
Risk Factors, Classifications and Pathogenesis of Diabetic Neuropathy
*Corresponding Author(s):
Gudisa BeredaDepartment Of Pharmacy, Negelle Health Science College, Guji, Ethiopia
Tel:+251 913118492, +251 919622717,
Email:gudisabareda95@gmail.com
Abstract
Diabetic neuropathies are the most prevalent chronic complications of diabetes. Diabetic neuropathy affects more than 50% of patients with diabetes and is defined as a set of clinical syndromes that affect different regions of the nervous system. Sensorimotor neuropathy involves muscular symptoms such as muscle weakness (not fatigue), atrophy, balance problems, ataxic gait; sensory symptoms such as pain, paresthesia, numbness, paralysis, cramping, nighttime falls, antalgic gait. The duration of diabetes and haemoglobin A1c (glycosylated hemoglobin) levels (a measurement of glycated haemoglobin as a surrogate for average daily glucose levels) are major predictors of diabetic neuropathy. Diabetic neuropathy is a unique neurodegenerative disorder of the peripheral nervous system that preferentially targets sensory axons, autonomic axons and later, to a lesser extent, motor axons. Progressive diabetic neuropathy involves retraction and ‘dying back’ of terminal sensory axons in the periphery, with relative preservation of the perikarya (cell bodies). Its ‘stocking and glove’ pattern of involvement reflects damage to the longest sensory axons first with, for example, loss of distal leg epidermal axons preceding loss in more proximal limbs; for this reason, diabetic neuropathy is considered a length-dependent neuropathy.
Keywords
Classifications; Diabetic neuropathy; Pathogenesis; Risk factors
Introduction
Diabetic neuropathy is one of the chronic microvascular complications of diabetes and is defined as a non-inflammatory impairment of the function and structure of peripheral somatic or autonomic nerves due to metabolic-vascular pathology. In the early stages of diabetes, hyperglycaemia causes changes in blood flow and vascular permeability. The damage of vasa nervorum is first manifested by functional changes, where vasoconstrictive factors predominate over vasodilators and coagulation is activated [1-3]. Diabetic neuropathies are the most prevalent chronic complications of diabetes. Diabetic neuropathy affects more than 50% of patients with diabetes and is defined as a set of clinical syndromes that affect different regions of the nervous system [3-6]. Painful diabetic neuropathy affects at least one in five adults with diabetes and has a major impact due to associated depression, anxiety and sleep disturbance [7,8]. Diabetic neuropathies include several distinct syndromes of which symmetric sensory polyneuropathy, often associated with autonomic polyneuropathy are the most common and are usually referred to as diabetic neuropathy [9]. Diabetic neuropathy can affect any part of the nervous system. This nerve disorder should be suspected in all patients with type 2 diabetes and in patients who have had type 1 diabetes for more than five years [10-12].
Classifications of diabetic neuropathy
The primary types of diabetic neuropathy are sensorimotor and autonomic. Sensorimotor neuropathy involves muscular symptoms such as muscle weakness (not fatigue), atrophy, balance problems, ataxic gait; sensory symptoms such as pain, paresthesia, numbness, paralysis, cramping, nighttime falls, antalgic gait. Autonomic neuropathy involves cardiovascular symptoms such as exercise intolerance, fatigue, sustained heart rate, syncope, dizziness, lightheadedness, balance problems; gastrointestinal symptoms such as dysphagia, bloating, nausea and vomiting, diarrhea, constipation, loss of bowel control; genitourinary symptoms such as loss of bladder control, urinary tract infection, urinary frequency or dribbling, erectile dysfunction, loss of libido, dyspareunia, vaginal dryness, anorgasmia; sudomotor (sweat glands) symptoms such as pruritus, dry skin, limb hair loss, calluses, reddened areas; endocrine symptoms such as hypoglycemic unawareness Other symptoms such as difficulty driving at night, depression, anxiety, sleep disorders, cognitive changes. A patient may have only one type of neuropathy or might develop different combinations of neuropathies. Sensory neuropathies can be classified as distal symmetric polyneuropathy, focal neuropathy (e.g., diabetic mononeuropathy), and diabetic amyotrophy. Motor neuropathies are identified by the muscles that are involved. Autonomic neuropathies may be classified by the system that is affected (e.g., endocrine, gastrointestinal, genitourinary). Distal symmetric polyneuropathy manifests with a ‘stocking and glove’ distribution, whereby the hands and lower limbs are commonly affected [13].
