
Journal of Stem Cells Research Development & Therapy Category: Medical
Type: Research Article
Use of Stem Cells in Intervention Dermatology and Trichology: A New Hope
*Corresponding Author(s):
Suruchi GargDepartment Of Dermatology, Aesthetic Surgery And Intervention Dermatology, Aura Skin Institute, Chandigarh, India
Tel:+91 9914253530,
Email:gargsuruchi01@gmail.com
Received Date: Mar 20, 2019
Accepted Date: Apr 05, 2019
Published Date: Apr 12, 2019
Abstract
Stem cells are undifferentiated cells that have capacity of self-renewal and differentiation in to cell lines of different lineages. The regeneration of the epidermis and the hair follicle is sustained by many different types of epidermal stem cell, which also participate in the repair of the skin after injuries. Extracutaneous sources of stem cells probably play a role in skin homeostasis and should be considered with respect to regenerative cell therapies in dermatology. These include mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, placenta, haematopoietic stem cells. Multipotent stem cells from the hair follicle and sebaceous glands are most commonly used stem cells for treatment of alopecias, and are known by several therapeutic strategies that they help in reversing the pathological mechanisms contributing to hair loss, regeneration of complete hair follicle from bulge derived stem cells, and neogenesis of hair follicle from a stem cell culture using tissue engineering techniques and have also been used in treatment of vitiligo more recently. Use of adipocyte derived stem cells is already an established surgical technique for scar revision and regeneration of damaged tissue. The article discusses case reports of use of stem cells in hair regrowth in Androgenetic alopecia and in scar revision of totally anesthetic post traumatic skin graft. Stem cell therapy has created a hope and opened new dimensions in treatment of difficult to treat disorders. Though most of the therapies are still in nascent stage and more substantial controlled trials are required in this field, but stem cell therapies hold a promising place in future of regenerative medicine.
Keywords
Stem Cells in Intervention Dermatology and Trichology
INTRODUCTION
Stem cells are undifferentiated cells that have capacity of self-renewal and differentiation in to cell lines of different lineages. The term Stem cell was first used by a Russian histologist, Alexander Maximow in 1908 [1]. The successful generation of offspring from adult and mammalian cells by cloning technology has gained popularity in 1997 with the famous sheep named Dolly born in United Kingdom. Shortly thereafter, in 1998, human embryonic stem cells were isolated from blastocysts at the University of Wisconsin, Madison [2]. Together with the description of an up to then unknown plasticity of stem cells, the generation of a human embryonic stem cell line lead to a great interest in stem cell biology, to repair and replace diseased tissues and organs.
Properties of stem cells
Based on the origin, stem cells can be of 2 types, embryonic stem cells and adult stem cells.
Adult stem cells are defined by specific properties [3,4]:
• They are relatively undifferentiated both morphologically and functionally
• They provide a source for continuous renewal of cells and long-term tissue maintenance
• In vivo, they have a slow generation time, but the process can be triggered by various stimuli like injury and growth factors
• Adult stem cells are located in a specialized micro environment called ‘niche’, which contributes to stem cell activity and behavior
The most appealing feature of a stem cell is plasticity, also called trans differentiation, which implies its ability to cross the germ lines and differentiate into cells types other than germ line lineage or tissue they were derived from [5]. Based on their ability to regenerate, stem cells can be classified as follows:
• Totipotent, as in they can differentiate into all cell lineages and embryonic tissue. Blastocyst up to 8 cell stage has this capacity
• Pluripotent cells which have the capacity to differentiate into all the 3 germ cell lineages, but not the extra-embryonic tissue
• Multipotent stem cells that can differentiate into different cells of same lineage
• Monopotent cells, as the name indicates can regenerate only one cell type
Functionally, a stem cell represents an undifferentiated cell that divides asymmetrically in to a stem cell, retaining its self-renewal capacity and a smaller cell called transient amplifying cells. Transient amplifying cells which have lost their capacity of continuous self-renewal, differentiates down a certain pathway. In mammals adult stem cells are identified in various locations of which common sites include hematopoietic system, central nervous system, cornea, skin. Among these, skin is the major source of stem cells due to its vast area, being the largest organ in the body and ease of accessibility. There are many different types of cells residing in the skin and these originate from multiple different embryonic sources [6]. The epidermis originates from neuroectodermal cells that remain at the surface of the embryo after gastrulation. The epidermis usually begins as a single layer of cells which then gives rise to the hair follicle, sebaceous glands and inters follicular epidermis. Dermal fibroblasts, vessels, nerves, arrector pili muscles, immune cells in the dermis, and mature adipocytes in the subcutis are derived from mesoderm. Melanocytes and sensory nerve endings originate from neural crest. During adult life, the maintenance of these various types of cells is the function of stem cells located at their particular ‘niches.’
Adult stem cells are defined by specific properties [3,4]:
• They are relatively undifferentiated both morphologically and functionally
• They provide a source for continuous renewal of cells and long-term tissue maintenance
• In vivo, they have a slow generation time, but the process can be triggered by various stimuli like injury and growth factors
• Adult stem cells are located in a specialized micro environment called ‘niche’, which contributes to stem cell activity and behavior
The most appealing feature of a stem cell is plasticity, also called trans differentiation, which implies its ability to cross the germ lines and differentiate into cells types other than germ line lineage or tissue they were derived from [5]. Based on their ability to regenerate, stem cells can be classified as follows:
• Totipotent, as in they can differentiate into all cell lineages and embryonic tissue. Blastocyst up to 8 cell stage has this capacity
• Pluripotent cells which have the capacity to differentiate into all the 3 germ cell lineages, but not the extra-embryonic tissue
• Multipotent stem cells that can differentiate into different cells of same lineage
• Monopotent cells, as the name indicates can regenerate only one cell type
Functionally, a stem cell represents an undifferentiated cell that divides asymmetrically in to a stem cell, retaining its self-renewal capacity and a smaller cell called transient amplifying cells. Transient amplifying cells which have lost their capacity of continuous self-renewal, differentiates down a certain pathway. In mammals adult stem cells are identified in various locations of which common sites include hematopoietic system, central nervous system, cornea, skin. Among these, skin is the major source of stem cells due to its vast area, being the largest organ in the body and ease of accessibility. There are many different types of cells residing in the skin and these originate from multiple different embryonic sources [6]. The epidermis originates from neuroectodermal cells that remain at the surface of the embryo after gastrulation. The epidermis usually begins as a single layer of cells which then gives rise to the hair follicle, sebaceous glands and inters follicular epidermis. Dermal fibroblasts, vessels, nerves, arrector pili muscles, immune cells in the dermis, and mature adipocytes in the subcutis are derived from mesoderm. Melanocytes and sensory nerve endings originate from neural crest. During adult life, the maintenance of these various types of cells is the function of stem cells located at their particular ‘niches.’
