
Physiologically relevant hematopoietic niche models
*Corresponding Author(s):
Eric GottwaldInstitute Of Functional Interfaces, Karlsruhe Institute Of Technology, Germany
Email:eric.gottwald@kit.edu
Keywords
Hematopoietic niche; Microcavity array; Oxygen measurement; 3D culture
We have previously shown that microcavity arrays can be used as a model system for the setup of an artificial hematopoietic stem cell niche [1,2]. For this, microcavity arrays were inoculated with human mesenchymal stromal cells and KG1a or CD34+ cells from human umbilical cord blood. This type of co-culture demonstrated that CD34+ cells not only survived but can be expanded from 41 cells per microcavity at day 1 to 216 cells at day 21 of the culture. At the same time, the number of CD38+ cells also increased to a certain extent (222 at day 1 to 601 at day 21) so that a portion of the CD34+ cells obviously differentiated towards the lymphocytic line. These data indicate that more factors, other than only 3D culture itself, are necessary for the proper construction of an artificial hematopoietic niche. Factors influencing the cellular behaviour in the niche comprise the cellular composition, soluble factors, mechanical cues, the material choice of the scaffolds used, and more. A factor that had to be neglected so far because it could not be measured properly, and although of utmost importance for the maintenance of stemness and differentiation capacity, is the oxygen concentration. Oxygen can be measured by various means but is very hard to determine in 3D cultures. Measurement approaches comprise the Winkler titration or electrochemical dissolved oxygen sensors such as the Clarke electrode [3]. However, the Winkler titration is not suitable for in vitro-approaches using cells because of the Mn(OH)2 that is built during the course of the reaction, is fatal for the culture. The Clarke electrode, due to its measurement principle, consumes oxygen that may lead to biased results in cases where the oxygen concentration is low and where the total amount of oxygen is limited [4]. Moreover, such electrodes are relatively large so that they cannot be used for small tissue aggregates. In a recent evolution, we have developed the microcavity array system further in a way that now the polymer used for the manufacturing of the microcavity arrays is able to measure oxygen. For this, the polymer is coated with a fluorophore cocktail comprising an oxygen-sensitive dye (red) and an oxygen-insensitive reference dye (green) so that optical ratiometric, non-analyte-consuming oxygen measurements in real-time can be performed [3,5]. The microcavity geometry, moreover, has been changed in way that they include a bevel at the top thus enabling the measurement of oxygen gradients so that this geometry is suited as a model system mimicking postcapillary diffusion. Since the whole microcavity surface area is oxygen-sensitive, oxygen can be measured at any point in the culture, so that e.g., in a confocal microscope modality oxygen in a 3D culture can be determined in 3D as well [Figure 1]. The procedure requires only images of the green and red fluorescence channel and, if desired, an additional channel for the cells, which then have to be quantified and the ratio of the intensity is calculated.
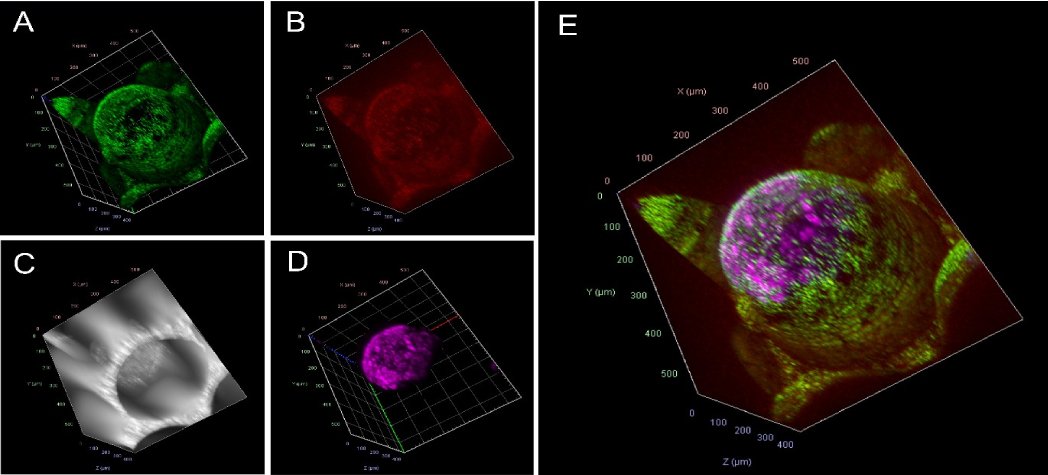 Figure 1: Confocal image of an oxygen sensitive microcavity. A) green channel corresponding to the reference dye signal B) Red channel corresponding to the oxygen-sensitive channel C) Bright field channel D) GFP channel of the stained cells displayed in magenta E) Overlay. By ratiometric comparison (red:green fluorescence intensity), after calibration, the oxygen concentration can be determined.
