
Monoclonal Anti-CD47 Therapy and its Haematological Effects, including Anaemia and Thrombocytopenia
*Corresponding Author(s):
Jackson DEDiscipline Of Laboratory Medicine, School Of Health And Biomedical Sciences, STEM College, RMIT University, Bundoora, Victoria, Australia
Tel:+61 39925 7392,
Fax:61 3 9925 7503
Abstract
Monoclonal anti-CD47 therapy is a new form of immunotherapy, which has been used in a clinical setting in recent years for the treatment of haematological and solid malignancies. Anti-CD47 functions by blocking the CD47-SIRPα interaction and promotes the phagocytosis of cancer cells, which attempt to invade the immune systems mechanisms via this interface. This review aims to highlight the significance of anaemia and thrombocytopenia associated with anti-CD47 therapy, as the expression of CD47 on healthy cells can lead to premature removal by phagocytes. Using the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines, eligible studies formed the basis of this systematic review. The screening process of the literature was conducted in multiple phases and studies were considered relevant if the data fit the specified eligibility criteria. Of the original 395 reports obtained, 7 eligible clinical trials were used as they contained relevant statistical data for comparison. Using a proportional, binary random-effects meta-analysis, the incidence of both anaemia and thrombocytopenia was considered significant with a p value < 0.001. Both plots were generated with a confidence interval of 95% [CI 0.207-0.445 anaemia; CI 0.075-0.259 thrombocytopenia]. Using the findings of this review, the occurrence of both anaemia and thrombocytopenia is significant in participants undergoing treatment with various forms of anti-CD47 therapies. However, large-scale studies should be considered in future to determine which specific anti-CD47 therapy can effectively promote the removal of cancer cells with limited adverse effects.
Introduction
- Structure and Function of CD47
CD47, also referred to as an integrin-associated protein, is a glycoprotein located on the surface of all cells including cancer cells. The role of CD47 is to regulate phagocytosis of the cell it is expressed upon, and does so by binding its ligand signal-regulatory protein alpha (SIRPα) present on macrophages [1]. The interaction between CD47 and SIRPα downregulates phagocytosis, providing the macrophage with a “do not eat” signal to extend cell survival [1].
The CD47 glycoprotein is linked to the Rh complex on red blood cells which can alter the CD47 expression depending on an individual’s Rh phenotype. It has been reported that individuals who are rr (D- ce/ce) have the highest expression of CD47 on the red cell surface, in comparison to R2R2 (DcE/DcE) individuals who have reduced expression of CD47 [2]. One case uncovered that individuals with the rare Rh null phenotype demonstrated an approximate 75% reduction in CD47 expression on their red cell surface [3].
- Therapeutic Use of Monoclonal Anti-CD47 Therapy
Targeting the CD47 glycoprotein has become of significant interest to pharmaceutical developers for new immunotherapy drugs for multiple cancer types due to its influence on cell survival [4]. Immunotherapies are being developed not only to aid standard cancer treatments including chemotherapy, radiation and surgical interventions, but to bind more specifically to tumour cells to maximise the drug’s efficacy. Anti-CD47 is most commonly used for patients with haematological and solid malignancies, some of which include multiple myeloma, various lymphomas, acute myeloid leukaemia and myelodysplastic syndromes [1].
Anti-CD47 monoclonal therapies including Hu5F9-G4 (Magrolimab) bind directly to the CD47 expressed on cancer cells and block its interaction with the SIRPα ligand on macrophages and dendritic cells [5]. By obstructing the CD47-SIRPα interaction, the macrophages will not receive an anti-phagocytic signal from the cancer cell and therefore it is marked for removal. This mechanism is known as an anti-tumour response as it results in the death of cancerous cells through either Antibody-Dependent Cell Mediated Cytotoxicity (ADCC) or Fc-independent CD47 intrinsic functions [3].
- Adverse Effects of Anti-CD47 Therapy
The clinical use of anti-CD47 therapy has not come without its challenges, including the premature removal of normal cells which has led to cases of anaemia and thrombocytopenia being reported. CD47 is also described as a marker of “self” when encountered by innate immune cells and acts as an immune checkpoint for phagocytosis [6]. When the CD47-SIRPα interaction is blocked by anti-CD47 antibodies on red blood cells, macrophages recognise the cell as foreign, resulting in anaemia [2]. Sensitised red cells are marked for destruction by splenic macrophages resulting in their decreased lifespan [7]. However, not only does the CD47-SIPRα complex inhibit platelet phagocytosis, CD47 also assists the regulation of platelet haemostasis and turnover [8]. As its target CD47 is widely expressed on all cells, including red blood cells and platelets, off target effects can cause haematological issues during the course of therapy [1]. Many patients beginning anti-CD47 treatment have known haematological disorders affecting their red cells, white cell and platelet counts. Patients should be monitored closely for drops in blood counts as they may require ongoing transfusion support.
