
The Effect of Low Intensity Laser Irradiation on Breast Cancer Cells and Breast Cancer Stem Cells
*Corresponding Author(s):
Heidi AbrahamseLaser Research Centre, Faculty Of Health Sciences , University Of Johannesburg, P.O. Box 17011, Doornfontein, 2028, South Africa
Tel:+27 115596550,
Fax:+27 115596884
Email:habrahamse@uj.ac.za
Abstract
The mechanism by which tumor proliferation, invasiveness and recurrence are sustained in malignant cancer has not been fully elucidated. Taking into account the findings of previous researches, one have strong reasons to believe that it might result from a small population of cells referred to as Cancer Stem Cells (CSCs). This study aimed to investigate and compare the photobiomodulative effect of Low Intensity Laser Irradiation (LILI) treatment on Breast Cancer Stem Cells (BCSCs) and Breast Cancer Cells (BCCs). BCSCs were isolated from the MCF-7 cell line based on their CD44+ phenotype using magnetic-activated cell sorting. CD44 antigen was detected in BCSCs using fluorescent microscopy. Cellular response to the treatment was evaluated based on their viability, proliferation and toxicity. Positive detection of CD44 confirmed the stemness of isolated BCSCs. Treated BCCs and BCSCs showed an increase in their proliferation and viability after being exposed to 5-40 J/cm2 using wavelengths of 636, 825 and 1060 nm. Membrane integrity assay revealed a decrease in cytotoxicity in both BCCs and BCSCs after treatment with low fluences of LILI. This study revealed that LILI did not have a bioinhibitory effect on both cell types.
Keywords
INTRODUCTION
Over the years, countless research projects have intended to find more responsive cancer treatments with improved outcome. Despite substantial therapeutic improvements, post-therapeutic loco regional and systemic recurrence remains a major issue encountered and new treatment strategies are urgently needed. As cancer recurrence would originate from residual therapy-resistant cells with the capacity to generate the original cancer phenotype, the lack of success of conventional therapeutic approaches to definitely cure the majority of solid cancers including breast cancer has re-ignited attention to the controversial Cancer Stem Cell (CSCs) theory of tumor initiation, therapeutic resistance and recurrence. The discovery of healthy stem cells and understanding of their properties has revived interest in the role that might play their cancerous counterparts in the repopulation of tumor sites after treatment. The ever-increasing advances in molecular biology have launched disruptive novel techniques such as molecular profiling (gene expression profiling) which have revolutionized our understanding of the heterogenic nature of breast cancer. As a result, CSCs have been identified as a minority of undifferentiated cell subtypes within a tumor mass, with stem-like properties and the ability of tumor regeneration. Healthy stem cell behaviour, also referred to as their stemness, is sustained by a group of signaling pathways, notably Notch, Hedgehog and Wnt, which turn out to play a similar role in CSCs behavior including self-renewal, differentiation, and fate determination [2]. Minor alterations in their regulation could induce drastic consequences and lead to malignant tumor formation [2,3]. It has been confirmed that these signaling pathways involved in the regulation of stemness of healthy stem cells happen to be altered in their cancerous counterpart [4]. In this regard, great understanding of their functioning will allow for a deeper understanding of the role that they could play in CSCs tumorigenic phenotype which could lead to the development of better therapeutic approaches [5].
