
Risk factors for Depression in Chinese youth population with untreated GD-A Retrospective Observational Study
*Corresponding Author(s):
Sumon Rahman ChowdhuryDepartment Of Diabetes, Endocrinology And Metabolism, Chittagong Diabetic General Hospital, Chittagong, Bangladesh
Email:sumonrahman79@yahoo.com
Abstract
Back ground
Graves’ disease (GD) is becoming more and more prominent among the young generation of China. This disease often causes psychiatric symptoms, but there are a few studies in this field. The aim of this article is to find out the risk factors for depression in Chinese youth population with untreated GD.
Methods
1158 patients aged 14 to 45 years, with newly diagnosed, untreated GD were enrolled. Epidemiological data was collected. Depression was defined by Hamilton Depression Scale (HAMD24) score above 20 points. Thyroid function was determined for all patients. A family questionnaire including Adaption, Partnership, Growth, Affection, Resolve (APGAR questionnaire) and a normal Chinese Life Event Stress questionnaire (LES questionnaire) were completed for the evaluation of family functioning and life stress respectively. The association between depression and the collected parameters were analyzed. Results: The overall incidence of depression was 54.6% (male 54.0%, female 54.7%). The prevalence of depression in the 40 to 45 years old group was 72.2%. Coincidentally, in the 40 to 45 years old group, the incidence of depression in female patients was 79.8%. In the less than 20 years old group, the prevalence of depression in male patients was 38.4%. The relative risk of age, marital status, level of free triiodothyronine (FT3), level of thyrotropin receptor antibodies (TR-Ab), Graves' Ophthalmopathy (GO), life events was 1.385, 1.412, 1.887, 2.469, 2.367, 1.003 respectively while the relative risk of income, level of thyroid stimulating hormone (TSH), and family functioning was 0.252, 0.361, 0.213 respectively.
Conclusion
Youth population with untreated GD in Shandong Province had a higher prevalence of depression. Older female patients with GD were more vulnerable to depression. Age, marital status, level of FT3, level of TR-Ab, GO, life events were risk factors of depression in young patients with GD while income, level of TSH and family functioning were protective factors.
Keywords
Depression; Epidemiology; Graves’ disease; Protective factors; Risk factors
INTRODUCTION
GD is the most common form of hyperthyroidism. Patients with GD especially young women usually present with psychiatric symptoms including emotional instability, impatience, irritability, insomnia, etc. In elder patients with GD, depression or the coexistence of anxiety and depression were observed [1]. 15.2% of the patients still showed mental disorders even once who were believed to be euthyroid [2]. In recent years, the incidence of GD in China had gradually increased. Patients with GD exhibited considerably more significant psychiatric symptoms when compared with other diseases. Sometimes psychiatric symptoms occurred as the first symptoms in patients with untreated GD, which delayed the correct diagnosis. According to the World Health Organization (WHO), 350 million people around the world suffer from depression, representing around 20% of the population. The incidence of depression in patients with hyperthyroidism was 31-69% [3,4]. In this article, depression and risk factors in youth population with untreated GD in Shandong Province would be analyzed.
SUBJECTS AND METHODS
Subjects
From July 2008 to December 2018, 1158 young inpatients and outpatients between 14 and 45 years old with newly diagnosed, untreated GD in our hospital were selected in the analysis (male 261, female 897, males/females 1:3.44, mean age ± standard deviation: 31.67±11.99 years). The study protocol was approved by the Committees on the Ethics of Human Research of Qilu Hospital of Shandong University and adhered to the tenets of declaration of the Helsinki. Written informed consents were obtained from all participants.
All of the subjects met the following criteria:
Inclusion criteria: (1) typical clinical manifestations of GD; (2) thyroid function: level of FT3>8.5pmol/L (2.0-8.5pmol/L) or (and) level of free tetraiodothyronine (FT4) >25.5pmol/L (9.5-25.5pmol/L), level of TSH <0.5uIU/ml (0.5-5uIU/ml), level of TR-Ab >1.75IU/L (0-1.75IU/L). The latter two were essential conditions;
Exclusion criteria: (1) exclude other causes of thyrotoxicosis; (2) all subjects that had not used anti-thyroid drugs and depression-related drugs; (3) exclude a history of thyroid diseases and mental diseases.