Risk factors of diabetic neuropathy
The duration of diabetes and haemoglobin A1c (glycosylated hemoglobin) levels (a measurement of glycated haemoglobin as a surrogate for average daily glucose levels) are major predictors of diabetic neuropathy. These two predictors commonly associate with other metabolic factors that are correlated with diabetic neuropathy, particularly in type 2 diabetes mellitus, such as insulin resistance and hypertension. Independent of glycosylated hemoglobin levels, the number of metabolic syndrome components, such as hypertriglyceridaemia, hypertension, abdominal obesity and low high-density lipoprotein levels is consistently associated with diabetic neuropathy in patients with type 2 diabetes mellitus. Other independent risk factors for the development of diabetic neuropathy include smoking, alcohol abuse, increased height and older age [14-20]. The risk of distal symmetric polyneuropathy and autonomic neuropathy increases with the following risk factors, indicators and co-morbidities such as duration of diabetes, glycaemic control (hyperglycaemia), arterial hypertension, peripheral artery disease, mönckeberg's medial sclerosis, diabetic retinopathy and nephropathy, depression, visceral obesity, hyperlipidaemia, alcohol and/or nicotine abuse, insufficient physical activity, demographic factors (age, height, weight) (Figure 1) [21].
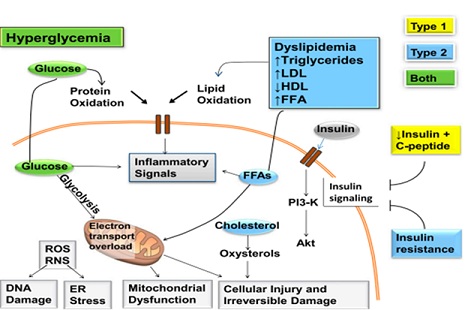 Figure 1: There may be multiple etiologies which account for the various neuropathic syndromes seen in patients with diabetes.
Figure 1: There may be multiple etiologies which account for the various neuropathic syndromes seen in patients with diabetes.
Pathogenesis of diabetic neuropathy
Mechanisms of diabetic neuropathy. Factors linked to type 1 diabetes (yellow), type 2 diabetes (blue), and both (green) cause DNA damage, endoplasmic reticulum stress, mitochondrial dysfunction, cellular injury, and irreversible damage. The relative importance of the pathways in this network will vary with cell type, disease profile, and time. ER, endoplasmic reticulum; FFA, free fatty acids; PI3-K, phosphatidylinositol-3 kinase; RNS, reactive nitrogen species; ROS, reactive oxygen species. Hyperglycemia clearly plays a key role in the development and progression of diabetic neuropathy as well as the other microvascular complications of diabetes. Diabetic neuropathy is a unique neurodegenerative disorder of the peripheral nervous system that preferentially targets sensory axons, autonomic axons and later, to a lesser extent, motor axons. Progressive diabetic neuropathy involves retraction and ‘dying back’ of terminal sensory axons in the periphery, with relative preservation of the perikarya (cell bodies). Its ‘stocking and glove’ pattern of involvement reflects damage to the longest sensory axons first with, for example, loss of distal leg epidermal axons preceding loss in more proximal limbs; for this reason, diabetic neuropathy is considered a length-dependent neuropathy. Diabetic neuropathy is thought to occur from hyperglycemia-induced damage to nerve cells per se and from neuronal ischemia caused by hyperglycemia-induced decreases in neurovascular flow [22-26]. Endoneurial capillaries often show signs of diabetic microangiopathy, with marked thickening of the basal lamina. The presence of multifocal nerve lesions and alterations of endoneurial capillaries have indicated a role for circulatory factors in symmetrical diabetic neuropathy. Dissociated sensory loss, severe autonomic dysfunction and predominant loss of unmyelinated axons cannot, however, be explained by nerve ischemia alone [27-29]. Oxidative and nitrosative stress, accumulation of glycation end products, microvascular insufficiency, derangements in normal metabolic homeostasis, and persistent hyperglycemia are some of the factors postulated to be involved in the pathogenesis of diabetic neuropathy [30,31]. Advanced protein oxidation or 3-nitrotyrosine products are used as protein markers and 8-hydroxy-2-deoxyguanosine as deoxyribonucleic acid damage markers. Alpha-lipoic acid is an option for the treatment of diabetic neuropathy mainly due to its ability to induce relief from annoying symptoms, which has been confirmed in several studies. Alpha-lipoic acid reduces oxidative stress by inhibiting the hexosamine pathway of glucose utilization and reduces advanced glycation end-products production. It further improves blood flow by increasing endothelial vasodilation via nitric oxide and contributes to improved signal conduction in the peripheral nervous system [32-38].