With undeniable advances in science, it is now possible to identify and isolate CSCs from an entire tumor mass cell population. With methods such as magnetic-activated cell sorting (MACS), breast CSCs can be identified as a minority of cells expressing the hyaluronic receptor CD44 and subsequently be isolated. CD44 is a commonly expressed, multifunctional, cell-surface trans-membrane antigen and the main receptor of Hyaluronic Acid (HA) which is one of the principal components of the Extracellular Matrix (ECM). This trans-membrane antigen has always been associated with the cell to cell interaction, cell adhesion, migration, stem cell homing and tumor metastasis [6]. It has also been identified as a cancer stem cell marker for several cancers including breast cancer [6]. The CD44+/CD24-/low phenotype supposedly differentiates tumorigenic (tumor initiating) from non-tumorigenic breast cancer cells within a malignant tumor mass [6,7]. Therefore, the possible involvement of this transmembrane glycoprotein in the carcinogenesis of various solid cancers has been intensively studied over the past years. Most published data have linked CD44 expression with CSCs phenotype and Epithelial-to-Mesenchymal Transition (EMT) [8]. Epithelial-to-mesenchymal transition is the basis of CSCs plasticity and has been observed in breast CSCs [9]. Studies have closely linked EMT to the metastasis and drug resistance observed in CSCs. In addition, EMT has been associated with the shifting of cancer cells from the differentiated to the undifferentiated state [9,10]. This supports other data that have associated high expression of CD44 in breast cancer with tumor initiation, growth, invasion, metastasis, therapeutic resistance and recurrence [11]. Hence, abnormal expression of CD44 has been highly linked to the increased efficiency of distant metastasis and the poor survival rate observed in patients with malignant breast cancer [6,12]. Interestingly, it has been revealed that blockage of CD44 expression seems to significantly diminish malignant phenotype in CSCs, slow their progression and reverse their resistance to conventional therapeutic approaches [8].
LILI, which involves the application of red or near infrared lasers irradiating between 600-1100 nm, was introduced as an alternative, non-invasive, therapeutic approach for various medical conditions such as osteoarthritis, rheumatoid arthritis, post-mastectomy lymphedema and chronic diabetic wound. The effectiveness of this phototherapy has been proven by the positive outcomes of findings and is no longer doubted [13,14]. Despite these positive findings, LILI remains a controversial treatment modality for some. The photobiomodulation effect of LILI relies on the sensitivity of specific cellular components (chromophores) to light photons applied. Studies have revealed that following photon absorption by photoacceptors of the mitochondrial respiratory chain, cellular activity could affect in one of the following ways: cell growth stimulation, production of anti-inflammatory response, enhanced cell regeneration, stimulation of long-term production of intracellular or extracellular reactive oxygen species (ROS overload), to name a few [15,16]. These photobiological responses to LILI don’t induce any significate elevation in temperature and highly depends on factors such as the intensity and wavelength of light applied, and the type of cell. Hence, LILI can be defined as a non-thermal photo-biomodulation technique using optical waves that usually correspond to the visible red or near-infrared (NIR) light and low fluencies to induce photobiological process at the cellular level [17,18].
Studies have revealed the biphasic dose effect of LILI on different types of non-cancerous and cancerous cells [18,19]. This refers to the ability of LILI to either induce cell proliferation or death depending on the light density applied. As CSCs are responsible for the therapeutic resistance that occurs when dealing with malignant cancers, their response to LILI might be different from other cells. Higher doses of light might be required to induce apoptosis in CSCs as compare to other cells [16]. The main objective of this investigative research study is to explore the possible therapeutic virtue of laser in the suppression of malignant breast cancer growth. Since cancer stem cells are thought to be directly involved in the therapeutic resistance observed in malignant cancer, they were our main focus.
MATERIALS AND METHODS
Cell culture
Magnetic-activated cell sorting
CD44 cell surface marker detection
LILI treatment
|
Parameters |
|
|
|
|
Laser type |
Semiconductor (Diode) |
|
|
|
Wavelength (nm) |
636 |
825 |
1060 |
|
Wave emission |
Continuous |
Continuous |
Continuous |
|
Power output (mW) |
± 75 |
± 94 |
± 75 |
|
Intensity (mW/cm2) |
8.26 |
10.35 |
8.26 |
|
Fluence (J/cm2) and corresponding exposure time |
5: 10 min 48 sec |
5: 8 min 18 sec |
5: 10 min 48 sec |
Prior to LILI treatment, the exposure time which corresponds to the amount of energy that is given to the cells depending on the fluences/doses required (5, 10, 20 or 40 J/cm2) were determined based on the laser power output, from which the intensity was calculated. The following formula was used to determine exposure time; Time (s) = Dose (J/cm2)/ Intensity (W/cm2). Intensity (W/cm2) = Power output (mW) / [(? x Diameter2)/ 4]. Readings of different lasers (636, 825 and 1060 nm diode lasers) power outputs were taken at cell (bench) level using a power meter. During LILI treatment, culture dishes containing cells were placed directly into the laser light beam area which was previously set at 3.3 cm diameter for equal distribution of the light energy into the cells inside the 3.3 cm diameter petri dishes.
