
Journal of Stem Cells Research Development & Therapy Category: Medical
Type: Short Commentary
The Tgf-B1 Over Expression has Major Impact on Multilineage Differentiation of Mesenchymal Stromal Cells
*Corresponding Author(s):
Hasan SALKINDepartment Of Medical Services Andtechnique, Programme Of Pathology Laboratory Techniques, Vocational School, Department Of Histology And Embryology, Faculty Of Medicine, Erciyes University, Büyükçekmece/Istanbul 34550, Kayseri, Turkey
Tel:+90 5301255808,
Fax:+90 2128722830
Email:hasansalkin@beykent.edu.tr
Received Date: Jul 24, 2019
Accepted Date: Aug 01, 2019
Published Date: Aug 08, 2019
Keywords
Mesenchymal Stromal Cells; adipocytes; osteoblasts; chondrocytes
Article
Mesenchymal Stromal Cells (MSCs) are multipotent stem cells with a superior capacity for self-renewal, and are capable of differentiating into adipocytes, osteoblasts, chondrocytes [1,2]. In these days, MSCs have been broadly used in regenerative medicine [3]. Dental Pulp-derived Mesenchymal Stem Cells (DPSCs) have become a popular research topic in that they are similar too the mesenchymal stem cells, easy to obtain and are used in regenerative medicine and tissue engineering [4]. In vitro and in vivo studies have been previously performed to understand the biology of DPSCs. The differentiation of mesenchymal stem cells usually involves the use of signaling factors as recombinant proteins or gene therapy that an functionally activate genes [5]. Transforming Growth Factor Beta 1 (TGF-β1) activates mainly SMAD transcription factors with complex cellular responses [6]. The superior proliferative ability and regenerative potential are primary phenotypes of MSCs [7].
Loss of therapeutic potential would limit their utilization in transplantation medicine. TGF-β1 has been reported to induce senescence in some tumor cells [8,9]. However, whether TGF-β affects senescence of DPSCs has still not been elucidated. Also, the effects on apoptosis, cell cycle and DNA damage of DPSCs of TGF-β1 over expression have not been investigated yet. In this study is to investigate the surface markers, multilineage differentiation, viability, apoptosis, cell cycle, DNA damage and senescence of Human Dental Pulp-Derived Mesenchymal Stromal Cells (hDPSC) which transfected by TGF-β1 gene. TGF-β1 gene transfer into hDPSCs was performed by electroporation method after the plasmid was prepared.
The transfection efficiency was achieved by using western blot and flow cytometry analyses and GFP transfection. Mesenchymal Stem Cell (MSC) markers, multilineage differentiation, cell proliferation, apoptosis, cell cycle, DNA damage and cellular senescence assays were performed by comparing the transfected and non-transfected cells. Strong expression of TGF-β1 in pCMV-TGF-β1-transfected hDPSCs was detected in flow cytometry analysis. TGF-β1 transfection efficiency was measured as 95%. Western blot analysis showed that TGF-β1 protein levels increased at third and sixth days in pCMV-TGF-β1-transfected hDPSCs. The continuous TGF-β1 overe xpression inhDPSCs did not influence the immunophenotype and surface marker expression of MSCs. Our results showed that TGF-β1 increased osteogenic and chondrogenic differentiation, but decreased adipogenic differentiation. Over expression of TGF-β1 increased the proliferation rate and decreased total apoptosis in hDPSCs. The number of cells at “S” phase was higher with TGF-β1 transfection. Cellular senescence decreased in TGF-β1 transfected group.These results reflect that TGF-β1 has major impact on MSC differentiation. TGF-β1 transfection has no effect on cell surface markers. TGF-β1 transfection has positive effects on proliferation, cell cycle and prevents cellular senescence and apoptosis. In further studies, it will be essential to determine whether TGF-β1 can play a role in attempts to use MSC for therapeutic approaches. With this study, cells were produced with increased differentiation potentials and strengthened biological features that may be used in regenerative medicine, tissue engineering, gene therapy and cellular therapy studies (Figure 1).
Loss of therapeutic potential would limit their utilization in transplantation medicine. TGF-β1 has been reported to induce senescence in some tumor cells [8,9]. However, whether TGF-β affects senescence of DPSCs has still not been elucidated. Also, the effects on apoptosis, cell cycle and DNA damage of DPSCs of TGF-β1 over expression have not been investigated yet. In this study is to investigate the surface markers, multilineage differentiation, viability, apoptosis, cell cycle, DNA damage and senescence of Human Dental Pulp-Derived Mesenchymal Stromal Cells (hDPSC) which transfected by TGF-β1 gene. TGF-β1 gene transfer into hDPSCs was performed by electroporation method after the plasmid was prepared.
