat.
Regenerative medicine and axial back pain
Chronic inflammatory flare is also one of the main pathophysiological processes that can cause back pain [61]. Unfortunately, chronic inflammation of the spinal column’s joints (i.e. sacroiliac and facet joint) or of the intervertebral disc is difficult to treat with current drugs and a mechanism-based diagnosis is mandatory [62]. As discussed above, it has been demonstrated that Mesenchymal Stromal Cells (MSCs) contained in lipoaspirate have important anti-inflammatory and immunomodulatory properties, that can last for several months in Micro-Fragmented Fat Tissue (MFAT) and that could therefore potentially be useful for back pain. Furthermore, MFAT in cellular experiments has displayed more anti-inflammatory activity than enzymatically derived stromal vascular fraction as it contains more pericytes that are responsible for modulation of the immune (especially NK cells that are strictly related with chronic pain) and inflammatory response [18]. These results are the basis to justify the use of regenerative medicine for chronic pain not only to “regenerate” cartilage or joint structures but also (and maybe most critically) to modulate inflammation more effectively than current pharmacological agents.
Hence, regenerative medicine in Low Back Pain (LBP) could be used not only for its “regenerative” properties, but also for its long lasting “immunomodulatory and antiinflammatory” properties. This possibility has been confirmed in a recent metanalysis [63] that summarizes all recent clinical trials and observational studies where regenerative medicine has been proved effective in treating back pain for all pain generators (facet joints, sacroiliac joint, discogenic pain). Currently the studies are more about Platelet Rich Plasma (PRP) and not about micro-fragmented fat. However, as recently discussed by American Society of Interventional Pain Physicians (ASIPP) guidelines [64], the level of scientific evidence still remains low, as randomized clinical trials are needed to demonstrate not only the effectiveness but also its effectiveness over time, as some studies have underlined that analgesia can last for several months after a single injection.
Finally, despite the promising results, we need more studies and data on who could benefit from regenerative medicine and who probably will not. Regenerative medicine could hence represent a mechanism-based treatment in many LBP patients with chronic inflammatory pain and it is only a matter of time before we get there.
Promising animal studies
Considering the great potential of immunomodulatory and regenerative activity of microfragmented fat tissue, in the last two years new important animal studies have suggested possible indications that need further exploration in order to attain clinical trial status.
Researchers from the Pasteur Institute [65] have analyzed in a Duchenne muscular dystrophy murine model the effect of micro fragmented fat injected in the muscle. Interestingly they found a reduction of fibrosis and necrosis with concomitant reduction of cytokines productions with final increase in muscle strength.
The same group has also investigated the antinflammatory and immunomodulatory activity of micro fragmented fat, compared not only to placebo but also to lipoaspirate, if administered systemically in a murine sepsis model [66]. They found not only a significant (compared both to placebo and lipoaspirate) reduction of systemic inflammatory response in the presence of micro-fragmented fat tissue but also an important improvement of outcome with reduction in mortality.
Finally, MFAT has been recently studied as a possible interesting new scaffold for drug delivery especially in cancer treatments as a possible vehicle of Paclitaxel to increase chemotherapy effectiveness (delivered directly to the tumor) whilst reducing systemic side effects [67]. The authors demonstrated also that in the drain bag during Lipogems® preparation, it is possible to find single isolated cells that can be expanded and loaded with Paclitaxel to administer chemotherapy [68]
CONCLUSION
This review updates the incredible amount of new scientific data about micro-fragmented fat tissue published in the last three years [27]. In fact evidence that justifies the stronger regenerative effect of micro-fragmented fat tissue is growing exponentially. Furthermore, also the clinical evidence, especially for orthopedic surgery, is increasing with new prospective and randomized clinical trials that are not only confirming previous results but are indicating new possible solutions for diseases that have not at this moment valid therapeutic solutions.
Lipogems® appears to be really promising in this arena and it is already approved by the FDA as an autologous fat tissue graft. More randomized clinical studies are needed but the rational of its paracrine activity could justify its use also in new clinical settings.
Finally, in order to improve even more effectiveness of regenerative medicine, it is important to understand that MSCs have to be considered Medicinal Signaling Cells that could help the modulation of inflammatory flare (even chronic) and of the immune system in order to restore physiological conditions in the area affected [69].