However, while anaemia and thrombocytopenia are noted in many clinical trials as adverse effects, the extent of these issues is not well described in the literature [9]. Further investigation into the degree of both anaemia and thrombocytopenia is essential for clinicians to assess the benefits of anti-CD47 therapy against the potential risk of transfusion dependence of packed red cell units and platelets. Another disadvantage of anti-CD47 therapy is the impact of the drug on serological techniques used within the transfusion laboratory. Drug interferences include strong positive reactions of 3+ or 4+ with screening cells in all stages of testing, including initial spin, at 37 °C and with Indirect Agglutination Technique (IAT). Anti-CD47 antibodies are also known to cause discrepancies between the reverse group and historical group for non-Group O individuals [1]. Many have reported on the importance of pre-transfusion testing, including extended phenotyping and genotyping in reference laboratories before treatment commences [1]. As anti-CD47 therapy jeopardises patient safety when issuing compatible blood products, having prior knowledge of existing underlying antibodies and antigens present on patient red cells can minimise alloimmunisation events and transfusion reactions. Reports also suggest that cancer cells are evading clearance by macrophages by upregulating the expression of CD47 on their cell surface [1,10]. Increased expression would require a higher dose of anti-CD47 to be administered in order to disrupt the CD47-SIRPα interaction and therefore stimulate cell death mechanisms.
Scope of Review
As monoclonal anti-CD47 therapy is a relatively new form of immunotherapy, it is important to understand the clinical significance of its side effects on patients. In particular, anaemia and thrombocytopenia should be monitored and more extensively investigated as these issues may cause patients to become transfusion dependent and require additional support from the laboratories. Therefore this review examined what are the effects of Monoclonal Anti-CD47 Therapy on Anaemia and Thrombocytopenia. Using multiple clinical trials involving patients with various types of haematological malignancies and solid tumours, the aim of this systematic review was to investigate the adverse events of anaemia and thrombocytopenia caused by the use of different forms of monoclonal anti-CD47 therapy.
Materials and Methods
- Study Design
To identify and evaluate relevant literature for this systematic review and meta-analysis, the Preferred Reporting Item for Systematic Review and Meta-Analysis (PRISMA) protocols were followed.
- Search Strategy
Reputable online databases including PubMed, Web of Science, ProQuest, Cochrane Library and Google Scholar were used to systematically review the literature relevant to monoclonal anti-CD47 therapy. Broad search criteria were initially used due to the limited research surrounding anti-CD47 treatment as it is a newly recognised and clinically applicable immunotherapy. Criteria initially included English speaking and peer reviewed articles and terms such as “monoclonal anti-CD47 therapy”, “anaemia”, “thrombocytopenia” and “platelets”, while alternating between the use of “AND” and “OR” to yield maximum results. Manual screening techniques were then used to identify articles with relevant information for data analysis and comparison.
- Selection Criteria
a. Types of Studies
Studies featured in this systematic review include clinical trials, as there are no case-control studies yet published. As seen in Table 3, two studies specify that they are open-label studies, meaning the assessors and participants are aware of who is receiving the drug [11]. One of these studies also specifies that the study is a nonrandomized clinical trial and therefore the participants are not assigned to a group by chance but intentionally selected [12]. However, as all studies evaluated are clinical trials, all are assumed to be open-label and nonrandomized trials as there are no specific control or treatment groups.
b. Types of Participants
As seen in Table 3, all eligible participants had existing malignancies or high risk Myelodysplastic Syndrome (MDS). These pre-existing conditions included Acute Myeloid Leukaemia (AML), non-Hodgkin’s lymphoma such as diffuse large B-cell (DLBCL) or follicular lymphoma and others with various forms of relapsed and refractory lymphomas or solid malignancies. While not all trials specified the age of participants, some highlighted how individuals must be 18 years or older and meet certain haematological parameters prior to their involvement in the trial.
c. Types of Interventions and Comparators
While all articles used were clinical trials, participants were intravenously infused with different types of monoclonal anti-CD47 antibodies, some of which are currently on the market and others which are still being analysed for their efficacy and safety. Studies were also included regardless of the priming or maintenance doses administered, length of cycles or if results were outstanding in later phases of the trial. All studies included must report the incidence of anaemia and thrombocytopenia amongst the enrolled participants after receiving at least one dose of the anti-CD47 treatment. Each study either reported the number of participants experiencing these haematological issues or the percentage frequency of participants.
d. Exclusion Criteria
Studies excluded from analysis included those which mentioned the ability of anti-CD47 therapies to target red blood cells and platelets, causing anaemia and thrombocytopenia but did not provide statistical evidence. Those studies which mentioned various grades of anaemia and thrombocytopenia with no extractable data were also dismissed.
e. Data Extraction and Management
After the exclusion of articles bases on their title and date of publication, full texts were reviewed to assess the relevance of each study to the topic of interest. Table 3 summarises the characteristics of each study, specifically its design, status, country of origin, number of participants and the specific monoclonal anti-CD47 therapy used throughout the trial. While many studies reported a range of haematological issues associated with anti-CD47 therapy, only data relating the cases of anaemia and thrombocytopenia were extracted for the purpose of this meta-analysis.