Viability
Proliferation
Cytotoxicity
Statistics
RESULTS
CD44 cell surface marker detection
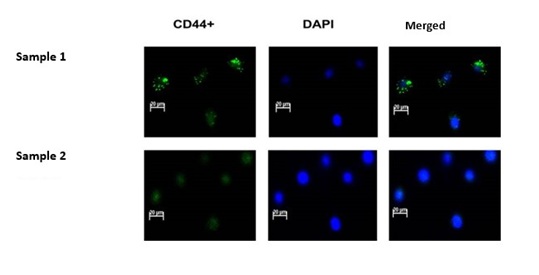 Figure 1: CD44 positive cells identification. Nuclear staining with DAPI was used to identify cells within slides. All DAPIlabelled cells appeared blue. Sample 1 represents isolated CD44 positive cells which have been labelled with CD44-FITC and have been identified as BCSCs. Green dots (fluorescence) representing the CD44 antigenic proteins could be observed on CD44-FITC positively labelled BCSCs. Sample 2 represents cells in which CD44-FITC staining results were negative. Only the blue DAPI nuclear dye could be seen in CD44 negative BCCs. Negative expression of CD44 confirmed the non-stem nature of BCCs.
Figure 1: CD44 positive cells identification. Nuclear staining with DAPI was used to identify cells within slides. All DAPIlabelled cells appeared blue. Sample 1 represents isolated CD44 positive cells which have been labelled with CD44-FITC and have been identified as BCSCs. Green dots (fluorescence) representing the CD44 antigenic proteins could be observed on CD44-FITC positively labelled BCSCs. Sample 2 represents cells in which CD44-FITC staining results were negative. Only the blue DAPI nuclear dye could be seen in CD44 negative BCCs. Negative expression of CD44 confirmed the non-stem nature of BCCs.Post-irradiation cell proliferation
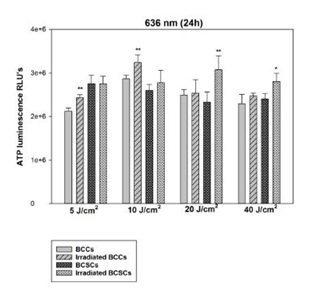 Figure 2: Changes in proliferation of BCCs and isolated BCSCs using 5, 10, 20 and 40 J/cm at 636 nm. In BCCs exposed to a 636nm diode laser statistically significant increases in ATP production were observed at 5 and 10 J/cm2 with a p-value of 0.01 (**). However, In BCSCs, statistical significance was only observed at 20 J/cm2 with a p value of 0.01 (**) and 40 J/cm2 with a p value of 0.05 (*). In both cell types, the ATP production did not decrease at any fluence.
Figure 2: Changes in proliferation of BCCs and isolated BCSCs using 5, 10, 20 and 40 J/cm at 636 nm. In BCCs exposed to a 636nm diode laser statistically significant increases in ATP production were observed at 5 and 10 J/cm2 with a p-value of 0.01 (**). However, In BCSCs, statistical significance was only observed at 20 J/cm2 with a p value of 0.01 (**) and 40 J/cm2 with a p value of 0.05 (*). In both cell types, the ATP production did not decrease at any fluence.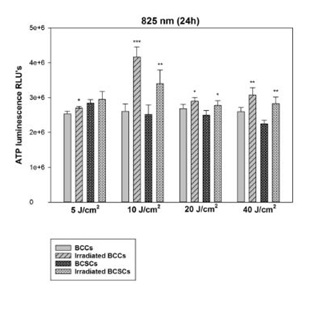 Figure 3: Changes in proliferation of BCCs and isolated BCSCs using 5, 10, 20 and 40 J/cm at 825 nm. After exposure to 825 nm, BCCs showed statistically significant increases in ATP production at 5 and 20 J/cm2 with a p-value of 0.05 (*) also at 10 with a p value of 0.001 (***) and 40 J/cm2 with p<0.01 (**). In BCSCs, statistical significance was observed at 10 and 40 J/cm2 with a p value of 0.01 (**), also at 20 J/cm2 with a p value of 0.05 (*). No decrease in ATP production could be seen in both BCCs and BCSCs, indicating that high fluences of 20 and 40 J/cm2 did not prevent cellular proliferation.