CONFLICTS OF INTEREST
Carlo Tremolada is a founder of Lipogems International SpA. Mark Slevin is a consultant of Lipogems. Massimo Allegri declines any conflict of interest relative this topic.
REFERENCES
- Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI (1995) Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant 16: 557-564.
- Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213: 341-347.
- Lennon D, Solchaga LA, Somoza RA, Schluchter MD, Margevicius S, et al. (2018) Human and Rat Bone Marrow-Derived Mesenchymal Stem Cells Differ in Their Response to Fibroblast Growth Factor and Platelet-Derived Growth Factor. Tissue Eng Part A 24: 1831-1843.
- Caplan AI (1991) Mesenchymal Stem Cells. J Orthop Res 9: 641-650.
- Caplan AI, Bruder SP (2001) Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 7: 259-264.
- Frese L, Dijkman PE, Hoerstrup SP (2016) Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus Med Hemother 43: 268-274.
- Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, et al. (2005) Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54: 132-141.
- Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J (2012) Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 21: 2724-2752.
- Ren H, Sang Y, Zhang F, Liu Z, Qi N, et al. (2016) Comparative analysis of human mesenchymal stem cells from umbilical cord, dental pulp, and menstrual blood as sources for cell therapy. Stem Cells Int 2016: 3516574.
- Fraser JK, Schreiber R, Strem B, Zhu M, Alonso Z, et al. (2006) Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nat Clin Pract Cardiovasc Med 1: 33-37.
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, et al. (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13: 4279-4295.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211-228.
- Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, et al. (2002) Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun 290: 763-769.
- Halvorsen YC, Wilkison WO, Gimble JM (2000) Adipose-derived stromal cells--their utility and potential in bone formation. Int J Obes Relat Metab Disord 24: 41-44.
- Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, et al. (2001) Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng 7: 729-741.
- Huang JI, Beanes SR, Zhu M, Lorenz HP, Hedrick MH, et al. (2002) Rat extramedullary adipose tissue as a source of osteochondrogenic progenitor cells. Plast Reconstr Surg 109: 1033-1041.
- Si Z, Wang X, Sun C, Kang Y, Xu J, et al. (2019) Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed Pharmacother 114: 108765.
- Vezzani B, Shaw I, Lesme H, Yong L, Khan N, et al. (2018) Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Transl Med 7: 876-886.
- Murray IR, Peault B (2015) Q&A: Mesenchymal stem cells — where do they come from and is it important? BMC Biol 13: 99.
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, et al. (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301-313.
- van den Brink SC, Sage F, Vértesy Á, Spanjaard B, Peterson-Maduro J, et al. (2017) Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat Methods 14: 935-936.
- Garcia-Contreras M, Messaggio F, Jimenez O, Mendez A (2015) Differences in exosome content of human adipose tissue processed by non-enzymatic and enzymatic methods. CellR4 3: 1423.
- Phinney DG, Pittenger MF (2017) Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 35: 851-858.
- Elahi FM, Farwell DG, Nolta JA, Anderson JD (2019) Concise Review: Preclinical Translation of Exosomes Derived from Mesenchymal Stem/Stromal Cells. Stem Cells Pg no: 1-10.
- Lombardi F, Palumbo P, Augello FR, Cifone MG, Cinque B, et al. (2019) Secretome of Adipose Tissue-Derived Stem Cells (ASCs) as a Novel Trend in Chronic Non-Healing Wounds: An Overview of Experimental In Vitro and In Vivo Studies and Methodological Variables. Int J Mol Sci 20: 3721.
- Tremolada C, Ricordi C, Caplan AI, Ventura C (2016) Mesenchymal Stem Cells in Lipogems, a Reverse Story: from Clinical Practice to Basic Science. Methods Mol Biol 1416: 109-122.
- Tremolada C, Colombo V, Ventura C (2016) Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr Stem Cell Rep 2: 304-312.
- Tremolada C (2015) Lipogems International Spa. US patent US9044547 B2.
- Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, et al. (2013) A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 22: 2063-2077.
- Giori A, Tremolada C, Vailati R, Navone SE, Marfia G, et al. (2015) Recovery of function in anal incontinence after micro-fragmented fat graft (Lipogems) injection: Two years follow up of the first 5 cases. CellR4 3:1544.
- Oberbauer E, Steffenhagen C, Wurzer C, Gabriel C, Redl H, et al. (2015) Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen 4: 7.