Statistical Analysis
Using the OpenMeta [Analyst] software, a proportional forest plot was constructed to compare the incidence of anaemia and thrombocytopenia as reported by eligible clinical trials. As all of the studies involved one group of interest being treated with the anti-CD47 therapy, proportional data could only be generated using the incidence of haematological events in comparison to the number of participants involved. The arcsine square root method was used with binary random effects and maximum likelihood parameters selected. Results with a p value of < 0.05 were considered significant using a 95% confidence interval range. The I2 value was also calculated to evaluate to presence or lack of heterogeneity between each of the studies.
Results
- Study Selection
As seen in Figure 1, 395 potential records were identified using the PRISMA protocols. Of these articles 115 articles were retrieved from PubMed, 117 from both Web of Science and ProQuest, 40 from Google Scholar and 6 clinical trials from the Cochrane Library. After duplicates were removed, 342 records were screened using title and date parameters. The 44 reports remaining contained “anti-CD47” within the document title and were published between January 1st, 2017 and July 1st, 2022. These reports were further screened for their eligibility and excluded on the basis of irrelevance to the topic (n=17), inability to access full text (n=9), no relevant statistical data (n=10) and trials which were not yet completed (n=1). Post screening, 7 studies were included in the systematic review as they contained relevant statistical information for assessment.
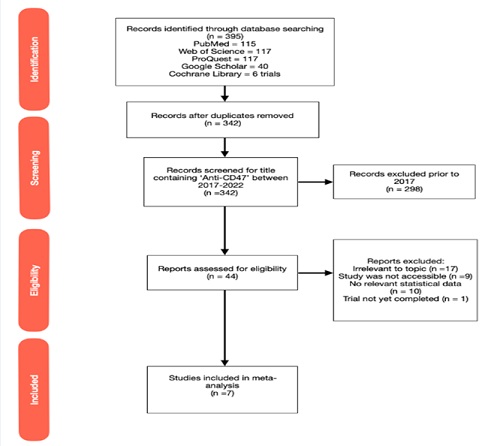 Figure 1: PRISMA Flowchart for Study Selection and Screening Processes. 395 potential records were identified using the PRISMA protocols. Of these articles 115 articles were retrieved from PubMed, 117 from both Web of Science and ProQuest, 40 from Google Scholar and 6 clinical trials from the Cochrane Library. 7 studies were included for quantitative assessment for this systematic review as they contained relevant statistical information.
Figure 1: PRISMA Flowchart for Study Selection and Screening Processes. 395 potential records were identified using the PRISMA protocols. Of these articles 115 articles were retrieved from PubMed, 117 from both Web of Science and ProQuest, 40 from Google Scholar and 6 clinical trials from the Cochrane Library. 7 studies were included for quantitative assessment for this systematic review as they contained relevant statistical information.
- Assessment of Study Quality and Risk of Bias
The quality of 7 eligible studies was assessed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist [13] as seen in Table 1. These studies were available in full text, clearly defined the number of participants and stated the eligibility criteria of patients who were involved in the study. Multiple studies also included details about the participants while maintaining anonymity, including details such as ethnicity, gender, age, previous therapies, stage of disease diagnosis, tumour type and their Eastern Cooperative Oncology (ECOG) Performance Score which relates to a patient’s ability to care for themselves [14].
|
STROBE Criteria |
Advani, 2018 (15) |
Ansell, 2021 (16) |
Berlin, 2020 (17) |
David, 2020 (18) |
Lakhani, 2020 (19) |
Sikic, 2019 (20) |
Zeidan, 2022 (21) |
|
Provides eligibility criteria and method of selection of participants |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
|
Detailed method assessment |
Y |
Y |
Y |
N |
Y |
Y |
Y |
|
Addresses potential sources of bias |
N |
Y |
N |
N |
N |
Y |
Y |
|
Reports number of participants |
Y |
Y |
Y |
Y |
Y |
Yd |
Y |
|
Provides characteristics of study participants |
Y |
Y |
N |
N |
N |
Y |
Y |
|
Provides outcome data |
Ya |
Yb |
Ya |
Yc |
Y |
Y |
Ye |
|
Discusses potential limitations |
Y |
Y |
N |
N |
N |
Y |
Y |
Table 1: Assessment of Quality of 7 Eligible Studies in Accordance with STROBE Checklist.
Y = criteria fulfilled, N = criteria not fulfilled
a - Phase 2 results not yet released
b - Phase 4 results not yet released
c - Estimates of association provided but no mention of variability or uncertainty parameters
d - Only Cohorts A, B and C included in analysis as cohort D was a biopsy cohort with no relevant data
e - Discontinued due to lack of monoclonal antibody activity or evidence of anti-drug antibodies to warrant Phase 2
Each study also provided a detailed method of how the trial was conducted and included key features such as the different doses administered at set intervals over varying cycle lengths. All trials provided outcomes which were relevant to this investigation however some trials are currently ongoing with the results of later stages not yet published. Limitations were also discussed amongst a number of studies [15-21]. One study was discontinued and therefore did not progress to later stages due to the lack of efficacy displayed by the CC-90002 antibody selected. Acknowledgements of potential sources of bias were only documented in 3 studies as seen in Table 1.