Methods
Epidemiological data
Demographic characteristics including gender, age, marital status, occupation, education level, income, alcohol consumption, smoking, family history of thyroid diseases, body mass index (BMI = weight / height2) and other demographic characteristics were collected. Thyroid function was assessed by the measurement of FT3, FT4, TSH, and TR-Ab in fresh fasting plasma (3ml, collected at 8am ~ 10am) with electrochemiluminescence actual immunity analyzer (Elecsys.2010). Intra-assay and inter-assay coefficient of variability was <10%.
Proptosis was assessed using a Hertel exophthalmometer. The diagnosis of GO was confirmed in patients with NOSPECS II-IV according to NOSPECS classification [5]. Goiter size was graded according to the criteria recommended by WHO 1994 (Indicators for assessing iodine deficiency disorders and their control through salt iodization. Geneva: World Health Organization & United Nations Children’s Fund & International Council for the Control of Iodine Deficiency Disorders.)
Family functioning
Family functioning was assessed with family APGAR questionnaire; a closed questionnaire, designed by Smilkstein in 1978 [6].
Life Event Stress (LES)
Life event stress was assessed with the Chinese version of the Perceived Stress Scale-10 [7]. Major life events experienced within 12 months before the onset of GD were provided by patients. Corresponding Life Event Unit (LEU) was selected according to the age of subjects, and then the total score of LEU was accumulated.
Depression
Depression status in patients one week before the survey was assessed by two trained raters applying Hamilton Depression Scale (HAMD24). According to the Demarcation criteria (Davis JM), depression was confirmed when score >20 points.
STATISTICAL ANALYSIS
Statistical analysis was conducted using SPSS software package (version 20.0). Descriptive statistical analysis was used to describe more than two proportions. Chi square test of contingency table was applied for the comparison among more than two proportions. Linear Regression was applied for bivariate association analysis for quantitative traits. The analysis of rank correlation (Spearman correlation) was applied for ranked data. Binary non-conditional logistic regression analysis was performed for analysis of risk factors using likelihood ratio estimation (forward selection).
RESULTS
Demographic characteristics
Gender distribution: The survey included 1158 young patients with GD, 897 women (77.5%) and 261 men (22.5%). Among patients with GD, the constituent ratios of marital status, education level, income and other demographic characteristics between genders were not significantly different (p>0.05) except age (χ2=14.41, p=0.00). A total of 632 patients with GD were associated with depression (54.6%) including 491 women (54.7%), 141 men (54.0%), which indicated that it was not significantly different between genders (χ2=0.04, p=0.84).
Age Distribution: As can be seen from the tables, GD mainly occurred in young people between 30 and 40 years old, among which women were significantly prone to GD than men (χ2=14.41, p=0.00). The survey screened young subjects according to WHO criteria (14-45 years old), so there were relatively less patients with GD in the 40-45 years old group. However, the prevalence of depression in this age group was significantly higher than other age groups (p=0.00). There was a significant difference between each age group for the prevalence of GD associated with depression in youth population (χ2=122.80, p=0.00). Age was positively correlated to depression scores (r=0.45, p<0.05).
In subgroups split by gender, the prevalence of depression in male and female patients had their own characteristics: 1) in young female patients with GD, the prevalence of depression between each age group was different (χ2=132.814, p=0.00). The prevalence of depression in male patients was not correlated to age (χ2=2.09, p=0.55). 2) In the age group (2=8.92, p=0.00). In the age group (20-30 years old), the prevalence of GD associated with depression was not different between genders. In the age group (30-40 years old and 40-45 years old), the prevalence of GD associated with depression in female patients was higher than male patients respectively (χ2=5.19, p=0.02; χ2=4.06, p=0.04).
Marital status: In the group of divorced patients with GD, the prevalence of depression was up to 72.9% which was significantly higher than groups of married and unmarried patients, and there was a significant difference between each group (χ2=97.41, p=0.00). Among all groups, the prevalence of depression in the group of unmarried patients was the lowest. In each subgroup there was no distinct difference between genders (χ2=1.05, p=0.59).