Identification of epidermal stem cells:
There are several methods to identify the stem cells among other residing cells in the epidermis including transient amplifying cells and terminally differentiated cells:
• All stem cells have the property of quiescence at normal conditions, and the multiplication rate triggered by various stimuli and growth factors [3,4], and this property has been utilized in their identification. Using this method all actively dividing cells within the epidermis are pulse-labelled with injections of a DNA precursor, such as tritiated thymidine or bromodeoxyuridine. This is then followed by a chase period (4–10 weeks) during which the label is lost from rapidly proliferating cells such as the transient amplifying cells as a result of proliferation-associated dilution, while the rarely dividing stem cells retain the label for prolonged periods and are therefore called label- retaining cells.
• The other method which makes use of this high proliferative capacity of epidermal stem cells is Colony Forming assay. Using this method the proliferative potential of cultured cells is assessed by examining the clonogenicity of individual cells through serial passage or colony forming efficacy. Based on this method, cells with a clonogenic or high proliferative capacity (stem cells) have been identified.
• Although these two methods help in the identification of epidermal stem cells, they do not allow for the easy isolation of living stem cells for further analysis therefore several epidermal stem cell markers have been identified.
• All stem cells have the property of quiescence at normal conditions, and the multiplication rate triggered by various stimuli and growth factors [3,4], and this property has been utilized in their identification. Using this method all actively dividing cells within the epidermis are pulse-labelled with injections of a DNA precursor, such as tritiated thymidine or bromodeoxyuridine. This is then followed by a chase period (4–10 weeks) during which the label is lost from rapidly proliferating cells such as the transient amplifying cells as a result of proliferation-associated dilution, while the rarely dividing stem cells retain the label for prolonged periods and are therefore called label- retaining cells.
• The other method which makes use of this high proliferative capacity of epidermal stem cells is Colony Forming assay. Using this method the proliferative potential of cultured cells is assessed by examining the clonogenicity of individual cells through serial passage or colony forming efficacy. Based on this method, cells with a clonogenic or high proliferative capacity (stem cells) have been identified.
• Although these two methods help in the identification of epidermal stem cells, they do not allow for the easy isolation of living stem cells for further analysis therefore several epidermal stem cell markers have been identified.
Epidermal stem cells
The epidermis is a multilayered epithelium that is composed of hair follicles, sebaceous glands and interfollicular epidermis. The regeneration of the epidermis and the hair follicle is sustained by many different types of epidermal stem cells, which also participate in the repair of the skin after injuries.
Hair follicle stem cells
The adult hair follicle consists of an upper portion that is permanent and a lower portion that constantly remodels during the hair cycle. Maintenance of the hair follicle cycle is largely dependent on different stem cell populations capable of giving rise to the different epithelial components of the hair follicle. The bulge region of the hair follicle, defined as the portion of the outer root sheath of the hair follicle at the insertion site of the arrector pili muscle, is currently the best characterized site of epidermal stem cell population [7]. Bulge keratinocyte stem cells, in addition to being quiescent, have been shown to have all of the characteristics of stem cells. Cytokeratin 15, a keratin intermediate filament, has a very high expression in the bulge stem cell. CD34, although was identified in rat bulge cells, has not been significantly found in human bulge cells. CD200, a membrane glycoprotein of immunoglobulin subfamily, was identified as authentic bulge cell marker in humans. Although previous studies indicate that bulge stem cells give rise to all components of the epidermis, there is more recent evidence to indicate that in normal states, they do not contribute to the reconstitution of the interfollicular epidermis. Injury to the epidermis results in migration of the bulge cells to the epidermis where they then contribute to wound repair.
In each hair cycle, at the transition from the anagen to catagen phase, melanocytes in the hair bulb matrix undergo apoptosis with their reconstitution occurring at the beginning of the next anagen phase. Evidence from murine and human studies indicates that this reconstitution process is made possible by a population of follicular bulge stem cells committed to melanocyte differentiation. These melanocyte stem cells are usually quiescent but become activated and proliferate at the onset of the anagen phase leading to the repopulation of the hair follicle matrix with melanocytes that generate melanin leading to pigmentation of the hair shaft. In addition, the defective self-maintenance of these melanocytes stem cells, which is thought to be part of physiological ageing, may be the underlying cause of hair greying.
The melanocyte stem cell markers include Pax3 and MITF, also known as melanocyte master transcriptional regulator. Pax3 has been shown to maintain the undifferentiated state of stem cells while simultaneously functioning in initiation of the melanogenic cascade. MITF, which may play a role in stem cell maintenance within the bulge through an antiapoptotic effect mediated by induction of Bcl-2 expression, has been shown to be highly expressed in the human bulge and is believed to serve as a potential marker of this stem cell population.
A hair growth hypothesis has been proposed by Garg et al depicting the model of ‘Golden anchorage and molecular locking of ectodermal and mesenchymal components for hair follicle integrity and survival.’ The ‘Golden anchorage’comprises of stem cells, ectodermal basement membrane and portion of arrector pili muscle attached to bulge region. After stimulatory nephronectin signals from basement membrane, the ‘molecular locking’ of α8B1 integrin receptor with nephronectin takes place in stem cell and bulge portion of arrector pili muscle,which is essentialfor further migration of stem cells towards dermal papilla and aid in hairfollicle formation in anagen phase. The lock and key arrangement of nephronectin-integrin molecules also helps in holding the ectodermal and mesenchymal components together for follicular integrity and survival as depicted in figure 1[8].
In each hair cycle, at the transition from the anagen to catagen phase, melanocytes in the hair bulb matrix undergo apoptosis with their reconstitution occurring at the beginning of the next anagen phase. Evidence from murine and human studies indicates that this reconstitution process is made possible by a population of follicular bulge stem cells committed to melanocyte differentiation. These melanocyte stem cells are usually quiescent but become activated and proliferate at the onset of the anagen phase leading to the repopulation of the hair follicle matrix with melanocytes that generate melanin leading to pigmentation of the hair shaft. In addition, the defective self-maintenance of these melanocytes stem cells, which is thought to be part of physiological ageing, may be the underlying cause of hair greying.
The melanocyte stem cell markers include Pax3 and MITF, also known as melanocyte master transcriptional regulator. Pax3 has been shown to maintain the undifferentiated state of stem cells while simultaneously functioning in initiation of the melanogenic cascade. MITF, which may play a role in stem cell maintenance within the bulge through an antiapoptotic effect mediated by induction of Bcl-2 expression, has been shown to be highly expressed in the human bulge and is believed to serve as a potential marker of this stem cell population.