Figure 3: Changes in proliferation of BCCs and isolated BCSCs using 5, 10, 20 and 40 J/cm at 825 nm. After exposure to 825 nm, BCCs showed statistically significant increases in ATP production at 5 and 20 J/cm2 with a p-value of 0.05 (*) also at 10 with a p value of 0.001 (***) and 40 J/cm2 with p<0.01 (**). In BCSCs, statistical significance was observed at 10 and 40 J/cm2 with a p value of 0.01 (**), also at 20 J/cm2 with a p value of 0.05 (*). No decrease in ATP production could be seen in both BCCs and BCSCs, indicating that high fluences of 20 and 40 J/cm2 did not prevent cellular proliferation.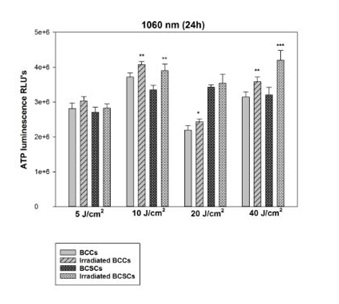 Figure 4: Changes in proliferation of BCCs and isolated BCSCs using 5, 10, 20 and 40 J/cm at 1060 nm. BCCs showed statistically significant increases in ATP production at 10 and 40 J/cm2 with a p-value of 0.01 (**) also at 20 J/cm2 with p<0.05 (*). On the other hand, BCSCs showed a statistically significant increase in their ATP production when using 10 J/cm2 with a p value of 0.01 (**) and 40 J/cm2 with a p value of 0.001 (***). No decrease in ATP production could be seen in both BCCs and BCSCs at high fluences of 20 and 40 J/cm2.
Figure 4: Changes in proliferation of BCCs and isolated BCSCs using 5, 10, 20 and 40 J/cm at 1060 nm. BCCs showed statistically significant increases in ATP production at 10 and 40 J/cm2 with a p-value of 0.01 (**) also at 20 J/cm2 with p<0.05 (*). On the other hand, BCSCs showed a statistically significant increase in their ATP production when using 10 J/cm2 with a p value of 0.01 (**) and 40 J/cm2 with a p value of 0.001 (***). No decrease in ATP production could be seen in both BCCs and BCSCs at high fluences of 20 and 40 J/cm2.Post-irradiation percentage viability
|
|
|
636 nm |
|
825 nm |
|
1060 nm |
|
|
5 J/cm2 |
BCCs control |
92 |
±1.581 |
96 |
±1.190 |
96 |
±0.408 |
|
|
BCCs test |
93 |
±1.601 |
95 |
±0.854 |
97 |
±0.408 |
|
|
BCSCs control |
95 |
±0.957 |
96 |
±0.750 |
94 |
±0.479 |
|
|
BCSCs test |
96 |
±0.323 |
97 |
±0.408 |
96 |
±0.854 |
|
10 J/cm2 |
BCCs control |
96 |
±479 |
96 |
±0.707 |
96 |
±0.479 |
|
|
BCCs test |
97 |
±408 |
96 |
±0.629 |
96 |
±0.408 |
|
|
BCSCs control |
96 |
±0.479 |
97 |
±0.479 |
96 |
±0.854 |
|
|
BCSCs test |
97 |
±707 |
98 |
±0.479 |
97 |
±0.707 |
|
20 J/cm2 |
BCCs control |
97 |
±0.866 |
96 |
±0.479 |
96 |
±0.479 |
|
|
BCCs test |
97 |
±0.629 |
97 |
±0.479 |
96 |
±0.289 |
|
|
BCSCs control |
97 |
±0.456 |
97 |
±0.408 |
96 |
±0.854 |
|
|
BCSCs test |
96 |
±0.479 |
98 |
±0.250 |
97 |
±0.750 |
|
40 J/cm2 |
BCCs control |
96 |
±0.479 |
97 |
±0.645 |
97 |
±0.645 |
|
|
BCCs test |
96 |
±0.479 |
95 |
±0.750 |
97 |
±0.629 |
|
|
BCSCs control |
97 |
±0.408 |
97 |
±0.289 |
96 |
±0.854 |
|
|
BCSCs control |
97 |
±0.408 |
97 |
±0.629 |
97 |
±0.816 |
At 636 nm using all four fluences, a slight yet not significant increase in the percentage of viable BCCs and BCSCs was observed. The same thing could be seen at 825 and 1060 nm. No statistically significant decrease in the percentage of viable cells could be noticed in both cell types. Hence, one could say that LILI treatment even using high fluences of 20 and 40 J/cm2 did not trigger cell death.
