
Synergistic antiproliferative effect of mechlorethamine and amyloid beta-peptide on the human lung cancer cell line H441
*Corresponding Author(s):
Georgi L. LukovFaculty Of Sciences, Brigham Young University-Hawaii, Laie, HI, United States
Tel:808-675-3812,
Email:georgi.lukov@byuh.edu
Abstract
Mechlorethamine (ME) is an antineoplastic agent which has been in clinical use for more than six decades. ME therapy is associated with high toxicity, and it is often in combination with other therapeutics. The use of additional antiproliferative agents could allow the use of ME at lower concentrations, which could lead to comparable therapeutic results, but with lower toxicity. Recent findings have shown that the amyloid β-peptide (Aβ) has antiproliferative properties toward the human cancer cell line, H441 among others. In this study, the cooperative antiproliferative effect of Aβ and ME on the human cell line, H441, isolated from papillary adenocarcinoma lung tissue, was measured. Cells were cultured for 72 hours under standard culturing conditions in growth media containing either Aβ (2 µM), a very low dose of ME (0.2 µM), or both agents combined. We observed a 48% decrease in cell proliferation when Aβ and ME were used simultaneously, compared to 30% and 33% when ME or Aβ, respectively, were used independently. The study demonstrates that the antiproliferative properties of Aβ can augment the anticancer effect of toxic chemotherapeutics such as ME when used at lower doses.
Keywords
amyloid; mechlorethamine; cancer; chemotherapy; nitrogen mustard
Introduction
In 2020, there were approximately 20 million new cases and 10 million deaths, worldwide, attributed to cancer [1]. Recent projections indicate that there will be more than four million deaths related to lung cancer alone in the United States, from 2015-2065 [2]. Current treatments of many cancers utilize a wide array of chemotherapeutic agents [3, 4]. The alkylating agents, and more specifically the nitrogen mustard compounds, are a part of the anticancer chemotherapeutics. The highly reactive and cytotoxic properties of the nitrogen mustard have led to multiple modifications of the original compound with the intent to minimize the generalized toxic effects and to increase selectivity and specificity for given cancer cells [5]. Mechlorethamine (ME) is a nitrogen mustard compound, developed as an antineoplastic agent. As an alkylating agent, ME crosslinks the two DNA strands, which prevents DNA replication, and leads to cell death [5, 6]. ME has been in clinical use for more than six decades and has often been paired with other therapeutic agents to treat many forms of cancers such as lymphomas, leukemias, polycythemia vera and lung cancer [5-7]. Previous studies have found ME to be toxic for in vitro cultured A-431 skin cells at concentrations of 25 µM [8]. When used to treat neuroblastoma cell cultures, a 10 µM ME treatments led to more than 90% cell death [9]. Further in vitro cell studies indicated that treatments with 10-20 µM of ME caused irreparable DNA damage, resulting in irreversible cell cycle arrest [10]. Co-treatments with ME and ebselen or other related organoselenium analogs suggest that the toxicity of ME can be reduced when used in conjunction with other therapeutic agents.
Alzheimer’s disease (AD) is a common form of dementia which affects more than 44 million individuals globally [11-14]. The accumulation of amyloid-β peptides (Aβ) in the brain leads to the formation of β-amyloid plaques. The heightened neuroinflammatory state induced by the Aβ production has been linked to the onset of AD [13, 14]. Aβ has also been identified as an antimicrobial peptide (AMP) [15]. In vitro studies with endothelial cells infected with gram-negative bacillus bacterium, demonstrate that Aβ was produced following the infection [16]. Studies showing the AMP properties of Aβ, support the theory that infections causing increased production of Aβ may lead to AD. AMPs, including Aβ, have also been shown to act as anticancer peptides (ACPs) killing not only infected host cells but also cancerous cells [17-20]. These properties make ACPs an interesting field of study for their potential use as antiproliferative agents in conjunction with chemotherapeutics to augment their effectiveness.