A hair growth hypothesis has been proposed by Garg et al depicting the model of ‘Golden anchorage and molecular locking of ectodermal and mesenchymal components for hair follicle integrity and survival.’ The ‘Golden anchorage’comprises of stem cells, ectodermal basement membrane and portion of arrector pili muscle attached to bulge region. After stimulatory nephronectin signals from basement membrane, the ‘molecular locking’ of α8B1 integrin receptor with nephronectin takes place in stem cell and bulge portion of arrector pili muscle,which is essentialfor further migration of stem cells towards dermal papilla and aid in hairfollicle formation in anagen phase. The lock and key arrangement of nephronectin-integrin molecules also helps in holding the ectodermal and mesenchymal components together for follicular integrity and survival as depicted in figure 1[8].
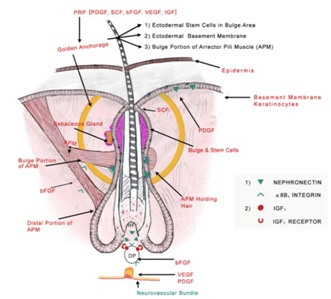 Figure 1: Proposed model of “Golden anchorage and molecular locking of ectodermal and mesenchymal components for hair follicle integrity and survival”. The role of various PRP growth factors acting on different targets for hair follicle survival is also highlighted. (Photo courtesy: stem cell investigation.2017:4; 64).
Figure 1: Proposed model of “Golden anchorage and molecular locking of ectodermal and mesenchymal components for hair follicle integrity and survival”. The role of various PRP growth factors acting on different targets for hair follicle survival is also highlighted. (Photo courtesy: stem cell investigation.2017:4; 64).A new population of stem cells, Bulge neural crest-derived stem cells, has been identified within the murine hair follicle bulge. These stem cells, with markers that differentiate them from other stem cells in the bulge, apparently have the ability to differentiate in vitro to keratinocytes, neurons, melanocytes, glial cells, smooth muscle cells and adipocytes. One of the markers which helped in the identification of this stem cell population is nestin, an intermediate filament protein expressed in the neuroepithelial stem cell cytoplasm and known to be a marker for neural stem cells. Although these nestin-positive cells do not contribute to the keratinocyte compartment in homeostatic conditions, they have been shown to enhance blood vessel formation during hair follicle growth.
Sebaceous gland progenitor cells
Sebaceous gland homeostasis necessitates the presence of a progenitor population of cells that gives rise to a continual flux of proliferating, differentiating and disintegrating sebocytes. Recent murine studies identified a resident basal sebocyte population with characteristics of progenitor cells suggesting that the sebaceous glands are capable of self-maintenance. Identification of Blimp1 (B lymphocyte-induced maturation protein 1) has helped characterize the progenitor cell population in the sebaceous glands of mice.
Stem cells in the interfollicular epidermis
The only mitotically active layer in the interfollicular epidermis, a layer of stratified squamous epithelium, is the basal layer. The interfollicular epidermis is dependent on multiple, functionally independent, hexagonal units, called the Epidermal Proliferative Units (EPU). These EPU ensure lifelong cell production to compensate for the continual loss of cells from the surface of the skin. Each epidermal proliferative unit consists of a single centrally located stem cell, its immediate transient amplifying cell progeny adjacent, with more differentiated keratinocytes lying directly above and mature, enucleated, squamous cells at the surface. Ghazizadeh et al. showed that there was no preferred site of origin of EPU in the basal compartment. Of the 200 EPU analyzed, 44% were traced to the relatively flat regions of the basement membrane, 17% to the side of rete ridges, 22% to the base of rete ridges, and 17% to the tip of dermal papillae [9]. Markers of potential utility in the identification of these cells include a8-integrin, b1-integrin and CK15, CK10, CD71, and desmosomal proteins.
Sweat gland stem cells
Adult human nestin-positive stem cells from the sweat gland mesenchyme have been isolated.
Skin Mesenchymal Stem Cells (MSCs)
Mesenchymal Stem Cells (MSCs) are multipotent stem cells and can be isolated from dermis and its components and adipose tissue in subcutis. MSCs are able to differentiate into adipocytes, osteocytes, chondrocytes, smooth muscle cells and hematopoietic supportive stroma [10]. In a steady state and/or in response to injury, turnover of stromal tissue occurs through the participation of a population of stem cells found in the stromal tissue [11].
Adipose Tissue Derived Stem Cells (AdSCs)
Adipose tissue derived stem cells owing to their property of multipotency, the capacity to differentiate into various cell lines including vascular cells, thus promoting angiogenesis, easy accessibility and lack of immunogenic properties, are considered as the ideal population for use in regenerative medicine. They also activate the epithelial stem cells by secreting various growth factors like VEGF, PDGF, IGF-1 and immunomodulatory cytokines like PGE2, Leucocyte inhibitory factor, Kynurenine [12,13]. Hair follicles are surrounded by subcutis, dermis and inter follicular epidermis forms a macro environment and promotes signaling mechanisms, which helps in maintenance of follicular stem cell reserve in the bulge region [12-14].
Extracutaneous sources of stem cells probably play a role in skin homeostasis and should be considered with respect to regenerative cell therapies in dermatology. These include mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, placenta, haematopoietic stem cells. Induced Pluripotent Stem Cells (iPSCs) are primary keratinocytes that can be reprogrammed into pluripotent stem cells, with at least 100-fold higher efficiency and twice as quickly compared with fibroblasts.
Extracutaneous sources of stem cells probably play a role in skin homeostasis and should be considered with respect to regenerative cell therapies in dermatology. These include mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, placenta, haematopoietic stem cells. Induced Pluripotent Stem Cells (iPSCs) are primary keratinocytes that can be reprogrammed into pluripotent stem cells, with at least 100-fold higher efficiency and twice as quickly compared with fibroblasts.
OUR EXPERIENCES
Androgenetic alopecia
A 30 year old male presented to us with androgenetic alopecia and was treated with 5 monthly sessions of platelet rich plasma therapy, followed by a single session of follicular stem cell suspension prepared from 50 hair follicle units from the occipital area of scalp.