Education level: The education level of subjects with GD in the survey were collected (junior middle school: 43.8%, senior middle school: 28.7%, college degree: 21.2%, postgraduate degree: 6.1%) and there was no difference between the genders (χ2=4.92, p=0.17). The prevalence of GD patients with depression was 59.4% (junior middle school), 56.2% (senior middle school), 48.3% (college degree), and 33.8% (postgraduate degree) which indicated that there was significant difference between different education levels (χ2=22.82, p=0.00). In the female subgroup, the prevalence of depression in patients with different education level was significantly different (χ2=15.42, p=0.00) as well as in the male subgroup (χ2=12.08, p=0.00). The patients with the same education level were not statistically significant (p> 0.05) between genders.
Income: The lowest per capita disposable income of families which conformed to the well-off standards of living in Shandong Province was 2400 Yuan per month per person. In the survey, patients with GD whose incomes were less than 1240 Yuan per month (the minimum living security line) accounted for only 16.7% of all subjects, but its prevalence of depression was 58.2%. The number of patients with GD who didn’t come up to the well-off standard of living was 682 (58.9%), and the prevalence of depression in these patients was 61.7% which was much higher than patients with incomes > 2400 Yuan per month (affluent population). There were significant differences between each subgroup (χ2=49.6, p=0.00) while in the subgroups the difference between genders wasn’t statistically significant (p>0.05). Linear correlation analysis indicated that income was negatively correlated to depression scores (r=-0.351, p<0.05) [Table 1].
|
Number of subjects |
Number of patients with depression (prevalence of depression) |
||||||
|
female |
male |
total |
female |
male |
total |
||
|
Age(years) |
|||||||
|
<20 |
89(9.9) |
13(5.0) |
102(8.8) |
9(10.1) |
5(38.5) |
14(13.7) |
p<0.00 |
|
20-30 |
225(25.1) |
48(18.4) |
273(23.6) |
88(39.1) |
24(50.0) |
112(41.0) |
|
|
30-40 |
464(51.7) |
153 (58.6) |
617(53.3) |
299(64.4) |
87(56.9) |
386(62.6) |
|
|
40-45 |
119 (13.3) |
47(18.0) |
166 (14.3) |
95(79.8) |
25(53.2) |
120(72.3) |
|
|
Marital status |
|||||||
|
Unmarried |
154(17.2) |
33(12.6) |
187(16.1) |
37(24.0) |
10(30.3) |
47(25.1) |
p<0.00 |
|
Married |
575(64.1) |
167(64.0) |
742(64.1) |
329(57.2) |
89(53.3) |
418(56.3) |
|
|
Divorced |
168(18.7) |
61(23.4) |
229(19.8) |
125(74.4) |
42(68.9) |
167(72.9) |
|
|
Education |
|||||||
|
≤Junior |
391(43.6) |
117(44.8) |
508(43.9) |
234(59.8) |
68(58.1) |
302(59.4) |
p<0.00 |
|
Senior |
264(29.4) |
69(26.4) |
333(28.8) |
145(54.9) |
42(60.9) |
187(56.2) |
|
|
Middle |
|||||||
|
School |
|||||||
|
College |
194(21.6) |
52(19.9) |
246(21.2) |
93(47.9) |
26(50.0) |
119(48.4) |
|
|
Postgraduat |
48(5.4) |
23(8.8) |
71(6.1) |
19(39.6) |
5(21.7) |
24(33.8) |
|
|
E |
|||||||
|
Income |
|||||||
|
(yuan, per month <1240) |
152(16.9) |
42(16.1) |
194(16.8) |
87(57.2) |
26(61.9) |
113(58.2) |
p<0.00 |
|
per person) |
|||||||
|
1240-2400 |
377(42.0) |
111(42.5) |
488(42.1) |
239(63.4) |
69(62.2) |
308(63.1) |
|
|
2400-4000 |
272(30.3) |
81(31.0) |
353(30.5) |
130(47.8) |
37(45.7) |
167(47.3) |
|
|
>4000 |
96(10.7) |
27(10.3) |
123(10.6) |
35(36.5) 9(33.3) |
|
44(35.8) |
|
Table 1: The distribution of socio-demographic profile in Youth Graves’ disease patients with depression
Family functioning assessment: The data of family functioning was collected from patients with GD in the survey (good 20.7%, moderate disorder 32.6%, severe disorder 46.7%), and the prevalence of depression was 27.9% (good), 43.0% (moderate disorder), 74.5% (severe disorder) respectively. The prevalence of depression between the subgroups was significantly different (χ2=169.88, p=0.00) while it was not significantly different in subgroups between genders (p>0.05). APGAR score was negatively correlated to depression score (r= 0.583, p<0.00) [Table 2].