The H441 cells (ATCC, HTB-174) are an epithelial cell line, which has been isolated from human lung papillary adenocarcinoma tissue. In this study, H441 cells were used to measure the synergistic antiproliferative effect of mechlorethamine and the beta-amyloid protein. The antiproliferative properties of Aβ appear to augment the anticancer effect of mechlorethamine, when ME is used at very low doses.
Methods
Cell Culture:
H441 cells were cultured in complete growth media DMEM/Ham’s F-12 50/50 with L-glutamine & 15 mM HEPES (Corning, 10-092-CV), supplemented with 10% by volume fetal bovine serum (ThermoFisher, A3160402) under standard conditions (37°C, in a humidified, 5% CO2 incubator). The cells were subcultured regularly to maintain active growth.
Mechlorethamine (ME) solution preparation:
Solid Mechlorethamine Hydrochloride (Sigma Aldrich, 122564) powder was removed from the protective canister and used to prepare 1 mg/mL ME solution, in serum-free DMEM/Ham’s F-12 50/50 media. The solution was gently triturated until the powder was completely dissolved. The homogeneous solution was then placed into a sterile 3 mL Luer lock syringe and filter sterilized, by passing it through a 0.2 μm syringe filter into a sterile tube. This sample became the stock solution used for the treatment assays.
β Amyloid (Aβ) peptide solution preparation:
In accordance with previous published protocols [21], 3 mL of 0.1 M NaOH and 3 mL of 1X PBS buffer were sterilized by 0.2 µm syringe disk filtration. 82.5 μL of the filtered 0.1 M NaOH were added to a vial containing 1 mg Aβ (residues 1–42), pre-treated with Hexafluoro-2-propanol (HFIP) (Sigma-Aldrich, AG968-1MG). The peptide was solubilized completely by gentle swirling and then incubated for 15 min. After that, 2 mL of the filtered PBS buffer were added, and the resulting solution was mixed and left undisturbed, at room temperature for 24 hours. The samples were then stored at 4°C until further use.
Treatment with ME and/or Aβ:
The cell concentration of an H441 cell suspension was determined with the help of a hemocytometer. In each test-well of a 96 well plate, 15,000 cells in 200 µL of complete growth media were cultured overnight, under standard conditions. On the next day, for the ME or Aβ individual treatments, the desired test concentration was achieved by adding the appropriate amount of the prepared ME or Aβ solution to each well. For the dual, ME and Aβ treatments, the cells in each well were incubated in media containing 0.2 µM ME and 2 µM Aβ. The total volume of each well was kept at 200 µL. The treated cells were incubated for 72 hours under standard conditions. Following the incubation period, the cells were counted and the total number of cells in each well determined.
Counting Cells:
The cells in each well were trypsinized and resuspended to a single cell suspension in 75 µL of media. The cell suspension was then mixed with an equal volume of Trypan Blue and incubated for 5 min to distinguish live from dead cells. The mixture was then loaded on a hemocytometer and the concentration and total number of live cells for each sample was determined.
Results
In order to investigate whether or not the amyloid β-peptide can potentiate the antiproliferative effect of mechlorethamine, the used concentration of ME should be sufficiently low, and has a partial effect on cell proliferation. To identify such low ME concentration, the proliferation of the lung cancer cell line, H441, was studied in the presence of variable concentrations of ME. The levels used in the initial assays were 0.0 µM (control sample), 2.5 µM, 5.0 µM, 10.0 µM, and 20.0 µM. For each trial, 15,000 cells were plated per well of a 96 well plate. On the next day, ME was added at the specified concentrations, and the cells were cultured for 72 hours under standard conditions. Then, the number of cells in each well was determined (Figure 1). The observed effect was significant and substantial even at 2.5 µM of ME.