Method of preparation of follicular stem cell suspension
Informed consent was taken from the patient and approval was taken from institution ethics committee. Fifty follicular unit grafts are extracted from the occipital area of scalp (known to be resistant in androgenetic alopecia, thus ideal source) mixed with 5ml of trypsin EDTA solution. The solution containing grafts is incubated at a temperature of 40 degree Celsius for 2 hours with frequent shaking in between. Later, it is mechanically agitated, filtered and centrifuged at 3200rpm for 10 min. The supernatant is discarded; the pellet formed is mixed with platelet poor plasma and subjected to a second centrifugation at 3200 rpm for 10 min. After discarding the supernatant, lower portion of the solution along with pellet is mixed with platelet rich plasma to produce a uniform suspension. It is then taken into 1ml syringes and 0.025 to 0.05 ml injected at a distance of 1 cm apart in the area that is to be treated (Figure 2).
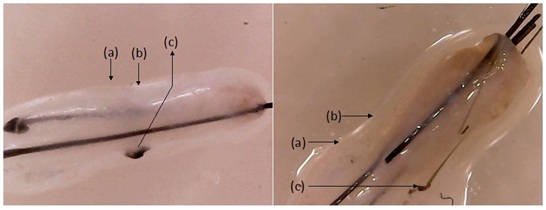 Figure 2: Video microscopic (500X) pictures of hair follicular unit before (left) and after (right) trypsinization to be followed by mechanical agitation. (a) bulge region of outer root sheath containing stem cells; (b) isthmus (c) hair root. There is dissolution of extracellular matrix, outer root sheath bulge and fragmented appearance of hair root which can be easily separated mechanically.
Figure 2: Video microscopic (500X) pictures of hair follicular unit before (left) and after (right) trypsinization to be followed by mechanical agitation. (a) bulge region of outer root sheath containing stem cells; (b) isthmus (c) hair root. There is dissolution of extracellular matrix, outer root sheath bulge and fragmented appearance of hair root which can be easily separated mechanically.Results
The improvement from baseline was significantly higher, in terms of hair texture and filling effect in frontotemporal angles, after single session of follicular stem cell suspension with compared to the response with 5 sessions of platelet rich plasma therapy (Figure 3).
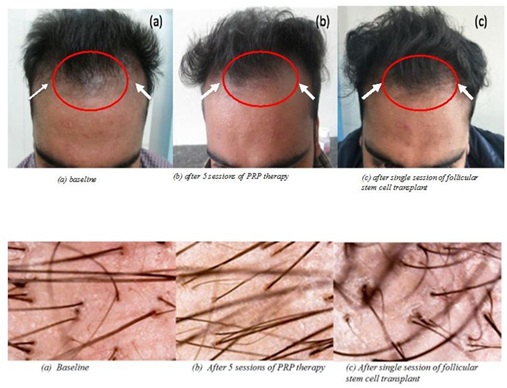 Figure 3: Patient treated with 5 sessions of platelet rich plasma followed by a gap of 6 months and then a single session of autologous hair follicle suspension comprising of stem cells along with extracellular matrix admixed with platelet rich plasma for androgenetic alopecia. There is noticeable improvement in the texture of hair and filling effect in the frontotemporal angles, with finevellus hair in the baseline picture and relatively increases in proportion of coarse, terminal hair after PRP therapy. The third picture shows more increase in coarse terminal hair and a definite change in hair line following autologous hair follicle cell suspension (white arrows). Improvement in the hair line is better with autologous hair follicle suspension compared to PRP therapy (red circles). Videomicroscopy images have also been shown which show more vellus hair in baseline image (a terminal: vellus::14:10 ) while number of terminal hair count has increased after 5 sessions of PRP therapy in image (bterminal:vellus::16:7) ; there are multiple coarse, terminal and secondary hair sprouting along with primary follicular units after autologous stem cell transplant in image (cterminal:vellus::22:5).
Figure 3: Patient treated with 5 sessions of platelet rich plasma followed by a gap of 6 months and then a single session of autologous hair follicle suspension comprising of stem cells along with extracellular matrix admixed with platelet rich plasma for androgenetic alopecia. There is noticeable improvement in the texture of hair and filling effect in the frontotemporal angles, with finevellus hair in the baseline picture and relatively increases in proportion of coarse, terminal hair after PRP therapy. The third picture shows more increase in coarse terminal hair and a definite change in hair line following autologous hair follicle cell suspension (white arrows). Improvement in the hair line is better with autologous hair follicle suspension compared to PRP therapy (red circles). Videomicroscopy images have also been shown which show more vellus hair in baseline image (a terminal: vellus::14:10 ) while number of terminal hair count has increased after 5 sessions of PRP therapy in image (bterminal:vellus::16:7) ; there are multiple coarse, terminal and secondary hair sprouting along with primary follicular units after autologous stem cell transplant in image (cterminal:vellus::22:5).Post traumatic scar
A twenty-eight year old male patient reported in our tertiary care skin institute for the treatment of post-traumatic scar with trophic ulcer on his left heel following a history of road side accident a year back. He was offered pedicle graft surgery by treating plastic surgeon at that time and the area healed as a hyper pigmented scar, with complete loss of sensations secondary to crush injury to cutaneous tibial and sural nerves, leading to recurrent trophic ulcers.
He was given a cocktail therapy of pixel erbium YAG laser, platelet rich plasma therapy and autologous fat transplantation at our institute with the idea of scar revision [15].
He was given a cocktail therapy of pixel erbium YAG laser, platelet rich plasma therapy and autologous fat transplantation at our institute with the idea of scar revision [15].
METHODS
Informed consent was taken from the patient and approval for cocktail treatment was taken from institution ethics committee. Monthly sessions of pixel erbium YAG laser to resurface the scar in combination with PRP injections. Pixel Er:YAG laser was done at a fluence of 1400mJ/p with 5 passes each on long pulse mode. Platelet rich plasma was prepared by taking 13.5cc of blood from patient’s ante cubital vein, mixed with 1.5cc of ACD solution, and subjected to centrifugation at 3200rpm for 4 min. During the fourth session, 15ml of autologous fat harvested from the medial aspect of thigh was injected to provide a cushioning effect to the heel.
RESULTS
At the end of 5 sessions, there was complete healing of trophic ulcer, with 40% improvement in touch and pain sensations over the heel. On his subsequent visit almost by the end of two and a half years after the completion of last session, patient reported ongoing improvement in the texture of the scar, with decreased pigmentation and better merging of the scar with surrounding area. Also, there was further improvement in the sensations, with a focal area of anesthesia left only at the center of heel seen as shiny white area (Figure 4). The advantage of using adipose stem cells and PRP therapy is that the cocktail keeps on building up the beneficial effects even after months and years of stopping the active treatment sessions as is evident in these sequential pictures.