- Characteristics of Included Studies
Table 2 demonstrates the characteristics of each of the 7 studies included in the meta-analysis of either incidence of anaemia or thrombocytopenia while on anti-CD47 therapy. Three studies also incorporated the use of other immunotherapies to measure the effectiveness of the anti-CD47 treatment in the presence of other medications. Some of these include Rituximab and Nivolumab which are forms of monoclonal antibodies which work to block the CD20 receptor and programmed cell death protein respectfully [22,23]. One study used anti-CD47 antibody Magrolimab (Hu5F9-G4) in conjunction with Azacitidine (AZA) which functions as an inhibitor of DNA methyltransferase and therefore downregulates tumour cell growth [24].
|
Included Studies |
Study Design |
Status of Study |
Country of Origin |
Study Size |
Specific Anti-CD47 Therapy |
|
Advani, 2018 (15) |
Clinical trial |
Incomplete, Phase 2 results pending |
United States of America |
22 participants |
Magrolimab (Hu5F9-G4) in combination with Rituximab (Anti-CD20 antibody) |
|
Ansell, 2021 (16) |
Open-label, phase I clinical trial |
Incomplete, Phase 4 results pending |
United States of America and Canada |
164 participants |
TTI-621 (SIRPα -IgG1 Fc)
Combined with Rituximab in patients with relapsed and refractory B-cell non-Hodgkin lymphoma (B-NHL) |
|
Berlin, 2020 (17) |
Clinical trial |
Incomplete, Phase 2 results pending |
United States of America |
20 participants |
Lemzoparlimab |
|
David, 2020 (18) |
Clinical trial |
Incomplete, Phase 3 results pending |
United States of America |
39 participants |
Magrolimab (Hu5F9-G4) in combination with Azacitidine (AZA) |
|
Lakhani, 2020 (19) |
Clinical trial |
Completed 2020 |
United States of America |
20 participants |
IBI188 |
|
Sikic, 2019 (20) |
Open-label, nonrandomized, first-in-human dose escalation phase I trial |
Completed 2019 |
United States of America |
62 total participants (44 participants on Anti-CD47 therapy) |
Magrolimab (Hu5F9-G4) |
|
Zeidan, 2022 (21) |
First clinical, phase I dose-escalation and expansion study |
Discontinued, 2022 |
United States of America |
28 participants |
CC-90002 |
Table 2: Characteristics of Eligible Studies.
As summarised in Table 3, each of the studies were conducted in a variety of phases or parts, many to determine the optimal priming and maintenance doses of the drug while measuring the response rate between participants and documenting a range of side effects. While there was much variation amongst the timing of each infusion, the doses ranged was between 1- 45 mg/kg depending on the phase of each trial. Other high incidence adverse effects of anti-CD47 therapy uncovered in the eligible studies included infusion-related-reactions, chills, fatigue and headaches.
|
Study |
Eligible Patients |
Phases / Cohorts |
Haematological Events of Interest Reported (Events/Total) |
Other Adverse Events Reported |
Average Haemo-globin Decrease |
Doses |
Infusion Period |
|
Advani, 2018 (15) |
Patients with relapsed or refractory non-Hodgkin’s lymphoma including diffuse large B-cell lymphoma (DLBCL) or follicular lymphoma. Patient parameters must fit within haematological guidelines prior to the study commencing |
Phase 1: Administer priming dose of Magrolimab in combination with rituximab and determine phase 2 dose |
Anaemia: 9/22 participants
Thrombocytopenia: 3/22 participants |
• Chills • Headache • Infusion-related reactions |
0.9 g/dL |
Phase 1: • Priming dose of 1 mg/kg • 10 mg/kg • 20 mg/kg • 30 mg/kg
Rituximab: • 375 mg/m2 |
Phase 1: Priming dose week 1 then maintenance dose every week following for 28-day cycle
Rituximab: Weekly after Week 1 in cycle 1 and monthly in cycles 2-6 |
|
Ansell, 2021 (16) |
Patients over 18 years with a range of relapsed and refractory lymphomas |
Phase 1: Escalation of Anti-CD47 therapy to determine maximum tolerated dose (MTD) Phase 2: Determine overall response rate and measure adverse effects Phase 3: Focused dose expansion Phase 4: Dose optimisation |
Anaemia: 22/164 participants
Thrombocytopenia: 43/164 participants |
• Infusion-related reactions • Chills • Fatigue |
NA |