Since the 2.5 µM concentration of ME still had a pronounced effect on the proliferation of the H441 cells, the cell assays were repeated with even lower concentrations of ME, 0.2, 0.4, 0.6, 0.8, 1.0, and 2.5 µM. Figure 2 shows the average number of H441 cells in each well, after 72 hours of ME exposure. For the lowest ME concentration used in this assay, the number of cells decreased by 35% compared to the control. The 0.2 µM concentration of ME, which is 50 to 125 times lower than the reported concentrations used to suppress cell proliferation, was selected for future studies because it produced a reasonable effect in suppressing H441 cell proliferation and provided a sufficient range to demonstrate whether or not Aβ would add to that effect.
Prior studies have indicated that 2 µM of Aβ can decrease the proliferation of the H441 cells as well. To test if Aβ can augment its antiproliferative effect to that of ME, an assay was performed where 2.0 µM of Aβ and 0.2 µM of ME were used individually and combined. This assay was repeated 5 times, in duplicates, following the same protocol for treating H441 cells with ME. The average number of cells in each well, following 72 hours of treatment, is shown in Figure 3. Each individual, ME or Aβ, treatment decreased the number of cells in the treated wells by approximately 30% (33% for Aβ, and 30% for ME). The obtained results were statistically significant with p values < 0.05, as determined by a paired t-test. The combined treatment of ME and Aβ caused a further decrease in the number of cells when compared to the individual treatments. Wells treated with ME and Aβ contained 48% less cells compared to the non-treated control. If the treatment with ME on its own is considered as a 100% reference point, the addition of Aβ causes an additional 25% decrease in the number of cells. The observed data is statistically significant, with a p value < 0.01, based on a paired t-test. These observations suggest that ME and Aβ can work together in suppressing cell proliferation, offering a viable option to explore in terms of finding less toxic, yet effective ways of suppressing cancer cell proliferation.
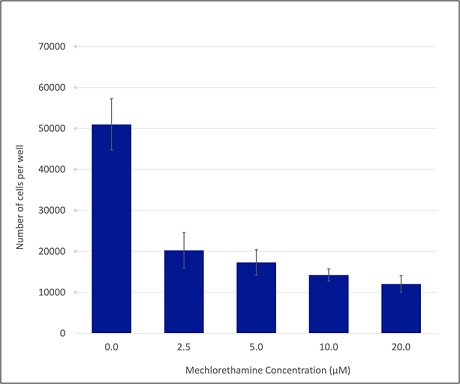 Figure 1: Mechlorethamine concentration-dependent effect on the number of H441 cells. H441 cells were treated with the specified ME concentrations for 72 hours under standard conditions. Following the incubation, the number of cells in each well was determined. The bar graph represents the average number of cells per well, from 4 trials. Each individual assay was done in duplicates. The error bars show the corresponding experimental error, calculated as the standard error of the mean (SEM).
Figure 1: Mechlorethamine concentration-dependent effect on the number of H441 cells. H441 cells were treated with the specified ME concentrations for 72 hours under standard conditions. Following the incubation, the number of cells in each well was determined. The bar graph represents the average number of cells per well, from 4 trials. Each individual assay was done in duplicates. The error bars show the corresponding experimental error, calculated as the standard error of the mean (SEM).
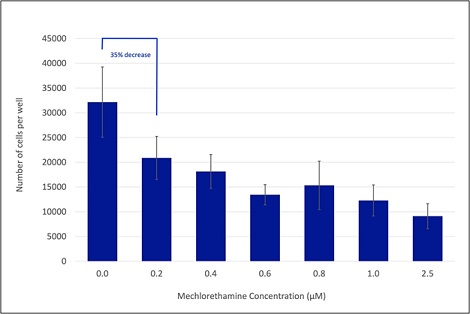 Figure 2: Mechlorethamine concentration-dependent effect on the number of H441 cells, at lower ME concentrations. H441 cells were treated with the specified ME concentrations for 72 hours under standard conditions. Following the incubation, the number of cells in each well was determined. The bar graph represents the average number of cells per well, from 4 trials. Each individual assay was done in duplicates. The error bars show the corresponding experimental error, calculated as the standard error of the mean (SEM). The percent difference between the number of cells in the 0.2 µM and the non-treated (0.0 µM) control is also designated.