Conclusion
Painful diabetic neuropathy affects at least one in five adults with diabetes and has a major impact due to associated depression, anxiety and sleep disturbance. The primary types of diabetic neuropathy are sensorimotor and autonomic. The duration of diabetes and haemoglobin A1c (glycosylated hemoglobin) levels (a measurement of glycated haemoglobin as a surrogate for average daily glucose levels) are major predictors of diabetic neuropathy. Oxidative and nitrosative stress, accumulation of glycation end products, microvascular insufficiency, derangements in normal metabolic homeostasis, and persistent hyperglycemia are some of the factors postulated to be involved in the pathogenesis of diabetic neuropathy.
Acknowledgment
The author would be grateful to anonymous reviewers by the comments that increase the quality of this manuscript.
Data Sources
Sources searched include Google Scholar, Research Gate, PubMed, NCBI, NDSS, PMID, PMCID, Scopus database, Scielo and Cochrane database. Search terms included: risk factors, classifications and pathogenesis of diabetic neuropathy.
Funding
None.
Availability of Data and Materials
The datasets generated during the current study are available with correspondent author.
Competing Interests
The author has no financial or proprietary interest in any of material discussed in this article.
References
- Ma cakova D, Plchova M, Cibi ckova L, Krystynik O, Karasek D, (2022) Association of Oxidative Stress Markers with Vascular Stiffness Parameters in Patients with Diabetic Neuropathy. BioMed 2: 1-12.
- American Diabetes Association (2020) Introduction: Standards of Medical Care in Diabetes-2020. Diabetes Care 43: 1-2.
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813-820.
- Oliveira-Abreu K, Cipolla-Neto J, Leal-Cardoso JH (2022) Effects of Melatonin on Diabetic Neuropathy and Retinopathy. Int J Mol Sci 23: 100.
- Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, et al. (2019) Diabetic neuropathy. Nat Rev Dis Prim 5: 41.
- Pourhanifeh MH, Hosseinzadeh A, Dehdashtian E, Hemati K, Mehrzadi S (2020) Melatonin: New insights on its therapeutic properties in diabetic complications. Diabetol Metab Syndr 12: 30.
- Kalteniece A, Ferdousi M, Azmi S, Ullah Khan S, Worthington A, et al. (2021) Corneal nerve loss is related to the severity of painful diabetic neuropathy. Eur J Neurol 29: 1-9.
- Abbott CA, Malik RA, Van Ross ER, Kulkarni J, Boulton AJ (2011) Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 34: 2220-2224.
- Birukov A, Cuadrat R, Polemiti E, Eichelmann F, Schulze MB (2021) Advanced glycation end-products, measured as skin autofluorescence, associate with vascular stiffness in diabetic, pre-diabetic and normoglycemic individuals: A cross-sectional study. Cardiovasc Diabetol 20: 110.
- Novella SP, Inzucchi SE, Goldstein JM (2001) The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve 24: 1229-12231.
- Singleton JR, Smith AG, Bromberg MB (2001) Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care 24: 1448-1453.
- Aring AM, Jones DE, Falko MJ (2005) Evaluation and Prevention of Diabetic Neuropathy. Am Fam Physician 71: 2123-2128.
- Callaghan BC, Kerber KA, Lisabeth LL, Morgenstern LB, Longoria R, et al. (2014) Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA Neurol 71:1143-1149.
- Andersen ST, Witte DR, Dalsgaard EM, Andersen H, Nawroth P, et al. (2018) Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: A ddition-Denmark. Diabetes Care 41: 1068-1075.
- Callaghan BC, Gao L, Yufenf L, Zhou X, Reynolds E, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol 5: 397-405.
