
Solid Pseudopapillary Neoplasm of the Pancreas: Case Report
*Corresponding Author(s):
Martin Kukucka3rd Department Of Surgery, Comenius University, Bratislava, Slovakia
Tel:+ 421-2- 57887 401,
Email:kukuckamartin@yahoo.com
Abstract
Solid pseudopapillary neoplasm of the pancreas is a rare pancreatic tumor with low malignant potential, typically affecting young women. In literature this tumor may be referred to as Frantz tumor, solid tumor, cystic tumor, papillary-cystic tumor or solid pseudopapillary tumor. According to the current WHO classification from 2010 these tumors are considered low-grade malignant epithelial tumors of pancreas. In this case report we present a case of 19-years old women with cystic lesion located in left hypogastrium. After thorough evaluation and subsequent multidisciplinary consultation, the patient was indicated to radical resection of the lesion. Final histological evaluation of the surgical specimen revealed the diagnosis of solid-pseudopapillary neoplasm.
INTRODUCTION
Solid pseudopapillary neoplasm of the pancreas (SPN), sometimes also referred to as solid-cystic tumor, papillary-cystic tumor, Homoudi tumor or Frantz tumor, was first described in 1959 by V. K. Frantz [1]. At the time of the diagnosis, the SPN is usually localized within the pancreas, with only 10-15% cases presenting with distant metastases [2]. The diagnosis of SPN relies mainly on imaging techniques like ultrasonography or endoscopic ultrasonography, CT, MRI or PET/CT [3]. Radical surgical resection of the tumor is currently considered the treatment of choice, with excellent outcome [4,5].
CASE REPORT
We present a case of a 19-year-old female patient admitted to surgical clinic for planned resection of CT-verified extensive cystic formation in left hypogastrium. Over the period of past three years the patient described various unspecific symptoms including epigastric discomfort with stabbing pain, nausea and loss of appetite. A year prior to the surgery, abdominal ultrasonography revealed and unspecified cystic lesion located in left hypogastric region. For further evaluation of the abdominal mass, the patient underwent a CT examination, which confirmed the presence of extensive septated cystic lesion located in left hypogastrium, measuring 10×5×10 cm with thickness of the wall reaching up to 3 mm. The mass was located in retroperitoneum, the lower portion of the lesion reached to the upper pole of the left kidney, while the upper portion dislocated the spleen upwards. However, the exact tissue from which the tumor was growing was not possible to diagnose more accurately at this time.
Upper midline laparotomy with omental bursa incision exposed a well demarcated retrogastric tumor, adhering firmly to splenic hilar region, reaching the size of 12×12×8 cm. Tumor with the intact capsule was carefully dissected from the splenic hilus, with ligation of several marginal arteries to avoid splenectomy. Only the dissection of the mass forms the splenic hilus revealed the origin of tumor in the tail of pancreas.
In the immediate postoperative period, the patient presented with fever and the blood tests showed leucocytosis. Ultrasonographic examination revealed subcapsular splenic fluid collection, without splenic and portal vein thrombosis. In spite of prompt antibiotic therapy, the fever persisted while the leukocyte count showed mild decrease. Subsequent CT examination confirmed extensive infarction of the caudal portion of the spleen while the splenic artery was intact. On the 7th postoperative day relaparotomy was performed, which confirmed spleen infarction with small amount of turbid fluid in the perisplenic region, and lead to splenectomy.
Following the surgery, the fever persisted, and drains started to derive turbid fluid. Ultrasonography pointed out possible subphrenic abscess development. Targeted antibiotic therapy with meropenem was initiated, taking into account microbiological culture results. The microbiological culture from drain turbid fluid showed Klebsiella pneumoniae and Escherichia coli. Finally, the fever subsided, inflammatory parameters reached reference values, the function of gastrointestinal tract was resumed and the surgical suture healed by first intention. Histologic examination of the surgical specimen confirmed the diagnosis of solid-pseudopapillary neoplasm of pancreas. 24 days after initial hospitalization the patient was released from hospital, remaining in to the oncological follow-up care (Figure 1,2).
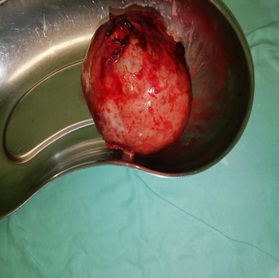 Figure 1: Resected tumor specimen.
Figure 1: Resected tumor specimen.
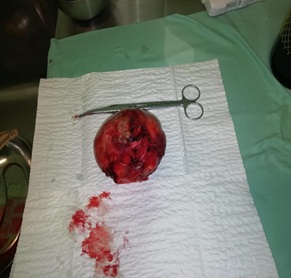 Figure 2: Resected tumor specimen.
Figure 2: Resected tumor specimen.