Post-irradiation cytotoxicity
|
|
|
636 nm |
|
825 nm |
|
1060 nm |
|
|
5 J/cm2 |
BCCs control |
0.662 |
±0.0041 |
0.663 |
±0.0039 |
0.682 |
±0.0329 |
|
|
BCCs test |
0.380 |
*** |
0.396 |
*** |
0.394 |
*** |
|
|
BCSCs control |
0.598 |
±0.0337 |
0.573 |
±0.0296 |
0.525 |
±0.0147 |
|
|
BCSCs test |
0.521 |
±0.0142 |
0.488 |
* |
0.518 |
±0.0025 |
|
10 J/cm2 |
BCCs control |
0.553 |
±0.0237 |
0.525 |
±0.0257 |
0.686 |
±0.0318 |
|
|
BCCs test |
0.362 |
*** |
0.340 |
*** |
0.357 |
*** |
|
|
BCSCs control |
0.507 |
±0.0133 |
0.503 |
±0.0128 |
0.579 |
±0.0195 |
|
|
BCSCs test |
0.333 |
*** |
0.451 |
* |
0.470 |
* |
|
20 J/cm2 |
BCCs control |
0.469 |
±0.0193 |
0.479 |
±0.0157 |
0.700 |
±0.0319 |
|
|
BCCs test |
0.412 |
* |
0.449 |
±0.0123 |
0.417 |
*** |
|
|
BCSCs control |
0.466 |
±0.0068 |
0.469 |
±0.00709 |
0.535 |
±0.0124 |
|
|
BCSCs test |
0.468 |
±0.0185 |
0.523 |
±0.0468 |
0.430 |
*** |
|
40 J/cm2 |
BCCs control |
0.590 |
±0.0091 |
0.592 |
±0.0089 |
0.612 |
±0.0037 |
|
|
BCCs test |
0.442 |
*** |
0.434 |
*** |
0.527 |
* |
|
|
BCSCs control |
0.518 |
±0.0108 |
0.515 |
±0.0119 |
0.654 |
±0.0103 |
|
|
BCSCs test |
0.459 |
** |
0.463 |
* |
0.539 |
*** |
At 636 nm, LILI treated BCCs portrayed a significant decrease in their LDH production with the highest changes with p2. Treatment with 20 J/cm2induced a drop in the LDH level with a p value of 0.05 (*). BCSCs on the other hand only had their LDH production dropped when using 10 and 40 J/cm2 with respective p values of 0.001 (***) and 0.01 (**).At 825 nm, a statistically significant decrease of LDH was observed in BCCs after treatment with 5, 10 and 40 J/cm2 all with p values of 0.001 (***). BCSCs had their LDH level lower following LILI treatment with 5, 10 and 40 J/cm2 all with p values of 0.05 (*). At 1060 nm, BCCs had their lowest level of LDH when treated with 5, 10 and 20 J/cm2 all with p values of 0.001 (***). Treatment with 40 J/cm2 induced a significant decrease in the level of LDH with p2. Treatment with 10 J/cm2induced a decrease in LDH with a p value of 0.05 (*). Note that no increase of LDH corresponding to the altered membrane integrity due to LILI treatment could be seen either in BCCs or in BCSCs irradiated samples.