- Carelli S, Messaggio F, Canazza A, Hebda DM, Caremoli F, et al. (2015) Characteristics and properties of mesenchymal stem cells derived from microfragmented adipose tissue. Cell Transplant 24: 1233-1252.
- Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, et al. (2013) Nanofat grafting: Basic research and clinical applications. Plast ReconstrSurg 132: 1017-1026.
- Yu B, Zhang X, Li X (2014) Exosomes derived from mesenchymalstem cells. Int J Mol Sci 15: 4142-4157.
- Rani S, Ryan AE, Griffin MD, Ritter T (2015) Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol Ther 23: 812-823.
- Nava S, Sordi V, Pascucci L, Tremolada C, Ciusani E, et al. (2019) Long-Lasting Anti- Inflammatory Activity of Human Microfragmented Adipose Tissue. Stem Cells Int 5901479.
- Carelli S, Colli M, Vinci V, Caviggioli F, Klinger M, et al. (2018) Mechanical activation of adipose tissue and derived mesenchymal stem cells: novel anti-inflammatory properties. International Journal of Molecular Sciences 19: 267.
- Lonardi R, Leone N, Gennai S, TrevisiBorsari G, Covic T, et al. (2019)Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: A randomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res Ther 10: 223.
- Del Papa N, Di Luca G, Andracco R, Zaccara E, Maglione W, et al. (2019) Regional grafting of autologous adipose tissue is effective in inducing prompt healing of indolent digital ulcers in patients with systemic sclerosis: Results of a monocentric randomized controlled study. Arthritis Res Ther 21: 7.
- Del Papa N, Caviggioli F, Sambataro D, Zaccara E, Vinci V, et al. (2015) Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell Transplant 24: 63-72.
- Ceserani V, Ferri A, Berenzi A, Benetti A, Ciusani E, et al. (2016) Angiogenic and anti-inflammatory properties of micro-fragmented fat tissue and its derived mesenchymal stromal cells. Vasc Cell 8: 3.
- Laureti S, Gionchetti P, Cappelli A, Vittori L, Contedini F, et al. (2019) Refractory Complex Crohn's Perianal Fistulas: A Role for Autologous Microfragmented Adipose Tissue Injection. Inflamm Bowel Dis.
- Cestaro G, De Rosa M, Massa S, Amato B, Gentile M (2015) Intersphincteric anal lipofilling with micro-fragmented fat tissue for the treatment of faecal incontinence: Preliminary results of three patients. WideochirInne Tech Maloinwazyjne 10: 337-341.
- Cortes JS, Velez D (2019) Evaluation of fat grafting technique using autologous microfragmented adipose tissue (Lipogems®) in female patients suffering from stress urinary incontinence. Int Clin Med 3: 1-4.
- Casarotti GA, Chiodera P, Tremolada C (2018) Menopause: New frontiers in the treatment of urogenital atrophy. Eur Rev Med Pharmacol Sci 22: 567-574.
- Raffaini M, Tremolada C (2014) Micro framgmented and purified adipose tissue graft (Lipogems) can improve the orthognatic surgery outcomes both aesthetically and in postoperative healing. Cell R4 2: 1118.
- Benzi R, Marfia GM, Bosetti M, Beltrami G, Magri AS, et al. (2015)Microfracturedlipoaspirate may help oral bone and soft tissue regeneration: A case report. Cell R4 3: 1583.
- Desando G, Bartolotti I, Martini L, Giavaresi G, NicoliAldini N, et al. (2019) Regenerative Features of Adipose Tissue for Osteoarthritis Treatment in a Rabbit Model: Enzymatic Digestion Versus Mechanical Disruption. Int J Mol Sci 20.
- Paolella F, Manferdini C, Gabusi E, Gambari L, Filardo G, et al. (2019) Effect of micro-fragmented adipose tissue on osteoarthritic synovial macrophage factors. J Cell Physiol 234: 5044-5055.
- SchiavonePanni A, Vasso M, Braile A, Toro G, De Cicco A, et al. (2019) Preliminary results of autologous adipose-derived stem cells in early knee osteoarthritis: Identification of a subpopulation with greater response. Int Orthop 43: 7-13.
- Panchal J, Malanga G, Sheinkop M (2018) Safety and Efficacy of Percutaneous Injection of Lipogems Micro-Fractured Adipose Tissue for Osteoarthritic Knees. Am J Orthop (Belle Mead NJ) 47.