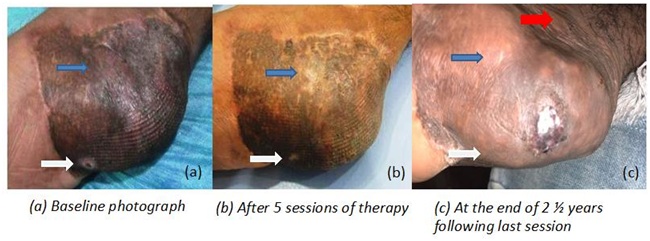 Figure 4: Post traumatic scar with recurrent trophic ulcer treated with a cocktail therapy of pixel erbium YAG laser, platelet rich plasma therapy and autologous fat transplantation. There is improvement in the texture, pigmentation of the scar (blue arrows), healing of trophic ulcer (white arrows) and better merging of the scar with surrounding tissues (red arrows).
Figure 4: Post traumatic scar with recurrent trophic ulcer treated with a cocktail therapy of pixel erbium YAG laser, platelet rich plasma therapy and autologous fat transplantation. There is improvement in the texture, pigmentation of the scar (blue arrows), healing of trophic ulcer (white arrows) and better merging of the scar with surrounding tissues (red arrows).DISCUSSION
Androgenetic alopecia
Androgenetic alopecia, also called as patterned hair loss is a multifactorial, hormonal dependent condition and is the most common cause of hair loss in males [16]. The prevalence of AGA has been on rise in the recent years. A population base study in India including 1005 men showed the prevalence of 58% among 30-50 year age group [17]. However, epidemiological studies in females are fewer in number. Androgenetic Alopecia (AGA) is characterized by miniaturization of hair follicles with decreased anagen phase, increased duration of telogenic (resting) phase, and infiltration of lymphocytes and mast cells in the follicular bulge region [7,18,19]. Though the number of stem cells is normal in the affected areas, the number of actively proliferating cells is reduced indicating either a lack of growth factors or excess inhibitors of hair follicle growth [20]. Evaluation of stem cell markers by immunohistochemistry revealed normal CK15 levels and decrease levels of CD34 and CD200 in the affected areas [20-23].
Various treatment modalities have been used for the management of patterned hair loss like topical minoxidil solution, oral finasteride, peptide based therapies, Low Level Light Therapy (LLLT), Platelet Rich Plasma Therapy (PRP) etc, out of which minoxidil and finasteride have been approved by USFDA [9,23-25]. Since the response to these treatments is often inadequate, the hope lies on the regenerative medicine which uses the therapeutic potential of stem cell therapy.
Stem cells of various origins like follicular stem cells, bone marrow stem cells, adipose tissue, skin stem cells have been used in the management of alopecia. However, multipotent stem cells from the hair follicle and sebaceous glands are most commonly used, and are known by several therapeutic strategies that they help in reversing the pathological mechanisms contributing to hair loss, regeneration of complete hair follicle from bulge derived stem cells, and neogenesis of hair follicle from a stem cell culture using tissue engineering techniques [27-29]. Occipital hair, which are resistant to hormonal influences in androgenetic alopecia are considered the best source for extraction of stem cells [30].
Gentile et al., evaluated the effect of autologous hair follicle stem cell suspension over placebo in 11 patients, aged from 38-61 years with androgenetic alopecia and found that there was 29 ± 5% increase in the hair density in the treatment group as compared to < 1% in the group treated with placebo [29].
A double blinded, randomized controlled trial compared the efficacy of single session of intradermal injections of autologous bone marrow derived mononuclear cells versus follicular stem cell culture in resistant cases of alopecia areata and androgenetic alopecia of 20 patients each. There was very good improvement in both the groups, which is classified as >50-75% improvement in hair density, along with significant improvement in immunohistochemistry for stem cell markers and digital dermoscopy, at the end of 23 weeks after the session [31].
Sixty patients of androgenetic alopecia were randomized in to 2 groups to receive 3 monthly sessions of either autologous adipose tissue derived stem cell versus platelet rice plasma (PRP) therapy by Kadry et al. It was shown that the mean increase in hair density as evaluated by increase in the terminal and intermediate hair count was significantly higher compared to baseline in the group treated with adipose tissue derived stem cells (p<0.001 and p<0.001 respectively). However, terminal hair count was significantly higher in PRP therapy group (p=0.037), but no significant improvement in intermediate hair count. Mild side effects with post session headache, erythema and pain were more in the group treated with adipose tissue derived stem cells [32].
Various treatment modalities have been used for the management of patterned hair loss like topical minoxidil solution, oral finasteride, peptide based therapies, Low Level Light Therapy (LLLT), Platelet Rich Plasma Therapy (PRP) etc, out of which minoxidil and finasteride have been approved by USFDA [9,23-25]. Since the response to these treatments is often inadequate, the hope lies on the regenerative medicine which uses the therapeutic potential of stem cell therapy.
Stem cells of various origins like follicular stem cells, bone marrow stem cells, adipose tissue, skin stem cells have been used in the management of alopecia. However, multipotent stem cells from the hair follicle and sebaceous glands are most commonly used, and are known by several therapeutic strategies that they help in reversing the pathological mechanisms contributing to hair loss, regeneration of complete hair follicle from bulge derived stem cells, and neogenesis of hair follicle from a stem cell culture using tissue engineering techniques [27-29]. Occipital hair, which are resistant to hormonal influences in androgenetic alopecia are considered the best source for extraction of stem cells [30].
Gentile et al., evaluated the effect of autologous hair follicle stem cell suspension over placebo in 11 patients, aged from 38-61 years with androgenetic alopecia and found that there was 29 ± 5% increase in the hair density in the treatment group as compared to < 1% in the group treated with placebo [29].
A double blinded, randomized controlled trial compared the efficacy of single session of intradermal injections of autologous bone marrow derived mononuclear cells versus follicular stem cell culture in resistant cases of alopecia areata and androgenetic alopecia of 20 patients each. There was very good improvement in both the groups, which is classified as >50-75% improvement in hair density, along with significant improvement in immunohistochemistry for stem cell markers and digital dermoscopy, at the end of 23 weeks after the session [31].
Sixty patients of androgenetic alopecia were randomized in to 2 groups to receive 3 monthly sessions of either autologous adipose tissue derived stem cell versus platelet rice plasma (PRP) therapy by Kadry et al. It was shown that the mean increase in hair density as evaluated by increase in the terminal and intermediate hair count was significantly higher compared to baseline in the group treated with adipose tissue derived stem cells (p<0.001 and p<0.001 respectively). However, terminal hair count was significantly higher in PRP therapy group (p=0.037), but no significant improvement in intermediate hair count. Mild side effects with post session headache, erythema and pain were more in the group treated with adipose tissue derived stem cells [32].