|
Number of patients with depression (prevalence of depression) |
|||||||
|
Number of subjects |
|||||||
|
female |
male |
total |
female |
male |
total |
||
|
APGAR Scores |
|||||||
|
7-10 |
198(22.1) |
42(16.1) |
240(20.7) |
56(28.3) |
11(26.2) |
67(27.9) p<0.00 |
|
|
4-6 |
282(31.4) |
95(36.4) |
377(32.6) |
117(41.5) |
45(47.4) |
162(43.0) |
|
|
0-3 |
417(46.5) |
124(47.5) |
541(46.7) |
318(76.3) |
85 (68.5) |
403(74.5) |
|
Table2: APGAR Scores: Family adaption, partnership, growth, affection, resolve questionnaire scores.
Binary multivariate logistic regression analysis: With depression scores as the dependent variable, with age, occupation, marital status, education level, income, alcohol consumption, smoking, family history of thyroid diseases, BMI, level of FT3, level of FT4, level of TSH, level of TR-Ab, GO, goiter degree and APGAR scores as the independent variable, correlation analysis was performed between two variables which indicated that age, marital status, education level, income, level of FT3, level of TSH, level of TR-Ab, GO, life events and family functioning were correlated to depression. Then binary non-conditional logistic regression analysis was performed between related variables which suggested that age, marital status, level of FT3, level of TR-Ab, GO and life events were risk factors of depression in patients with GD while income, level of TSH and family functioning were protective factors [Table 3].
|
Independent variable X |
Partial-regression coefficient B |
Standard err S.E. |
P-value Sig. |
Relative risk Exp(B) |
||
|
X2 |
Age |
0.326 |
0.012 |
0 |
1.385 |
|
|
X4 |
Marital status |
0.345 |
0.452 |
0 |
1.412 |
|
|
X6 |
Income |
-1.377 |
0.119 |
0.002 |
0.252 |
|
|
X11 |
Level of FT3 |
0.635 |
0.368 |
0.008 |
1.887 |
|
|
X13 |
Level of TSH |
-1.02 |
0.01 |
0.048 |
0.361 |
|
|
X15 |
Level of TR-Ab tTR-Ab |
0.904 |
0.429 |
0 |
2.469 |
|
|
X16 |
GO |
0.862 |
0.301 |
0.004 |
2.367 |
|
|
X18 |
Life events |
0.003 |
0.007 |
0.004 |
1.003 |
|
|
X19 |
Family functioning |
-1.548 |
0.324 |
0 |
0.213 |
|
Table 3: The Logistic regression in Graves’ disease with depression
Logistic regression equation:
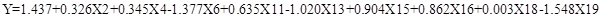
DISCUSSION
Parry described mental disorders in patients with thyroid disorders in 1825. Disturbances of thyroid metabolism in the mature brain may profoundly alter mental function which could affect cognition and emotion. GD is the most frequent cause of hyperthyroidism (excess hormone production) [8]. Genetic and environmental factors play roles in the pathogenesis of GD, reflected by an increased association with other autoimmune disorders and syndromes, both in the individual patient and in other family members [9-12]. Many reports concluded that emotional stress was related to the onset of GD [13-15]. Some mechanisms mentioned below are related to depression in patients with GD. Firstly, neuropsychiatric disorders are thought to be caused by the thyrotoxic condition and could be improved after treatment because thyroid hormones have chemical effects to psychological states including reinforcement of β-adrenergic effects. In fact, in small studies, only anti-thyroid drugs (ATD) or β blockers improved depression and anxiety associated with GD [16,17]. Secondly, long-term emotional stress, depression, intense grief were often the predisposing factors of GD [18]. Thirdly, the patients’ characters: GD patients’ characters were introversion and emotional lability. Data suggested that the mental disorders of the GD patients were caused by not only thyrotoxicosis but also the patients’ own character and emotional stresses [19].