- Hudetz D, Bori? I, Rod E, Jele? Ž, Kunovac B, et al. (2019) Early results of intraarticular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: a prospective study. Croat Med J 60: 227-236.
- Russo A, Screpis D, Di Donato SL, Bonetti S, Piovan G, et al. (2018)Autologous microfragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis: an update at 3 year follow-up. J Exp Orthop 5: 52.
- Hudetz D, Bori? I, Rod E, Jele? Ž, Radi? A, et al. (2017) The Effect of Intra-articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes (Basel) 8.
- Polancec D, Zenic L, Hudetz D, Boric I, Jelec Z, et al. (2019) Immunophenotyping of a Stromal Vascular Fraction from Microfragmented Lipoaspirate Used in Osteoarthritis Cartilage Treatment and Its Lipoaspirate Counterpart. Genes (Basel) 10.
- D'Ambrosi R, Indino C, Maccario C, Manzi L, Usuelli FG (2018) Autologous Microfractured and Purified Adipose Tissue for Arthroscopic Management of Osteochondral Lesions of the Talus. J Vis Exp 131.
- Jannelli, E, Fontana A (2017) Arthroscopic treatment of chondral defects in the hip: AMIC, MACI, micro fragmented adipose tissue transplantation (MFAT) and other options. SICOT J 3: 43.
- Mautner K, Bowers R, Easley K, Fausel Z, Robinson R. Functional Outcomes Following Microfragmented Adipose Tissue Versus Bone Marrow Aspirate Concentrate Injections for Symptomatic Knee Osteoarthritis. Stem Cells Transl Med.
- Jones IA, Wilson M, Togashi R, Han B, Mircheff AK, et al. (2018) A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol. BMC Musculoskelet Disord Oct 19: 383.
- Zeira O, Scaccia S, Pettinari L, Ghezzi E, Asiag N, et al. (2018) Intra-Articular Administration of Autologous Micro-Fragmented Adipose Tissue in Dogs with Spontaneous Osteoarthritis: Safety, Feasibility, and Clinical Outcomes. Stem Cells Transl Med 7: 819-828.
- Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, et al. (2018) What low back pain and why we need to pay attention. Lancet 391: 2356-67.
- Allegri M, Montella S, Salici F, Valente A, Marchesini M, et al. (2016) Mechanisms of low back pain: a guide for diagnosis and therapy. Version 2 F1000Res F1000 Faculty Rev-1530.
- Sanapati J, Manchikanti L, Atluri S, Jordan S, Albers SL, et al. (2018) Do Regenerative Medicine Therapies Provide Long-Term Relief in Chronic Low Back Pain: A Systematic Review and Metaanalysis. Pain Physician 21: 515-540.
- Navani A, Manchikanti L, Albers SL, Latchaw RE, Sanapati J, et al. (2019) Responsible, Safe, and Effective Use of Biologics in the Management of Low Back Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 22: 1-74.
- Bougle A, Rocheteau P, Briand D, Hardy D, Verdonk F, et al. (2019) Beneficial role of adipose-derived mesenchymal stem cells from microfragmented fat in a murine model of duchenne muscular dystrophy. Muscle Nerve 60: 328-335.
- Bougle A, Rocheteau P, Hivelin M, Haroche A, Briand D, et al. (2018) Microfragmented fat injection reduces sepsis-induced acute inflammatory response in a mouse model. Br J Anaesth 121: 1249-1259.
- Alessandri G, Cocce V, Pastorino F, Paroni R, Dei Cas M, et al. (2019) Microfragmented human fat tissue is a natural scaffold for drug delivery: Potential application in cancer chemotherapy. J Control Release 302: 2-18.
- Cocce V, Brini A, Gianni AB, Sordi V, Berenzi A, et al. (2018) A Nonenzymatic and Automated Closed-Cycle Process for the Isolation of Mesenchymal Stromal Cells in Drug Delivery Applications. Stem Cells Int 4098140.
- Caplan AI (2019) Medicinal signalling cells: they work, so use them. Nature 566: 39.
Citation: Tremolada C, Allegri M, Selvin M (2019) Mesenchymal Stromal Cells and Micro Fragmented Adipose Tissue: New Horizons of Effectiveness of Lipogems. J Stem Cell Res Dev Ther 5: 017.
Copyright: © 2019 Carlo Tremolada, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