Figure 2: Mechlorethamine concentration-dependent effect on the number of H441 cells, at lower ME concentrations. H441 cells were treated with the specified ME concentrations for 72 hours under standard conditions. Following the incubation, the number of cells in each well was determined. The bar graph represents the average number of cells per well, from 4 trials. Each individual assay was done in duplicates. The error bars show the corresponding experimental error, calculated as the standard error of the mean (SEM). The percent difference between the number of cells in the 0.2 µM and the non-treated (0.0 µM) control is also designated.
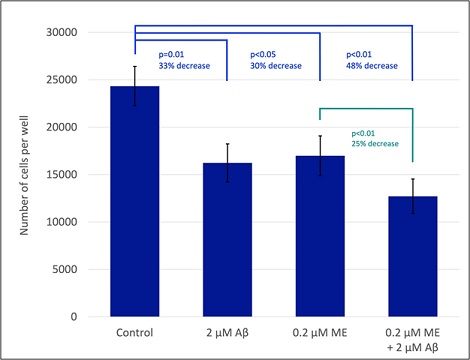 Figure 3: Individual and combined effect of ME and Aβ treatments on the number of H441 cells. H441 cells were treated with the specified ME and/or Aβ concentrations for 72 hours under standard conditions. Then, the number of cells in each well was determined. The bar graph represents the average number of cells per well, from 5 trials, where each individual assay was done in duplicates. The error bars show the corresponding experimental error, calculated as the standard error of the mean (SEM). The percent difference and the corresponding statistical significance, p values, between two various samples is also designated. The p values were calculated using paired t-tests.
Figure 3: Individual and combined effect of ME and Aβ treatments on the number of H441 cells. H441 cells were treated with the specified ME and/or Aβ concentrations for 72 hours under standard conditions. Then, the number of cells in each well was determined. The bar graph represents the average number of cells per well, from 5 trials, where each individual assay was done in duplicates. The error bars show the corresponding experimental error, calculated as the standard error of the mean (SEM). The percent difference and the corresponding statistical significance, p values, between two various samples is also designated. The p values were calculated using paired t-tests.
Discussion
The anticancer properties of mechlorethamine and the associated high toxicity of ME therapy are well established. Because of that, any efforts to decrease that toxicity while still providing adequate treatment are considered valuable. In more recent years, the antiproliferative effect of Aβ has been revealed. It is then plausible that ME and Aβ could work together to provide adequate suppression of cell proliferation, but with reduced side effects, by using doses lower than usual. In order to investigate if ME and Aβ can act synergistically in suppressing cellular proliferation, much lower than the recommended, 10 – 25 µM concentrations of ME were used in this study in conjunction with Aβ.
The obtained results indicate that a 30% decrease in the cell number of cultured H441 cells can be achieved with only 0.2µM ME, which is 50 to 125-fold lower than the reported treatment concentrations. This very low concentration of ME was then used in conjunction with Aβ, also shown to have a similar antiproliferative effect at a 2 µM concentration. When the low dose of ME was combined with that of Aβ, the cell numbers were further decreased compared to the non-treated control. The individual treatments resulted in approximately 30% decrease in the number of treated cells, while the combined, ME and Aβ, treatment caused a 48% decrease. The individual treatments were not completely additive when combined, but our results demonstrate that functionally, they complement each other. These observations strongly suggest that low doses of ME could achieve effective anticancer therapeutic outcomes, when combined with Aβ. These findings also indicate the potential synergistic nature of Aβ when used with other chemotherapeutic agents.
Further studies should be conducted to understand the mechanism of the antiproliferative effect of Aβ, and how exactly it acts synergistically with ME. [22] demonstrated that Aβ suppresses the function of the ABC transporters, which are known to expel foreign molecules, including medicinal agents, from stem, progenitor and cancer cells, increasing their survival and continued proliferation. It is likely that by inhibiting the ABC transporters, Aβ increases the therapeutic effectiveness of ME, thus further decreasing the number of the treated cells observed in this study. It will be interesting to investigate the potential Aβ has in supporting other anticancer therapeutics.