- Callaghan BC, Xia R, Banerjee M, Rekeneire ND, Harris BT, et al. (2016) Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care 39: 801-807.
- Callaghan BC, Xia R, Banerjee M, Rekeneire ND, Harris BT, et al. (2016) Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol 73: 1468-1476.
- Hanewinckel R, Drenthen J, Ligthart S, Dehghan A, Franco HO, et al. (2016) Metabolic syndrome is related to polyneuropathy and impaired peripheral nerve function: a prospective population-based cohort study. J Neurol Neurosurg Psychiatry 87: 1336-1342.
- Lu B, Hu J, Wen J, Zhang Z, Zhou L, et al. (2013) Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre-diabetes-shanghai diabetic neuRopathy Epidemiology and Molecular Genetics Study (SH-DREAMS). plos one 8: e61053.
- Tesfaye S, Selvarajah D (2009) The Eurodiab study: What has this taught us about diabetic peripheral neuropathy? Curr Diab Rep 9: 432-434.
- Callaghan BC, Price RS, Feldman EL (2015) Distal symmetric polyneuropathy: A review. JAMA 314: 2172-2181.
- Politi C, Ciccacci C, Amato CD, Novelli G, Borgiani P, et al. (2016) Recent advances in exploring the genetic susceptibility to diabetic neuropathy. Diabetes Res Clin Pract 120: 198-208.
- Dunnigan SK, Ebadi H, Breiner A, Katzberg HD, Lovblom LE, et al. (2013) Conduction slowing in diabetic sensorimotor polyneuropathy. Diabetes Care 36: 3684-3690.
- Gumy LF, Bampton ET, Tolkovsky AM (2008) Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol Cell Neurosci 37: 298-311.
- Mizisin AP, Shelton GD, Wagner S, Rusbridge C, Powell HC (1998) Myelin splitting, Schwann cell injury and demyelination in feline diabetic neuropathy. Acta Neuropathol 95: 171-174.
- Pan S, Chan JR (2017) Regulation and dysregulation of axon infrastructure by myelinating glia. J Cell Biol 216: 3903-3916.
- Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444: 860-867.
- Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M (2003) The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 60: 108-111.
- Leinninger GM, Edwards JL, Lipshaw MJ, Feldman EL (2006) Mechanisms of disease: Mitochondria as new therapeutic targets in diabetic neuropathy. Nat Clin Pract Neurol 2: 620-628.
- Zakin E, Abrams R, Simpson DM (2019) Diabetic Neuropathy. Semin. Neurol 39: 560-569.
- Vinik AI, Nevoret ML, Casellini C, Parson H (2013) Diabetic Neuropathy. Endocrinol Metab Clin N Am 42: 747-787.
- Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, et al. (2006) Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: The sydney 2 trial. Diabetes Care 29: 2365-2370.
- Ametov AS, Barinov A, Dyck PJ, Hermann R, Kozlova N, (2003) The sensory symptoms of diabetic polyneuropathy are improved with alpha-lipoic acid: The sydney trial. Diabetes Care 26: 770-776.
- Papanas N, Ziegler D (2014) Efficacy of α-lipoic acid in diabetic neuropathy. Expert Opin Pharmacother 15: 2721-2731.
- Vallianou N, Evangelopoulos A, Koutalas P (2009) Alpha-lipoic Acid and diabetic neuropathy. Rev Diabet Stud 6: 230-236.
- Prenner SB, Chirinos JA (2015) Arterial stiffness in diabetes mellitus. Atherosclerosis 238: 370-379.
- Sugiura T, Dohi Y, Takase H, Yamashita S, Fujii S, et al. (2017) Oxidative Stress is Closely Associated with Increased Arterial Stiffness, Especially in Aged Male Smokers without Previous Cardiovascular Events: A Cross-Sectional Study. J. Atheroscler. Thromb 24: 1186-1198.
- Kawamoto R, Ninomiyax D, Kusunoki T, Kasai Y, Ohtsuka N, et al. (2016) Oxidative stress is associated with increased arterial stiffness in middle-aged and elderly community-dwelling persons. J Clin Gerontol 7: 136-140.
Citation: Bereda G (2022) Risk Factors, Classifications and Pathogenesis of Diabetic Neuropathy. J Diabetes Metab Disord 9: 042.
Copyright: © 2022 Gudisa Bereda, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