At the onset of GD, various manifestations could be observed, and the neuropsychiatric symptoms usually manifested as sympathetic over activity including irritability, anxiety, uneasiness, insomnia, nervousness, loquacity, restlessness, impaired concentration, memory loss etc. which were much more obvious in GD than other diseases [2]. Young female patients were vulnerable to these neuropsychiatric symptoms above while elder patients usually presented with depression or the coexistence of anxiety and depression. Sometimes psychiatric symptoms occurred as the first symptoms in a few patients, which delayed the correct diagnosis of GD. In newly diagnosed patients with GD varied psychiatric symptoms usually occurred in the first few days after the onset of GD, no later than 17 months. There were 36.3 percent of patients with GD who showed mood disorders, 27.2 percent of patients who showed manic-like symptoms, and 15.2 percent of patients who mainly presented with schizophrenia [20]. After a 1-year follow-up study of patients with GD, researcher Lee in Taiwan found that among patients who developed psychiatric symptoms only half of them experienced a disappearance of psychiatric symptoms with the improvement of symptoms of GD, 34.6 percent of patients showed that part of the psychiatric symptoms could be cured, and 15.4 percent of the patients showed no significant improvements in psychiatric symptoms [21].
This survey included 1158 patients in youth population with untreated GD. Among these patients, 54.6 percent of them developed depression and there was no significant difference in the prevalence of depression between male and female. Depression mainly occurred in those young people who were older than 30. In the male group, the prevalence of depression in patients with GD tended to increase with age but there was no statistical significance between age groups, while in the female group, the prevalence of depression increased with age gradually, in up to 79.8% in patients older than 40. In a study including 13 hospitalized patients with newly diagnosed, untreated GD, Trzepace observed that 9 patients presented with depressive neurosis, similar to the results of this study [17]. Hein noticed that nearly 36.7 percent of patients with GD had mood disorders and some of them even showed mild mania and schizophrenia. What’s more, the onset of GD was significantly correlated to depression and anxiety [22]. Fahrenfort found that among 233 patients with GD nearly one third of patients presented with mood disorders. After 12 months of anti-thyroid drug therapy, one ninth of all subjects showed that mood disorders were still not alleviated, manifesting that attention, memory, analyzing ability, cooperative ability as well as the ability to solve complex problems significantly decreased when compared with the control group [23]. Over one third of female patients with GD who had a full-time job were unable to resume the same work even after witnessing an improvement in GD [24]. In this article, young people (14-45 years old, according to WHO criteria) who were the high-risk population of GD were observed. The prevalence of depression in this study was significantly higher than other studies which may have resulted from population selection. Researchers had shown that subclinical hyperthyroidism and overt hyperthyroidism were not risk factors for depression and anxiety [25], and changes in thyroid function did not affect the drug therapy for depression and anxiety [26]. However, changes of thyroid function in menopausal women were risk factors for depression [27], which agreed with our results.
Currently high divorce rates had become a social problem, and similarly in young patients with GD, the marital status had also become a risk factor for depression. The group of 229 divorced patients with GD accounted for only 19.8 percent of participants included in the study, while the prevalence of depression in this group was up to 72.9%, significantly higher than the non-divorced group. What’s more, the prevalence of depression in divorced women was higher than men, which indicated that divorced female patients with GD were more vulnerable to mental disorders. Canadian scholar Esms who conducted a study including 4053 subjects with previous history of thyroid diseases found that 177 divorced female patients and 110 non-divorced female patients presented with depression (p<0.001). This indicated that divorce could increase the prevalence of depression in female patients with thyroid diseases [28], which was similar to the conclusions of this study.