DISCUSSION
The presence of a minority of cells expressing the hyaluronic receptor CD44 CSC marker in malignant adenocarcinoma MCF-7 cell line has been confirmed in this study. The tumor initiation ability of these CD44positive cells has been confirmed in previous studies [6,11]. These highly tumorigenic cells that have previously been identified and isolated from breast cancer are thought to play a role in the poor prognosis and to be responsible for tumor malignancy and all the consequences thus arising [11].
Contrary to what is expected of an anti-cancer treatment, among other things the eradication of cancer cells, low fluence LILI treatment had a biostimulatory effect on the proliferation and viability of both BCCs and BCSCs. This finding is consistent with previous data from studies on lung CSCs [18]. Results deduced from the proliferation assay showed a statistically significant increase in the production of ATP in BCCs when applying light density of 5 and 10 J/cm2 at wavelengths of 636, 5-40 J/cm2 at 825 nm and 10-40 J/cm2 at 1060 nm. However, in BCSCs, the proliferative effect of LILI could be seen after cells were exposed to 20 and 40 J/cm2 at 636 nm, 10-40 J/cm2 at 825 nm and finally 10 and 40 J/cm2 at 1060 nm. Light density of 5 J/cm2 did not induce any bio-stimulatory response in BCSCs. Trypan blue dye exclusion test that was conducted to determine the percentage of viable cells post treatment revealed no statistically significant changes in that matter. Results from the cytotoxicity assay that was carried to assess the membrane integrity following irradiation revealed that neither BCCs nor BCSCs had membrane damage post treatment with fluences ranging from 5 to 40 J/cm2. Some of these data were similar to the ones found in a study done on lung cancer stem cells in which doses of light of 5, 10 and 20 J/cm2 did not have a damaging effect on the cells membrane [18,20]. However, a statistically significant increase in the cell membrane damage was observed following exposure of lung cancer stem cells to 40 J/cm2, which was not the case in the present study. Data showed a statistically significant decrease in the level of LDH production in BCCs when using fluences of 5 to 40 J/cm2 at 636 and 1060 nm and 5, 10 and 40 J/cm2 at 825 nm. In BCSCs, a statistically significant drop in the LDH production could be seen at 636 nm when applying fluences of 10 and 40 J/cm2, at 825 nm when applying 5, 10 and 40 J/cm2 and finally at 1060 nm after exposure to light densities of 10 to 40 J/cm2.
Data of the present study demonstrated that even high fluences of 20 and 40 J/cm2 were not sufficient to induce any bioinhibitory effect on both BCCs and BCSCs unlike human adipose derived stem cells (non-cancerous cells) and lung cancer in which high fluence of 40 J/cm2 did incite a bioinhibitory response on the cellular level [18,19]. Results revealed that BCCs have different cellular response when compared to BCSCs after being exposed to the same treatment. This shows that different cells even from the same tumor bulk may react in different ways to the same treatment condition. Furthermore, breast cancer, in general, might be more resistant to conventional therapeutic approaches as compared to other cancer such as lung cancer.
CONCLUSION
Generally, the hypothesis supporting the possible curative effect of laser treatment has also been questioned. However, the beneficial effects of LILI using specific light parameters in the treatment of cancer and other medical conditions have been revealed [17]. The use of LILI alone with the aim to eradicate cancer cells has still not yield convincing results. On the other hand, LILI used in Photodynamic Therapy (PDT), which is the application of LILI in combination with a photoactive drug referred to as a photosensitizer, has shown better effectiveness in the treatment of cancer [22]. A metallophthalocyanine photosensitizer called Zinc phthalocyanine (ZnPcSmix) has shown effective stimulation and initiation of apoptosis in breast cancer [23]. Therefore, it is important to emphasise that PDT could be a potential therapeutic approach to take into consideration in cancer treatment.
AUTHORS CONTRIBUTION
FUNDING AND GRANT DETAILS
CONFLICT OF INTEREST
REFERENCES
- World Health Organization (WHO) (2013) Latest world cancer statistics Global cancer burden rises to 14.1 million new cases in 2012: Marked increase in breast cancers must be addressed. IARC, Pg no: 1-3.