Phase 2: • 0.05 mg/kg • 0.1 mg/kg • 0.3 mg/kg • 1 mg/kg • 3 mg/kg • 10 mg/kg
Additional cohorts: • 375 mg/m2 of rituximab per week • 3mg/kg of Nivolumab every 2 weeks |
TTI-621: Weekly doses for 3-week period
TTI-621 + Rituximab: Weekly doses for 8-week period
TTI-621 + Nivolumab: Fortnightly doses for 8-week period
|
|
Berlin, 2020 (17) |
Patients with relapsed/ refractory solid tumours |
Phase 1a: Dose escalation with Lemzoparlimab Phase 1b: Combined dose escalation with Pembrolizumab and Rituximab Phase 2: Expansion study using tumour response data |
Anaemia: 6/20 participants
|
• Fatigue • Infusion-related reactions • Diarrhoea |
1.5 mg/dL in phase 1a |
• 1 mg/kg • 3 mg/kg • 10 mg/kg • 20 mg/kg • 30 mg/kg |
Phase 1: Weekly dose with 1-30mg/kg Lemzoparlimab |
|
David, 2020 (18) |
Patients with high risk myelodysplastic syndrome (MDS) as defined by Revised International Prognostic Scoring System (IPSS-R) |
Phase 1: Investigate potential benefits and risks of using Magrolimab in combination with Azacitidine (AZA) |
Anaemia: 17/39 participants
Thrombocytopenia: 2/39 participants |
• Fatigue • Infusion-related reactions • Neutropenia |
0.4 g/dL |
Phase 1: • Between 1-30mg/kg
AZA: • 75 mg/m2 on days 1-7 |
NA |
|
Lakhani, 2020 (19) |
Patients with advanced/ refractory solid tumours or lymphoma |
Part A: Determine optimal priming dose Part B: Determine optimal maintenance dose |
Anaemia: 3/20 participants
Thrombocytopenia: 1/20 participants |
• Infusion-related reactions • Nausea • Back pain • Vomiting • Blood bilirubin increase |
NA |
Part A: • 0.1 mg/kg • 0.3 mg/kg • 1.0 mg/kg
Part B: • 3 mg/kg • 10 mg/kg • 20 mg/kg • 30 mg/kg |
Part A: Priming dose
Part B: Maintenance dose received weekly over average 1.8-month period |
|
Sikic, 2019 (20) |
Patients over 18 years with advanced solid malignancy or lymphoma |
Part A: Determining priming dose Part B: Determine weekly maintenance dose Part C: Evaluate loading dose specific to Week 2 Part D: Tumour biopsy cohort |
Anaemia: 25/44 participants
Thrombocytopenia: 5/44 participants |
• Haemagglutination • Fatigue • Headache • Fever • Chills • Lymphopenia • Infusion-related reactions • Arthralgia • Hyperbilirubinemia |
1-2 g/dL in Part A |
Part A: • Between 0.1 - 3.0 mg/kg
Part B: • 3 mg/kg • 10 mg/kg • 20 mg/kg
Part C: • 20 mg/kg • 30 mg/kg • 45 mg/kg |
Part A: Weekly for 28-day cycle
Part B: Priming dose on Day 1 and maintenance dose on Day 8 of 28-day cycle
Part C: Loading dose on Day 11 and weekly maintenance dose during 28-day cycle |
|
Zeiden, 2022 (21) |
Patients with relapsed/ refractory acute myeloid leukaemia (AML) or high-risk myelodysplastic syndrome (MDS) |
Phase 1: Using dose escalation and expansion study to determine a recommended phase 2 dose Phase 2: Determine preliminary efficacy of Anti-CD47 therapy |
Anaemia: 9/28 participants
Thrombocytopenia: 11/28 participants |
• Diarrhoea • Febrile neutropenia • Asparate aminotransferase increase • Disseminated intravascular coagulation (DIC) • Cerebral haemorrhage • Purpura • Congestive cardiac failure • Acute respiratory failure • Sepsis |
NA |
Phase 1: • 0.1mg/kg • 0.3 mg/kg • 1.0 mg/kg • 2.0 mg/kg • 4.0 mg/kg |
Phase 1: Days 1, 8, 15 and 22 of each 42-day cycle for cycles 1-4
Maintenance phase: Day 1 of each 28-day cycle for cycles 5-24 |
Table 3: Methodology Comparison of Eligible Studies.
- Meta-analysis of Incidence of Anaemia
As seen in Figure 2, 91 of 337 participants (27%) across all 7 studies reported anaemia as an adverse event associated with monoclonal anti-CD47 therapy (95% CI, 0.207-0.445). Ansell [16] expressed the highest weight of 18.12% due to an increased sample size in comparison to the other studies. Heterogeneity, as expressed by an I2 value of 78.126%, shows high variability between each of the eligible trials. This meta-analysis highlights that the occurrence of anaemia in individuals on anti-CD47 therapy is statistically significant due to a p value of < 0.001.
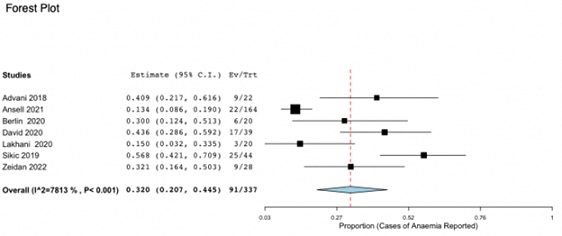 Figure 2: Forest Plot for Incidence of Anaemia due to anti CD47 therapy. 27% across all 7 studies reported anaemia as an adverse event associated with monoclonal anti-CD47 therapy (95% CI, 0.207-0.445;p < 0.001).