This study also found that the prevalence of depression in patients with GD who had low income, low education level was relatively higher. Further Logistic regression analysis suggested that education level was not a risk factor for depression which differed from the results of foreign researches [28] and it may be related to social habits and ethnic differences. It could also be seen in the regression analysis that age, marital status, level of FT3, GO and life events were significant risk factors for the coexistence of depression and GD. Lee IT studied 39 outpatients with thyrotoxicosis whose scores of depression and life events were significantly higher than euthyroid outpatients and healthy people [21]; which agreed with the results of this study. Researchers also found that the number and impact of negative Stressful Life Events (SLE) were significantly higher in GD compared to Toxic Nodular Goiter (TNG) and Control Groups (CG) which indicated that SLEs could be the causative factor for GD [29]. Patients with GO which is a characteristic manifestation of GD usually presented with photophobia, blurred vision, diplopia, ophthalmodynia, and sometimes even vision loss. Blindness could also be observed in severe cases. Proptosis and periorbital swelling could also affect the appearance and increase psychological burden on patients [30]. Lee H found that patients with GO had reduced health-related quality of life and the prevalence of depression in those patients were significantly higher than those without exophthalmos, especially in patients with a sight threatening condition or significant diplopia [31], which were similar to the results of this study.
LIMITATION OF THE STUDY
This study had one strength: it paid attention to the mental situation which played an important role in the development of disease and not only the disease itself. The study also had a limitation: it had revealed the risk factors of GD only and didn’t focus on the interventions to alleviate symptoms. So, in the future we can also do some studies about the interventions which may be helpful for the GD patients.
CONCLUSION
In conclusion, the prevalence of depression was higher in patients with GD, especially in older female patients with low income and low education. Age, marital status, level of FT3, GO, life events were risk factors for GD associated with depression. Income, level of TSH and family functioning were protective factors.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS CONTRIBUTION
Sumon Rahman Chowdhury contributed to the study conception and design, supervised the study, conducted data analysis and wrote the manuscript. Reza Haider Chy and Tasnuva Tanzil planned the study, were involved in data collection and prepared the first draft proposal. Satyajit Mallick, Abdullah Abu Sayeed and Sun Lei contributed on data analysis, supervised the study and critically revised the manuscript.
ACKNOWLEDGEMENT
This study was supported by The National Natural Science Foundation (No. 81000326) and the China Postdoctoral Science Foundation Grant (No. 2012M521354) of Shandong University Cheeloo School of Medicine, Shandong, China.
REFERENCES
- Gottfries CG (1998) Is there a difference between elderly and younger patients with regard to the symptomatology and aetiology of depression? Int Clin Psychopharmacol 13: 3-8.
- Lu CL, Lee YC, Tsai SJ, Hu PG, Sim CB (1995) Psychiatric disturbances associated with hyperthyroidism: an analysis report of 30 cases. Zhonghua Yi Xue Za Zhi (Taipei) 56: 393-398.
- Kathol RG, Delahunt JW (1986) the relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry 8: 23-8.
- Trzepacz PT, McCue M, Klein I, levey GS, Greenhouse J (1988) A psychiatric and neuropsychological study of patients with untreated Graves’ disease. Gen Hosp Psychiatry 10: 49-55.
- Eckstein AK, Plicht M, Lax H, Neuhauser M, Mann K, et al. (2006) Thyrotropin receptor autoantibodies are independent risk factors for Graves' ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab 9: 3464-3470.
- Smilkstein G (1978) the Family APGAR: A Proposal for a Family Function Test and Its Use by Physicians. The Journal of Family Practice 6: 1231-1239.
- Wei L, Qian B, Wenzheng W, Xiaoling W, Zhen W, et al. (2017) Chinese version of the Perceived Stress Scale-10: A psychometric study in Chinese university students. PLoS One 12: e0189543.
- Simone DL, Sun YL, Lewis EB (2016) Hyperthyroidism. Lancet 388: 906-918.
- Alessandro A, Silvia MF, Giusy E, Sabrina RP, Ilaria R, et al. (2020) Graves’ disease: Epidemiology, genetic and environmental risk factors and viruses. Best Practice & Research Clinical Endocrinology & Metabolism 76: 457-464.
- Brix TH, Hegedus L (2012) Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin Endocrinol (Oxf) 76: 457-464.
- Lee HJ, Li CW, Hammerstad SS, Stefan M, Tomer Y (2015) Immunogenetics of autoimmune thyroid diseases: a comprehensive review. J Autoimmun. 64: 82-90.
- Atsushi F, JuntaT, Takeshi A, MikaT, Toshio K, et al. (2020) Graves’ disease and mental disorders. Journal of Clinical & Translational Endocrinology 19: 100207.