Figure 1: Confocal image of an oxygen sensitive microcavity. A) green channel corresponding to the reference dye signal B) Red channel corresponding to the oxygen-sensitive channel C) Bright field channel D) GFP channel of the stained cells displayed in magenta E) Overlay. By ratiometric comparison (red:green fluorescence intensity), after calibration, the oxygen concentration can be determined.
This additional functionality will now enable us to improve the hematopoietic niche model according to physiological oxygen concentrations since in the vascular zone of the bone marrow, an oxygen concentration of 12% has been reported, whereas this concentration drops to 1 – 6% in the sinusoidal cavity [6,7]. The oxygen plays a crucial role in the regulation of the stem cell niche since hypoxia inducible factors are a family of master transcriptional regulators of the hypoxic response in HSCs [7].
So far, it was not possible to determine the oxygen concentration in artificial 3D niches needed for the cells to exert a proper function. Moreover, by being able to also regulate the oxygen concentration in such systems, we will be able to tune the system with regard to the desired function. In a more sophisticated view, this approach would also lead to a dynamic culture with a closed perfusion loop in which the measured oxygen signal regulates the culture conditions thereby effectively using the cells themselves as the regulator. This can be used e.g. to shed more light on healthy artificial niche function as well as on leukemic artificial niches because leukemic stem cells in the bone marrow are made responsible for the maintenance of several leukemia types and most likely are responsible for patient relapses. Moreover, not only niche models will profit from the new measurement technique but also any other 3D system for that oxygen control is crucial.
References
- Gottwald E, Nies C, Wuchter P, Saffrich R, Truckenmüller R, et al. (2019) A microcavity array-based 3D model system of the hematopoietic stem cell niche. InStem Cell Mobilization: Methods and Protocols 85-95.
- Nies C, Rubner T, Lorig H, Colditz V, Seelmann H, et al. (2019) A microcavity array-based 4D cell culture platform. Bioengineering 6: 50.
- Grün C, Pfeifer J, Liebsch G, Gottwald E (2023) O2-sensitive microcavity arrays: A new platform for oxygen measurements in 3D cell cultures. Frontiers in Bioengineering and Biotechnology 11: 1111316.
- Wei Y, Jiao Y, An D, Li D, Li W, et al. (2019) Review of dissolved oxygen detection technology: From laboratory analysis to online intelligent detection. Sensors 19: 3995.
- Gottwald E, Grün C, Nies C, Liebsch G (2023) Physiological oxygen measurements in vitro-Schrödinger’s cat in 3D cell biology. Frontiers in Bioengineering and Biotechnology 11: 1218957.
- Sayin E, Baran ET, Elsheikh A, Mudera V, Cheema U, et al. (2021) Evaluating oxygen tensions related to bone marrow and matrix for MSC differentiation in 2D and 3D biomimetic lamellar scaffolds. International journal of molecular sciences 22: 4010.
- Morikawa T, Takubo K (2016) Hypoxia regulates the hematopoietic stem cell niche. Pflügers Archiv-European Journal of Physiology 468: 13-22.
Citation: Gottwald E, Nies C, Grun C (2026) Physiologically relevant hematopoietic niche models. HSOA J Stem Cell Res Dev Ther 12: 119.
Copyright: © 2026 Eric Gottwald, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