Alopecia areata
Alopecia areata is an autoimmune condition characterized by patches of nonscarring alopecia on scalp and other body areas, with a prevalence of 0.7% of cases in dermatology cases [33,34]. The main pathogenesis involved in alopecia areata is loss of immune privilege and infiltration of CD4/CD8 lymphocytes and cytokines around the hair follicles [35]. Conventional treatment options include topical, intralesional, oral steroids and immunomodulatory agents. However, poor prognosis and treatment resistance has been observed in patients with alopecia universalis, totalis and those associated with risk factors. There are few animal and clinical studies demonstrating the efficacy of mesenchymal stem cell therapy in autoimmune conditions including alopecia areata, atopic dermatitis, rheumatoid arthritis, graft versus host disease etc. Stem cells exhibit anti- inflammatory properties thus resulting in immunomodulation and this property has been used for treatment of resistant and non-responsive cases of alopecia areata. Mesenchymal Stem Cells (MSCs) act by inhibiting the proliferation of T- cells and B-cells and alter the function of other immune cells. MSCs can increase the secretion of immunosuppressive molecules like IDO, PGE2 and TGF-β1[36,37,38]. They inhibit the maturation of dendritic cells (DCs) via IL-10 and activating the JAK1/STAT3 pathway and induce the differentiation of regulatory DCs, regulatory T cells (Treg).
An open labelled pilot study evaluating the efficacy of follicular stem cell suspension in eight patients of alopecia areata showed excellent (>50%) improvement in 62.5% patients, good (10-50%) improvement in 25% patients and poor (<10%) improvement in 1 patient at the end of 6 months of therapy [36].
It has been reported as a coincidental finding that a treatment resistant, long-lasting case of alopecia universals in a 40 year old male, completely recovered after allogeneic hematopoietic stem cell transplantation given to treat chronic myeloid leukemia [39].
A retrospective study of 20 patients with alopecia areata, used autologous stromal vascular fraction showed that there was increased hair growth and decreased pull test 3 and 6 months after the treatment [hair density (85.1 ± 8.7 vs 121.1 ± 12.5 hair/cm2, P< 0.0001), hair diameter (60.5 ± 1.8 vs 80.8 ± 2.4μ, P < 0.0001) and pull-test values (4.4 ± 0.3 vs 0.8 ± 0.2, P < 0.0001), untreated versus 6 months post-operative)] [37].
An open labelled pilot study evaluating the efficacy of follicular stem cell suspension in eight patients of alopecia areata showed excellent (>50%) improvement in 62.5% patients, good (10-50%) improvement in 25% patients and poor (<10%) improvement in 1 patient at the end of 6 months of therapy [36].
It has been reported as a coincidental finding that a treatment resistant, long-lasting case of alopecia universals in a 40 year old male, completely recovered after allogeneic hematopoietic stem cell transplantation given to treat chronic myeloid leukemia [39].
A retrospective study of 20 patients with alopecia areata, used autologous stromal vascular fraction showed that there was increased hair growth and decreased pull test 3 and 6 months after the treatment [hair density (85.1 ± 8.7 vs 121.1 ± 12.5 hair/cm2, P< 0.0001), hair diameter (60.5 ± 1.8 vs 80.8 ± 2.4μ, P < 0.0001) and pull-test values (4.4 ± 0.3 vs 0.8 ± 0.2, P < 0.0001), untreated versus 6 months post-operative)] [37].
Aging
Aging is a degenerative biological process characterized by loss of soft tissue, with decrease in the collagen content in the dermis. Regeneration and augmentation of soft tissue is one of the leading concerns in the field of aesthetics. Treatment modalities like biomaterials, composite flaps, dermal grafting, and fat grafting are in use [38]. However, there are inherent complications associated with biomaterials such as infections, fibrosis, tissue contracture and allergic reactions. Under normal circumstances, stem cell population in the dermis and subcutis, under the influence of various growth factors and cytokines, are involved in stimulating dermal fibroblasts and synthesis of collagen for skin rejuvenation [40]. The exact mechanism however has not been clear, but paracrine activation of dermal fibroblasts and stimulation of angiogenesis has been suggested [41].
Park et al demonstrated that combined ADSCs with autologous lipoaspirate cells administered intradermally to an aged skin patient resulted in an improvement in the texture of the skin and wrinkles and dermal thickness 8 weeks after treatment [41].
In a study by Amirkhani et al., 16 patients aged from 38-56 years underwent transplantation of autologous stromal vascular fraction in the nasolabial folds. They were assessed at the end of 6 months, and showed increased elasticity and density of dermis along with enrichment of vascular bed in the subcutis [42].
Park et al demonstrated that combined ADSCs with autologous lipoaspirate cells administered intradermally to an aged skin patient resulted in an improvement in the texture of the skin and wrinkles and dermal thickness 8 weeks after treatment [41].
In a study by Amirkhani et al., 16 patients aged from 38-56 years underwent transplantation of autologous stromal vascular fraction in the nasolabial folds. They were assessed at the end of 6 months, and showed increased elasticity and density of dermis along with enrichment of vascular bed in the subcutis [42].
Vitiligo
Vitiligo is an acquired autoimmune disorders affecting melanocytes, clinically characterized by hypopigmented and depigmented patches, with a world-wide prevalence of 0.3-0.5%. The pathogenesis is multifactorial with both genetic and non-genetic factors mediating autoimmune destruction of melanocytes [43]. Major clinical types include segmental and non-segmental vitiligo, having varied presentations and prognostic value [44]. Standard therapeutic options include topical and oral steroids, immunomodulatory agents and phototherapy. Surgical modalities like tissue grafts and cellular grafts are used to treat stable and resistant areas [45]. There have been a lot of progresses in the surgical techniques since 1992, when Gauthier and Bazielle proposed non cultured cell suspension transplantation for vitiligo [46]. Recent advance in the surgical techniques have incorporated the properties of regenerative medicine using stem cell therapy.
Kim et al. showed that co-culturing of melanocytes with Adipose Tissue Derived Stem Cells (AdSCs) had a better survival effect on melanocytes. Immunomodulatory properties of stem cells, owing to their ability to inhibit CD4/CD8 T cell infiltration and inflammatory cytokines have been suggested as a possible explanation for the above phenomenon [47].
An intrapatient comparison evaluating the efficacy of combined epidermal cell suspension and follicular cell suspension versus epidermal suspension alone in 5 patients with stable vitiligo, showed that the repigmentation was better with combination therapy, with 100% lesions showing >90% repigmentation in combination arm at the end of 16 weeks. Whereas, in the lesions treated with epidermal cell suspension alone, 80% lesions showed >75% repigmentation and 20% lesions had >90% repigmentation [48].