- Fukao A, Takamatsu J, Miyauchi A, Hanafusa T (2014) Endocrine diseases. iConcept Press; Stress and thyroid disease.
- Mizokami T, Wu LA, El-Kaissi S, Wall JR (2004) Stress and thyroid autoimmunity Thyroid 14: 1047-1055.
- Kathol RG, Delahunt J (1986) The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry 81: 23-28.
- Katohl RG, Turner R, Delahunt J (1986) Depression and anxiety associated with hyperthyroidism: response to antithyroid therapy. Psychosomatics 27: 501-505.
- Trzepacz PT, McCue M, Klein I, Greenhouse J, Levey GS (1988) Psychiatric and neuropsychological response to propranolol in Graves’ disease. Biol Psychiatry 23: 678-688.
- Gulseren S, Gulseren L, Hekimsoy Z, Cetinay P, Ozen C, (2006) Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch Med Res 37: 133-139.
- Kendler KS, Gardner CO (2016) Depressive vulnerability, stressful life events and episode onset of Major depression: a longitudinal model. Psychol Med 46: 1865-1874.
- Placidi GPA, Boldrini M, Patronelli A, Fiore E, Chiovato L, et al. (1998) Prevalence of Psychiatric Disorders in Thyroid Disease Patients. Neuropsychobiology 38: 222-225.
- Lee IT, Sheu WHH, Liau YJ, Lin SY, Lee WJ, et al. (2003) Relationship of Stressful Life Events, Anxiety and Depression to Hyperthyroidism in an Asian Population. Horm Res 60: 247-251.
- Hein MD, Jackson IM (1990) Review: thyroid function in psychiatric illness. Gen Hosp Psychiatry 12 :232-44.
- Fahrenfort JJ, Wilterdink AM, Veen EA (2000) Long-term residual complaints and psychosocial sequelae after remission of hyperthyroidism. Psychoneuroendocrinology 25: 201-11.
- Nexo MA, Watt T, Pedersen J, Bonnema SJ, Hegedüs L, et al. (2014) Increased Risk of Long-Term Sickness Absence, Lower Rate of Return to Work, and Higher Risk of Unemployment and Disability Pensioning for Thyroid Patients: A Danish Register-Based Cohort Study. J Clin Endocrinol Metab 99: 3184-
- Oomen HAPC, Schipperijn AJM, Drexhage HA (1996) The prevalence of affective disorder and in particular of a rapid cycling of bipolar disorder in patients with abnormal thyroid function tests. Clinical Endocrinology 45: 215-
- Eker SS, Akkaya C, Sarandol A, Cangur S, Sarandol E, et al. (2008) Effects of various antidepressants on serum thyroid hormone levels in patients with major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry 32: 955-
- Pae Chi-Un, Mandelli L, Han C, Ham BJ, Prakash S, et al. (2009) Thyroid hormones affect recovery from depression during antidepressant treatment. Psychiatry and Clinical Neurosciences 63: 305-
- Bjelland I, Krokstad S, Mykletun A, Dahl AA, Tell GS, et al. (2008) Does a higher educational level protect against anxiety and depression? The HUNT Study. Soc Sci Med 66:1334-45.
- Matos-Santos A, Nobre EL, Costa JGE, Nogueira PJ, Macedo A, et al. (2001) Relationship between the number and impact of stressful life events and the onset of Graves' disease and toxic nodular goiter. Clinical Endocrinology 55: 15-19.
- Wickwar S, McBain HB, Ezra DG, Hirani SP, Rose GE, et al. (2015) Which factors are associated with quality of life in patients with Graves' orbitopathy presenting for orbital decompression surgery? Eye (Lond) 29: 951-
- Lee H, Roh HS, Yoon JS, Lee SY (2010) Assessment of Quality of Life and Depression in Korean Patients with Graves' Ophthalmopathy. Korean J Ophthalmol 24: 65-7
Citation: Chowdhury SR, Chowdhury RH, Tanzil T, Mallick S, Sayeed AA, et al. (2020) Risk factors for Depression in Chinese youth population with untreated GD-A Retrospective Observational Study, J Diabetes Metab Disord 7: 033.
Copyright: © 2020 Sumon Rahman Chowdhury, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