The transfection efficiency was achieved by using western blot and flow cytometry analyses and GFP transfection. Mesenchymal Stem Cell (MSC) markers, multilineage differentiation, cell proliferation, apoptosis, cell cycle, DNA damage and cellular senescence assays were performed by comparing the transfected and non-transfected cells. Strong expression of TGF-β1 in pCMV-TGF-β1-transfected hDPSCs was detected in flow cytometry analysis. TGF-β1 transfection efficiency was measured as 95%. Western blot analysis showed that TGF-β1 protein levels increased at third and sixth days in pCMV-TGF-β1-transfected hDPSCs. The continuous TGF-β1 overe xpression inhDPSCs did not influence the immunophenotype and surface marker expression of MSCs. Our results showed that TGF-β1 increased osteogenic and chondrogenic differentiation, but decreased adipogenic differentiation. Over expression of TGF-β1 increased the proliferation rate and decreased total apoptosis in hDPSCs. The number of cells at “S” phase was higher with TGF-β1 transfection. Cellular senescence decreased in TGF-β1 transfected group.These results reflect that TGF-β1 has major impact on MSC differentiation. TGF-β1 transfection has no effect on cell surface markers. TGF-β1 transfection has positive effects on proliferation, cell cycle and prevents cellular senescence and apoptosis. In further studies, it will be essential to determine whether TGF-β1 can play a role in attempts to use MSC for therapeutic approaches. With this study, cells were produced with increased differentiation potentials and strengthened biological features that may be used in regenerative medicine, tissue engineering, gene therapy and cellular therapy studies (Figure 1).
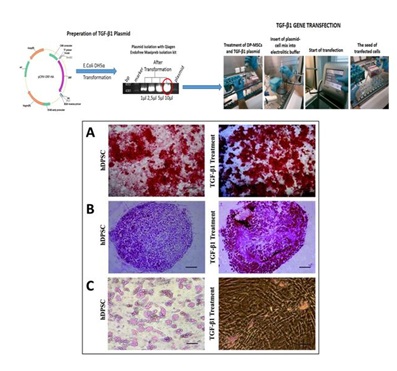 Figure 1: Osteogenic and chondrogenic differentiation was increased remarkable by over expression of TGF-β1 in DPSCs. However, adipogenic differentiation was reduced significantly in TGF-β1 transfected DPSCs compare with non-transfected hDPSCs. Particularly adipogenic differentiation was impaired by TGF-β1. Osteogenic differentiation and alizarin red staining.[10]
Figure 1: Osteogenic and chondrogenic differentiation was increased remarkable by over expression of TGF-β1 in DPSCs. However, adipogenic differentiation was reduced significantly in TGF-β1 transfected DPSCs compare with non-transfected hDPSCs. Particularly adipogenic differentiation was impaired by TGF-β1. Osteogenic differentiation and alizarin red staining.[10]A) Chondrogenic differentiation and safranin-ostaining.
B) Graphic show adipored assay fluorimetric measurement results for adipogenic differentiation.
C) Microscope magnifications are 4×.Scale bar is 100 μm. *p<0.05.
REFERENCES
- Zhang F, Hong Y, Liang W, Ren T, Jing S, et al. (2012) Co-culture with Sertoli cells promotes proliferation and migration of umbilical cord mesenchymal stem cells. Biochem Biophys Res Commun 427:86-90.
- Ferroni L, Gardin C, Tocco I, Epis R, Casadei A, et al. (2013) Potential for neural differentiation of mesenchymal stem cells. Adv Biochem Eng Biotechnol 129: 89-115.
- Patel DM, Shah J, Srivastava AS (2013) Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int 496218.
- Karaöz E, Do?an BN, Aksoy A, Gacar G, Akyüz S, et al. (2010) Isolationand in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol 133: 95-112.
- Rizk A, Rabie BM (2013) Electro poration for transfection and differentiation of dental pulp stemc ells. Biores Open Access 2: 155-162.
- Liang YY, Brunicardi FC, Lin X (2009) Smad3 mediates immediate early induction of Id1 by TGF-beta. Cell Res 19: 140-148.
- Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE (2009) Human mesenchymal stemcells self-renew and differentiate according to a deterministic hierarchy. PloSOne 4: 6498.
- Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, Cingoz B, Akcali KC, et al. (2010). Transforming growth factor-beta induces senescence in hepato cellular carcinoma cells and inhibits tumor growth. Hepatology 52: 966-974.
- Yu AL, Birke K, Moriniere J, Welge-Lüssen U (2010) TGF-{beta}2 induces senescence-associated changes in human trabecular mesh work cells. Invest Ophthalmol Vis Sci 51: 5718-5723.
- Salk?n H, Gönen ZB, Ergen E, Bahar D, Çetin M (2019) Effects of TGF-β1 Overexpression on Biological Characteristics of Human Dental Pulp-derived Mesenchymal Stromal Cells. Int J Stem Cells 12: 170-182.
Citation: SALKIN H (2019) The Tgf-β1 Over Expression has Major Impact on Multilineage Differentiation of Mesenchymal Stromal Cells. J Stem Cell Res Dev Ther 5: 013.
Copyright: © 2019 Hasan SALKIN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