An observer blinded, randomized controlled, intrapatient trial in 30 patients of stable vitiligo, where in the lesions were divided in to 2 groups, to receive a combination of epidermal cell suspension and follicular cell suspension transplant versus epidermal cell suspension alone. At the end of 16 weeks following therapy, the results were better with combination therapy in terms of mean repigmentation (76% vs 57%), rapidity of repigmentation (48% vs. 31%) and color match (73% vs. 61%) [49].
Kim et al. showed that co-culturing of melanocytes with Adipose Tissue Derived Stem Cells (AdSCs) had a better survival effect on melanocytes. Immunomodulatory properties of stem cells, owing to their ability to inhibit CD4/CD8 T cell infiltration and inflammatory cytokines have been suggested as a possible explanation for the above phenomenon [47].
An intrapatient comparison evaluating the efficacy of combined epidermal cell suspension and follicular cell suspension versus epidermal suspension alone in 5 patients with stable vitiligo, showed that the repigmentation was better with combination therapy, with 100% lesions showing >90% repigmentation in combination arm at the end of 16 weeks. Whereas, in the lesions treated with epidermal cell suspension alone, 80% lesions showed >75% repigmentation and 20% lesions had >90% repigmentation [48].
An observer blinded, randomized controlled, intrapatient trial in 30 patients of stable vitiligo, where in the lesions were divided in to 2 groups, to receive a combination of epidermal cell suspension and follicular cell suspension transplant versus epidermal cell suspension alone. At the end of 16 weeks following therapy, the results were better with combination therapy in terms of mean repigmentation (76% vs 57%), rapidity of repigmentation (48% vs. 31%) and color match (73% vs. 61%) [49].
Wound healing
Wound healing essentially occurs in 2 steps, early and late phase. In early phase of wound healing, there are cascade of events including homeostasis, inflammation, proliferation and remodeling [50]. This process normally takes 4-6 weeks, and delay due to any reason leads in the formation of a chronic wound. The late phase consists of constant remodeling to achieve near normal architecture of the tissues. Aberrations in this process may lead to excess fibrous tissue sometimes, resulting in formation of keloids and hypertrophic scars. Imbalance between the inflammatory and anti-inflammatory process, with more inflammatory cytokines and cells stimulates dermal fibroblasts leading to excess collagen production. Recent advances in the management of aberrant wound healing process are the use of stem cell therapy. Stem cells by their properties of cellular differentiation, regeneration, ability to release of growth factors and immunomodulatory properties can help in chronic non healing wounds. Among different sources of stem cells in the skin, stromal vascular fraction from subcutis is being used commonly, due to its ability to differentiate into various components like hematopoietic stem cells, fibroblasts, pericytes, endothelial cells and pre adipocytes, all of which favor the process of wound healing [51].
Ismail et al., studied the effect of autologous bone marrow stem cell therapy in 20 patients with reconstructable chronic critical lower limb ischemia with no distal run off, and showed that 55% patients had decrease in rest pain at the end of 1 month after therapy, and this increased to 75% after 1 year and 80% after 2 years of therapy. Also, there was a 80% limb salvage rate at the end of 1 year [52].
An open labelled, pilot study of 15 patients with chronic non healing ulcers secondary to peripheral arterial disease and diabetes, were treated with stromal vascular fraction derived from abdomen as local intramuscular injections in 2 sessions, 2 months apart. When followed up at the end of 12 months, all ulcers were healed with 86.7% improvement in walking distance [53].
Raposio et al., evaluated the efficacy of enriched PRP (PRP mixed with adipose tissue derived stem cells) versus standard wound care therapy in an open labelled, case control study in which a single session of 5 ml injection of e-PRP were given around and base of the ulcer. At the end of 18 month follow up period both groups showed similar healing rates, however, wound closure was higher in the group treated with e-PRP [54].
Ismail et al., studied the effect of autologous bone marrow stem cell therapy in 20 patients with reconstructable chronic critical lower limb ischemia with no distal run off, and showed that 55% patients had decrease in rest pain at the end of 1 month after therapy, and this increased to 75% after 1 year and 80% after 2 years of therapy. Also, there was a 80% limb salvage rate at the end of 1 year [52].
An open labelled, pilot study of 15 patients with chronic non healing ulcers secondary to peripheral arterial disease and diabetes, were treated with stromal vascular fraction derived from abdomen as local intramuscular injections in 2 sessions, 2 months apart. When followed up at the end of 12 months, all ulcers were healed with 86.7% improvement in walking distance [53].
Raposio et al., evaluated the efficacy of enriched PRP (PRP mixed with adipose tissue derived stem cells) versus standard wound care therapy in an open labelled, case control study in which a single session of 5 ml injection of e-PRP were given around and base of the ulcer. At the end of 18 month follow up period both groups showed similar healing rates, however, wound closure was higher in the group treated with e-PRP [54].
CONCLUSION
Stem cell therapy has created a hope and opened new dimensions in treatment of difficult to treat disorders. Though most of the therapies are still in nascent stage and more substantial controlled trials are required in this field, but stem cell therapies hold a promising place in future of regenerative medicine.
REFERENCES
- Konstantinov IE (2000) In search of Alexander A. Maximow: the man behind the unitarian theory of hematopoiesis. Perspect Biol Med 43: 269-276.
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282: 1145-1147.
- Verfaillie CM (2002) Adult stem cells: assessing the case for pluripotency. Trends Cell Biol 12: 502-508.
- Verfaillie C (2009) Pluripotent stem cells. Transfusion Clinique et Biologique 16: 65-69.
- Lodi D, Iannitti T, Palmieri B (2011) Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res 30: 9.
- Hu MS, Borrelli MR, Hong WX, Malhotra S, Cheung ATM, et al. (2018) Embryonic skin development and repair. Organogenesis 14: 46-63.
- Cotsarelis G, Sun TT, Lavker RM (1990) Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61: 1329-1337.
- Garg S, Manchanda S (2017) Platelet-rich plasma-an ‘Elixir’ for treatment of alopecia: personal experience on 117 patients with review of literature. Stem Cell Investig 4: 64.
- Ghazizadeh S, Taichman LB (2005) Organization of stem cells and their progeny in human epidermis. J Invest Dermatol 124: 367-372.
- Yagi H, Soto-Gutierrez A, Kitagawa Y, Tilles AW, Tompkins RG, et al. (2010) Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant 19: 823-830.
- Owen M (1988) Marrow stromal stem cells. J Cell Sci Suppl 10: 63-76.