Figure 2: Forest Plot for Incidence of Anaemia due to anti CD47 therapy. 27% across all 7 studies reported anaemia as an adverse event associated with monoclonal anti-CD47 therapy (95% CI, 0.207-0.445;p < 0.001).
- Meta-analysis of Incidence of Thrombocytopenia
Using the same method and parameters as previously described, the incidence of thrombocytopenia was compared across 6 eligible trials as seen in Figure 3. Of the 317 participants, 65 individuals suffered from variable drops in their platelet count over the course of anti-CD47 administration (CI 95%, 0.075-0.259). The heterogeneity was again highly variable with an I2 of 76.13% and Ansell [16] represented the highest weight of 21.384%. While only approximately 21% of participants experienced thrombocytopenia, the p value of < 0.001, which is < 0.05, is also considered statistically significant. Therefore, the prevalence of thrombocytopenia in individuals on anti-CD47 therapy is significant and therefore should be considered in the context of the patient’s medical history.
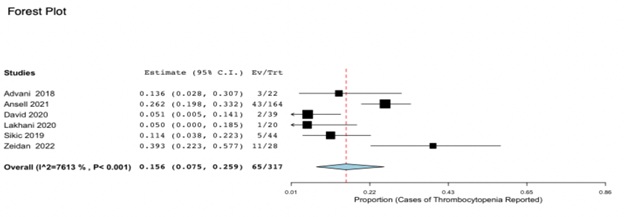 Figure 3: Forest Plot for Incidence of Thrombocytopenia due to anti CD47 therapy. 21% patients demonstrated variable degrees of thrombocytopenia over the course of anti-CD47 administration (CI 95%, 0.075-0.259;p < 0.001).
Figure 3: Forest Plot for Incidence of Thrombocytopenia due to anti CD47 therapy. 21% patients demonstrated variable degrees of thrombocytopenia over the course of anti-CD47 administration (CI 95%, 0.075-0.259;p < 0.001).
Discussion
- Prevalence of Haematological Risk
While new forms of immunotherapy, including monoclonal anti-CD47 therapy, prove to be a more targeted approach to treat relapsed and refractory solid tumours, lymphomas and high risk myelodysplastic syndromes, there can be foreseeable risk involved. As demonstrated in each of the relevant studies, 7 of which related to anaemia and 6 to cases of thrombocytopenia, healthy cells can be subjected to removal by different forms of anti-CD47 therapy. While it is known that blockage of the CD47-SIRPα interaction by anti-CD47 results in premature phagocytosis of red cells and platelets, the prevalence is uncovered in this meta-analysis. In both proportional meta-analysis plots, a p value of < 0.001 indicates that the events of anaemia and thrombocytopenia reported by participants is statistically significant and therefore participants should be monitored and receive ongoing transfusion support when required. Beyond these studies, oncology patients within the population may already be predisposed to anaemia, thrombocytopenia and other haematological malignancies. The risk of causing more severe anaemia and thrombocytopenia amongst these patients needs to be carefully considered by clinicians. In some cases, the adverse effects may outweigh the benefits provided by anti-CD47 therapy if alternative treatment options are available. Multiple studies reported the average decrease in haemoglobin across all participants, which ranged from 0.4-2 g/dL. However, none of the included studies specified what haemoglobin and platelet values justified the use red cell or platelet transfusions during treatment. It is also difficult to establish the numerical decrease in haemoglobin and platelet counts and therefore highlight the severity of these events. For example, Sikic [20] reported a total of 25 cases of anaemia, 6 of which were considered Grade 3.
Of the 5 cases of thrombocytopenia, these did not fall within the Grade 3 or 4 categories. However, the study did not report the haemoglobin or platelet ranges that were considered a severity of Grade 3 or Grade 4. Without defining the grades of anaemia or thrombocytopenia, it is difficult to determine the trigger values associated with required transfusion support.
Many of the studies used different forms of a monoclonal anti-CD47 antibody, some of which are clinically used and others which are still in testing phases. Sikic [20] recorded the highest prevalence of anaemia, affecting 57% of participants with Magrolimab (Hu5F9-G4) being the antibody of choice. In comparison to Ansell [16] which only reported anaemia amongst 13% of participants and utilised the TTI-621 antibody in combination with Rituximab throughout the trial. However, Zeidan [21] reported the highest incidence of thrombocytopenia at 39% while using antibody CC-90002. The lowest rates of thrombocytopenia were approximately 5% of the sample population in trials David [18] and Lakhani [19], which utilised Magrolimab and IBI188 antibodies respectfully. While the dosage administered and infusion rates varied amongst the trials, it cannot be determined which specific monoclonal anti-CD47 antibody is most detrimental to a patient’s haematological state, causing high prevalence of both anaemia and thrombocytopenia. Thaker [25] describes a new anti-CD47 therapy known as STI-6643 which may be a safer alternative, with a high efficacy for cancer cells resulting in minimal red cell and platelet destruction. Although this report does not include any statistical data in regard to the prevalence of different haematological issues, further testing is required to support these claims and to obtain approval for use in clinical settings.