- Sun H, Jia J, Wang X, Ma B, Di L, et al. (2013) CD44+/CD24- breast cancer cells isolated from MCF-7 cultures exhibit enhanced angiogenic properties. Clin Transl Oncol 15: 46-54.
- Regad T, Sayers T, Rees R (2015) Principles of Stem Cell Biology and Cancer: Future Applications and Therapeutics. Wiley-Blackwell Pg no: 376.
- Karamboulas C, Ailles L (2013) Developmental signaling pathways in cancer stem cells of solid tumors. Biochim Biophys Acta 1830: 2481-2495.
- Mohr M, Zänker KS, Dittmar T (2015) Cancer (stem) cell differentiation: An inherent or acquired property?. Med Hypotheses 85: 1012-1018.
- McFarlane S, Coulter JA, Tibbits P, O'Grady A, McFarlane C, et al. (2015) CD44 increases the efficiency of distant metastasis of breast cancer. Oncotarget 6: 11465-11476.
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci 100: 3983-3988.
- Xu H, Tian Y, Yuan X, Wu H, Liu Q, et al. (2015) The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targts Ther 8: 3783-3792.
- Luo M, Brooks M, Wicha MS (2015) Epithelial-mesenchymal plasticity of breast cancer stem cells: implications for metastasis and therapeutic resistance. Curr Pharm Des 21: 1301-1310.
- Mukherjee S, Mazumdar M, Chakraborty S, Manna A, Saha S, et al. (2014) Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/β-catenin negative feedback loop. Stem Cell Res Ther 5: 116.
- Basakran NS (2015) CD44 as a potential diagnostic tumor marker. Saudi Med J 36: 273-279.
- Isacke CM, Yarwood H (2002) The hyaluronan receptor, CD44. Int J Biochem Cell Biol 34: 718-721.
- Brosseau L, Welch V, Wells G, Tugwell P, de Bie R, et al. (2002) Low level laser therapy for osteoarthritis and rheumatoid arthritis: a metaanalysis. J Rheumatol 27: 1961-1969.
- Houreld N, Abrahamse H (2010) Low-Intensity Laser Irradiation Stimulates Wound Healing in Diabetic Wounded Fibroblast Cells (WS1). Diabetes Technol Ther 12: 971-978.
- Hamblin MR, Demidova TN (2006) Mechanisms of low level light therapy. SPIE BiOS, San Jose, California, USA, Pg no: 1-12.
- Kiro NE, Hamblin MR, Abrahamse H (2017) Photobiomodulation of breast and cervical cancer stem cells using low-intensity laser irradiation. Tumour Biol 39.
- Abrahamse H (2011) Inducing stem cell differentiation using low intensity laser irradiation: a possible novel therapeutic intervention. Cent Eur J Biol 6: 695.
- Crous AM, Abrahamse H (2016) High Fluence Low Intensity Laser Irradiation Bioinhibits Viability and Proliferation of Lung Cancer Stem Cells. J Stem Cell Res Ther 6: 368.
- Mvula B, Mathope T, Moore T, Abrahamse H (2008) The effect of low level laser irradiation on adult human adipose derived stem cells. Lasers Med Sci 23: 277-282.
- Crous AM, Abrahamse H (2013) Lung cancer stem cells and low-intensity laser irradiation: a potential future therapy?. Stem Cell Res Ther 4: 129.
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105-111.
- Mfouo-Tynga I, Houreld NN, Abrahamse H (2014) Induced cell death pathway post photodynamic therapy using a metallophthalocyanine photosensitizer in breast cancer cells. Photomed Laser Surg 32: 205-211.
- Venning FA, Wullkopf L, Erler JT (2015) Targeting ECM Disrupts Cancer Progression. Front Oncol 5: 224.
Citation: Kiro NE, Hamblin M, Adrahamse H (2019) The Effect of Low Intensity Laser Irradiation on Breast Cancer Cells and Breast Cancer Stem Cells. J Stem Cell Res Dev Ther: S1005.
Copyright: © 2019 Ndivito Elodie Kiro, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