Having in mind the role Aβ has in the development of Alzheimer’s disease, the potential side effects from the use of Aβ as a therapeutic should also be investigated.
Acknowledgements
This work was supported by research funds provided by Brigham Young University–Hawaii.
References
- Ferlay JEM,Lam F,Colombet M,et al(2020)Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer.
- Jeon J(2018)Smoking and Lung Cancer Mortality in the United States From 2015 to 2065: A Comparative Modeling Approach. Ann Intern Med 169(10):684-693.
- Chabner BA,T G Roberts Jr(2005)Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer 5(1): 65-72.
- Perez-Herrero E,A Fernandez-Medarde(2015)Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm 93:52-79.
- Singh RK(2018) Therapeutic journery of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. Eur J Med Chem 151: 401-433.
- Mechlorethamine,( 2012)in LiverTox: Clinical and Research Information on Drug-Induced Liver Injury: Bethesda (MD).
- Liner K.,C Brown, LY McGirt (2018)Clinical potential of mechlorethamine gel for the topical treatment of mycosis fungoides-type cutaneous T-cell lymphoma: a review on current efficacy and safety data. Drug Des Devel Ther 12:241-254.
- Tumu HCR,(2020) Ebselen oxide attenuates mechlorethamine dermatotoxicity in the mouse ear vesicant model. Drug Chem Toxicol 43(4):335-346.
- Kisby GE,N Springer,PS Spencer(2000) In vitro neurotoxic and DNA-damaging properties of nitrogen mustard. J Appl Toxicol 20:35-41.
- Jan YH(2019)Sulfur Mustard Analog Mechlorethamine (Bis(2-chloroethyl)methylamine) Modulates Cell Cycle Progression via the DNA Damage Response in Human Lung Epithelial A549 Cells. Chem Res Toxicol 32(6):1123-1133.
- Dumurgier J,S Sabia(2020)Epidemiology of Alzheimer's disease: latest trends.Rev Prat 70(2): 149-151.
- Mayeux R,Y Stern(2012)Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2(8).
- Alzheimer's disease facts and figures(2021). Alzheimers Dement 17(3):327-406.
- Alzheimer's A(2013) Alzheimer's disease facts and figures. Alzheimers Dement 9(2):208-245.
- Fulop T(2018) Can an Infection Hypothesis Explain the Beta Amyloid Hypothesis of Alzheimer's Disease? Front Aging Neurosci 10:224.
- Balczon R(2018) Methods for Detecting Cytotoxic Amyloids Following Infection of Pulmonary Endothelial Cells by Pseudomonas aeruginosa. J Vis Exp 137:57447.
- Deslouches,B,YP Di (2017)Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget 8(28): 46635-46651.
- Pavliukeviciene B(2019)Amyloid beta oligomers inhibit growth of human cancer cells. PLoS One 14(9).
- Veloria JR,(2018) Novel cell-penetrating-amyloid peptide conjugates preferentially kill cancer cells. Medchemcomm 9(1):121-130.
- Trevor Tuthill,JR,Emma Barry, Aisha Liongi,(2020)The effects of amyloid β-protein on the proliferation of human lung papillary adenocarcinoma. Impulse: The Premier Journal for Undergraduate Publications in the Neurosciences.
- Hellstrand E,(2010)Amyloid beta-protein aggregation produces highly reproducible kinetic data and occurs by a two-phase process. ACS Chem Neurosci 1(1):13-18.
- Molnar J, I Ocsovszki,R Pusztai(2018)Amyloid-beta Interactions with ABC Transporters and Resistance Modifiers. Anticancer Res 38(6):3407-3410.
Citation: Georgi L. Lukov. Synergistic antiproliferative effect of mechlorethamine and amyloid beta-peptide on the human lung cancer cell line H441. J Cancer Biol Treat 7: 017.
Copyright: © 2021 Lee B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