- Transfusion Laboratory Issues
Depending on the severity of either anaemia or thrombocytopenia, patients may require ongoing transfusion support throughout the course of their anti-CD47 treatment. However, the drug itself has proven to cause discrepancies within the transfusion laboratory including pan-agglutination with all antibody panel cells and altering the reverse group of Group O individuals. In order to determine if a patient has underlying antibodies prior to transfusing packed red cells, the anti-CD47 antibodies bound to the panel cells need to be eluted off. While many reports use different techniques to remove the drug antibody reactivity, using papain-treated allogeneic red blood cells or pooled plasma for multiple alloadsorptions of the patient’s plasma is proving to be the most promising so far [1]. This process does cause significance time delays within the laboratory in regard to testing patient samples and identifying alloantibodies and autoantibodies in order to source compatible units. An Immucor reagent known as Anti-IgG monoclonal Gamma-clone can also be used to detect underlying antibodies, as this monoclonal does not bind to the IgG4 subclass. This product is particularly useful for patients on Hu5F9-G4 therapy which contains the IgG4 subclass. Therefore, pan-agglutination is not observed in all panel cells and other clinically significant antibodies can be detected [1].
Many things should be considered prior to commencing anti-CD47 therapy, including the importance of pre-transfusion testing, the cost of monitoring patients during treatment and the overall risk to the patient’s wellbeing. Pre-transfusion testing is highly recommended to identify the patient’s blood group and existing antibodies prior to treatment due to the issues caused by anti-CD47. Clinicians will need to consider the welfare of patients as symptoms of fatigue could impact their daily functioning and result in loss of independence. Severe thrombocytopenia can also increase the risk of uncontrollable bleeding if the patient is to suffer a fall or become wounded.
- Strengths and Limitations of the Review
While this review highlights the significance of haematological issues such as anaemia and thrombocytopenia amongst individuals on monoclonal anti-CD47 therapy, there were limitations when conducting the analysis. As immunotherapy is a relatively new form of targeted treatment, there are very limited studies conducted which investigates these adverse effects. All of the eligible studies included were published between 2017-2022 as there is limited research currently available. Many of the trials were also not yet completed, so there is likely more literature to be published for review in the coming years. The mechanism of removal of both red blood cells and platelets for patients on anti-CD47 therapy at this stage is still unclear. While anaemia is present, indicators of haemolysis including increased bilirubin, lactate dehydrogenase and reticulocytes have not been detected [26]. Removal of red cells by phagocytic macrophages in the spleen, a process also known as extravascular haemolysis, is thought to be the main mechanism of premature elimination. A reduction in CD47 on the platelet cell surface when bound by the anti-CD47 is also believed to increase the phagocytosis of platelets in circulation [8]. However, further studies are required in order to uncover the true mechanisms driving red cell and platelet removal due to anti-CD47 drugs.
Another complication was that many studies regarding anti-CD47 therapy described the occurrences of anaemia and thrombocytopenia with varying severity, however did not provide any statistical data for comparison with other studies. This analysis also demonstrates the need for more data comparing the different forms of anti-CD47 antibodies in order to determine which has limited haematological effects on participants and therefore the greater population.
Conclusion
From the meta-analysis and systematic review of these eligible articles, the findings highlight the significance of both anaemia and thrombocytopenia for participants undergoing monoclonal anti-CD47 therapy. As forms of this treatment are currently used, it is important to acknowledge the haematological issues which can arise from treatment. These findings also highlight the need to further improve the specificity of existing drugs and evaluate the efficacy of drugs in their developmental stages to minimise the risk of not only anaemia and thrombocytopenia but other adverse effects related to treatment. By increasing the efficacy of anti-CD47 and limiting the off-target effects on healthy cells, this would reduce the cost of ongoing testing requirements to monitor patients during treatment.
Future studies would benefit from the use of a larger number of participants, using different forms of anti-CD47 therapy with consistent methodology related to dosage and infusion administration periods. Being able to assess the advantages and downfalls of each specific monoclonal anti-CD47 antibody would assist clinicians when making an informed decision about treatment options for patients with solid malignancies, lymphomas and myelodysplastic syndromes. Overall, the data reveals the impact haematological issues associated with anti-CD47 therapy on patient’s health as well as the inconsistences reported in the transfusion laboratory due to drug interaction.
Conflicts of Interest
Authors declare no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
- Velliquette RW, Aeschlimann J, Kirkegaard J, Shakarian G, Lomas-Francis C, et al. (2019) Monoclonal anti-CD47 interference in red cell and platelet testing. Transfusion 59: 730-737.
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, et al. (2000) Role of CD47 as a Marker of Self on Red Blood Cells. Science 288: 2051-2054.
- Lu Q, Chen X, Wang S, Lu Y, Yang C, Jiang G (2020) Potential New Cancer Immunotherapy: Anti-CD47-SIRPα Antibodies. Onco Targets Ther 13: 9323-9331.