DISCUSSION
Solid-pseudopapillary neoplasm of the pancreas is considered to be a rare tumor, with low-malignant potential and overall good prognosis. The incidence of this tumor is according to available sources low, and accounts for approximately 1-2% of all pancreatic neoplasms [6,7]. SPN is typically diagnosed in young female patients, the median age being 22 years, only minority of cases is diagnosed after the age of 40 and in males [8]. SPN is usually well demarcated round mass, reaching large dimensions (>10 cm). According to WHO there is no preferred site of occurrence within the pancreatic tissue, however, some authors point out increased incidence in head and tail of pancreas [9]. Patients often present with number of non-specific symptoms, like dyspepsia, abdominal discomfort or epigastric pain. In some cases, the course of the disease may remain completely asymptomatic. Lack of specific symptoms often leads to delayed diagnosis. Physical examination is generally negative, in some cases there might be palpable mass in epigastric region. Laboratory diagnostic tests usually show serum and urine amylases within reference values. The diagnosis of SPN relies on imaging techniques, mainly ultrasonography, CT or MR, which visualize tumorous mass within the pancreatic tissue. More precise diagnosis can be obtained with the use of endoscopic ultrasonography, PET/CT or core needle biopsy [3,5]. The primary choice of treatment in majority of cases is radical surgical resection of tumor, leading to approximately 10 % recurrence rate after 4 years. Current findings consider complete resection of tumor sufficient in the treatment of SPT [4,5]. Prognosis of the SPN patients is overall favorable. In majority of cases the tumor is limited to pancreatic tissue, only 10-15% of patients present with distant metastases at the time of diagnosis. The five-year survival rate is as high as 95% to 97%, with an estimated 10-year survival rate of approximately 93% [10]. Most common site of distant spread is to liver, peritoneum, mesentery, omentum and regional lymph nodes [11]. Even in the case of distant spread of the disease, the overall survival of the patients remains high [12].
CONCLUSION
Solid-pseudopapillary neoplasm of pancreas is considered to be a rare pancreatic tumor, with low-malignant potential, favorable prognosis and sporadic distant spread, with radical surgical resection being the sufficient treatment of choice in the case of localized disease.
REFERENCES
- Frantz VK (1959) Tumors of the pancreas. In: Atlas of Tumor Pathology. Washington DC: AFIP 1959: 32-33.
- Reddy S, Cameron J, Scudiere J, Hruban R, Fishman EK, et al. (2009) Surgical management of solid pseudopapillary neoplasms of the pancreas (Franz or Hamoudi Tumors): a large single institutional series. JAm Coll Surg 208: 950-957.
- Zhang H, Liang T, Wang W, Shen Y, Ren GP, et al. (2006) Diagnosis and treatment of solid pseudopapillary tumor of the pancreas. Pancreas 5: 454-458.
- Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO Classification of Tumours of the Digestive System. WHO.
- Sun CD, Lee WJ, Choi JS, Oh JT, Choi SH (2005) Solid pseudopapillary tumors of the pancreas: 14 years experience. ANZ J Surg 75: 684-689.
- Tipton S, Smyrk T, Sarr M, Thompson GB (2006) Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg 93: 733-737.
- Martin R, Klimstra D, Brennan M, Conlon K (2002) Solid pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol 9: 35-40.
- Rebhandl W, Felberbauer FX, Puig S, Paya K, Hochschorner S, et al. (2001) Solid - pseudopapillary tumor of the pancreas (Frantz tumor) in children: report of four cases and review of the literature. Surg Oncol 76: 289-296.
- Bektas H, Werner U, Kaaden S, Philippou S, Kloppel G, et al (1999) Solid - pseudopapillary tumor of the pancreas – a rare and frequently misdiagnosed neoplasm. Langenbecks Arch Surg 384: 39-43.
- Chen X, Zhou GW, Zhou HJ, Peng CH, Li HW (2005) Diagnosis and treatment of solid pseudopapillary tumors of the pancreas. Hepatobiliary Pancreat Dis Int 4: 456-459.
- Mao C, Guvendi M, Domenico DR, Kim K, Thomford NR, et al. (1995) Papillary cystic and solid tumors of the pancreas: a pancreatic embryonic tumor? Studies of three cases and cumulative review of the world’s literature. Surgery 118: 821-828.
- Sclafani LM, Reuter VE, Coit DG, Brennan MF (1991) The malignant nature of papillary and cystic neoplasm of the pancreas. Cancer 68: 153-158.
Citation: Kukucka M, Danihel L, Oravský M, Rajcok M, Schnorrer M (2020) Solid Pseudopapillary Neoplasm of the Pancreas: Case Report. J Emerg Med Trauma Surg Care 7: 051
Copyright: © 2020 Martin Kukucka, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