- Xu Z, Gao J, Yao J, Yang T, Wang D, et al. (2021) Preclinical efficacy and toxicity studies of a highly specific chimeric anti-CD47 antibody. FEBS Open Bio 11: 813-825.
- Ni H, Cao L, Wu Z, Wang L, Zhou S, et al. (2022) Combined strategies for effective cancer immunotherapy with a novel anti-CD47 monoclonal antibody. Cancer Immunol Immunother 71: 353-363.
- Narla RK, Modi H, Bauer D, Abbasian M, Leisten J, et al. (2022) Modulation of CD47-SIRPα innate immune checkpoint axis with Fc-function detuned anti-CD47 therapeutic antibody. Cancer Immunol Immunother 71: 473-489.
- Oldenborg PA, Gresham HD, Lindberg FP (2001) CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med 193: 855-862.
- Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg PA (2005) Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood 105: 3577- 3582.
- Li M, Yu H, Qi F, Ye Y, Hu D, et al. (2022) Anti-CD47 immunotherapy in combination with BCL-2 inhibitor to enhance anti-tumor activity in B-cell lymphoma. Hematol Oncol 40: 596-608.
- Reyland L, Dwight M, Bullock T, Latham T, Lord K, et al. (2020) Two case reports involving therapeutic monoclonal anti-CD47 (Hu5F9-G4), it's effect on compatibility testing and subsequent selection of components for transfusion. Transfus Med 30: 157-160.
- National Cancer Institute (2022) (United States of America): National Cancer Institute. Open Label Study.
- Cochrane Childhood Cancer (2022) London (United Kingdom): Cochrane Childhood Cancer; 2022. Non-randomised controlled (NRS) designs.
- Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD et al. (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 18: 806-808.
- ECOG-ARS1N (2022) Philadelphia (USA): ECOG-ARS1N Cancer Research Group. ECOG Performance Scale Score.
- Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, et al. (2018) CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin's Lymphoma. N Engl J Med 379: 1711-1721.
- Ansell SM, Maris MB, Lesokhin AM, Chen RW, Flinn IW, et al. (2021) Phase I Study of the CD47 Blocker TTI-621 in Patients with Relapsed or Refractory Hematologic Malignancies. Clin Cancer Res 27: 2190-2199.
- Berlin J, Harb W, Adjei A, Xing Y, Swiecicki P, et al. (2020) 385 A first-in-human study of lemzoparlimab, a differentiated anti-CD47 antibody, in subjects with relapsed/refractory malignancy: initial monotherapy results. Journal for Immunotherapy of Cancer 8: A233-A234.
- Sallman DA, Al Malki M, Asch A, Lee D, Kambhampati S, et al. (2020) The first-in-class anti-CD47 antibody magrolimab combined with azacitidine is well tolerated and effective in MDS patients: phase 1b results. Hemasphere 4: 49-52.
- Lakhani N, Orloff M, Fu SQ, Liu Y, Wang Y, et al. (2020) First-in-Human Phase I Trial of Ibi188, An Anti-Cd47 Targeting Monoclonal Antibody, in Patients with Advanced Solid Tumors and Lymphomas. J for Immunother of Can 8: A180.
- Sikic BI, Lakhani N, Patnaik A, Shah SA, Chandana SR, et al. (2019) First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients With Advanced Cancers. J Clin Oncol 37: 946-953.
- Zeidan AM, DeAngelo DJ, Palmer J, Seet CS, Tallman MS, et al. (2022) Phase 1 study of anti-CD47 monoclonal antibody CC-90002 in patients with relapsed/refractory acute myeloid leukemia and high-risk myelodysplastic syndromes. Ann Hematol 101: 557-569.
- Salles G, Barrett M, Foà R, Maurer J, O'Brien S, et al. (2017) Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv Ther 34: 2232-2273.
- Koppolu V, Rekha Vasigala VK (2018) Checkpoint immunotherapy by nivolumab for treatment of metastatic melanoma. J Cancer Res Ther 14: 1167-1175.
- Cancer Research UK (2022) London (United Kingdom); Cancer Research UK. Azacitidine (Vidaza).
- Thaker YR, Rivera I, Pedros C, Singh AR, Rivero-Nava L, et al. (2022) A Novel Affinity Engineered Anti-CD47 Antibody With Improved Therapeutic Index That Preserves Erythrocytes and Normal Immune Cells. Front Oncol 12: 884196.
- Brierley CK, Staves J, Roberts C, Johnson H, Vyas P, et al. (2019) The effects of monoclonal anti-CD47 on RBCs, compatibility testing and transfusion requirements in refractory acute myeloid leukemia. Transfusion 59: 2248-2254.
Citation: Clark B, Moon J, Jackson DE (2024) Monoclonal Anti-CD47 Therapy and its Haematological Effects, including Anaemia and Thrombocytopenia. J Emerg Med Trauma Surg Care 10: 070.
Copyright: © 2024 Clark B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