- Zhang P, Kling RE, Ravuri SK, Kokai LE, Rubin JP, Chai JK, et al. (2014) A review of adipocyte lineage cells and dermal papilla cells in hair follicle regeneration. J Tissue Eng 5: 2041731414556850.
- Zhu M, Zhou Z, Chen Y, Schreiber R, Ransom JT, et al. (2010) Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg 64: 222-228.
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, et al. (2011) Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146: 761-771.
- Garg S (2017) Post Traumatic Scar with Repeated Trophic Ulcers Treated With a Cocktail of Ablative Pixel Erbium YAG Laser, PRP Therapy and Autologous Fat Transplant - A Novel Case Report of Terminal Nerve Ending Regeneration through Regenerative Surgery. J Mol Genet Med 11: 303.
- Springer K, Brown M, Stulberg DL (2003) Common hair loss disorders. Am Fam Physician 68: 93-102.
- Krupa Shankar D, Chakravarthi M, Shilpakar R (2009) Male androgenetic alopecia: population-based study in 1,005 subjects. Int J Trichology 1: 131-133.
- Paus R, Cotsarelis G (1999) The Biology of Hair Follicles. N Engl J Med 341: 491-497.
- Jaworsky C, Kligman AM, Murphy GF (1992) Characterization of inflammatory infiltrates in male pattern alopecia: implications for pathogenesis. Br J Dermatol. 127: 239-246.
- Garza LA, Yang CC, Zhao T, Blatt HB, Lee M, He H, et al. (2011) Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 121: 613-622.
- Zhao L, Hantash BM (2014) Male androgenetic alopecia is due to hair follicle stem cell inactivation. Expert Rev Dermatol 6: 145-147.
- Fiuraskova M, Brychtova S, Kolar Z, Kucerova R, Bienova M (2005) Expression of beta-catenin, p63 and CD34 in hair follicles during the course of androgenetic alopecia. Arch Dermatol Res 297: 143-146.
- Li J, Jiang TX, Chuong CM (2013) The many paths to alopecia, with compromised hair stem cell regeneration. J Invest Dermatol. 133: 1450-1452.
- Garg S (2016) Outcome of Intra-operative Injected Platelet-rich Plasma Therapy During Follicular Unit Extraction Hair Transplant: A Prospective Randomised Study in Forty Patients. J Cutan Aesthetic Surg 9: 157-164.
- Rossi A, Anzalone A, Fortuna MC, Caro G, Garelli V, et al. (2016) Multi-therapies in androgenetic alopecia: review and clinical experiences. Dermatol Ther 29: 424-432.
- Varothai S, Bergfeld WF (2014) Androgenetic alopecia: an evidence-based treatment update. Am J Clin Dermatol 15: 217-230.
- Asakawa K, Toyoshima KE, Ishibashi N, Tobe H, Iwadate A, et al. (2012) Hair organ regeneration via the bioengineered hair follicular unit transplantation. Sci Rep 2: 424.
- Balañá ME, Charreau HE, Leirós GJ (2015) Epidermal stem cells and skin tissue engineering in hair follicle regeneration. World J Stem Cells 7: 711-727.
- Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V (2017) Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig 4:58.
- Teumer J, Cooley J (2005) Follicular Cell Implantation: An Emerging Cell Therapy for Hair Loss. Semin Plast Surg 19: 193-200.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, Abdou SM, El Attar YA, et al. (2018) Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J Dermatol Treat. 29: 431-440.
- Kadry MH, El-Kheir WA, Shalaby ME-S, Shahid ARE, Metwally HG (2018) Autologous Adipose Derived Stem Cell versus Platelet Rich Plasma Injection in the Treatment of Androgentic Alopecia: Efficacy, Side Effects and Safety. J Clin Exp Dermatol Res 9: 1-7.
- Seetharam KA (2013) Alopecia areata: An update. Indian J Dermatol Venereol Leprol 79: 563.
- Sharma VK, Dawn G, Kumar B (1996) Profile of alopecia areata in Northern India. Int J Dermatol 35: 22-27.
- McElwee KJ, Tobin DJ, Bystryn JC, King LE, Sundberg JP (1999) Alopecia areata: an autoimmune disease? Exp Dermatol 8: 371-379.
- Fawzy MM, Gabr HM, El Maadawi ZM (2011) Autologous progenitor cell implantation as a novel therapeutic intervention for alopecia areata. J Egypt Women’s Dermatol Soc 8: 11.
- Anderi R, Makdissy N, Azar A, Rizk F, Hamade A (2018) Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res Ther 9: 141.
- Eremia S, Newman N (2000) Long-term follow-up after autologous fat grafting: analysis of results from 116 patients followed at least 12 months after receiving the last of a minimum of two treatments. Dermatol Surg 26: 1150-1158.
- Seifert B, Passweg JR, Heim D, Rovó A, Meyer-Monard S, et al. (2005) Complete remission of alopecia universalis after allogeneic hematopoietic stem cell transplantation. Blood 105: 426–427.
- Park BS, Jang KA, Sung JH, Park JS, Kwon YH, et al. (2008) Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg 34: 1323-1326.
- Kim J-H, Jung M, Kim H-S, Kim Y-M, Choi E-H (2011) Adipose-derived stem cells as a new therapeutic modality for ageing skin. Exp Dermatol 20: 383-387.
- Amirkhani MA, Shoae-Hassani A, Soleimani M, Hejazi S, Ghalichi L, et al. (2016) Rejuvenation of facial skin and improvement in the dermal architecture by transplantation of autologous stromal vascular fraction: a clinical study. BioImpacts BI 6: 149-154.
- Njoo MD, Westerhof W (2001) Vitiligo. Pathogenesis and treatment. Am J Clin Dermatol 2: 167-181.
- Taïeb A, Picardo M (2009) Clinical practice. Vitiligo. N Engl J Med 360: 160-169.
- Bahadoran P, Ortonne JP (2007) Classification of Surgical Therapies for Vitiligo. Wiley, Pg no: 59-68.
- Gauthier Y, Surleve-Bazeille JE (1992) Autologous grafting with noncultured melanocytes: a simplified method for treatment of depigmented lesions. J Am Acad Dermatol 26: 191-194.
- Kim JY, Park CD, Lee JH, Lee CH, Do BR, et al. (2012) Co-culture of melanocytes with adipose-derived stem cells as a potential substitute for co-culture with keratinocytes. Acta Derm Venereol 92: 16-23.
- Razmi T M, Parsad D, Kumaran SM (2017) Combined epidermal and follicular cell suspension as a novel surgical approach for acral vitiligo. J Am Acad Dermatol 76: 564-567.
- Razmi T M, Kumar R, Rani S, Kumaran SM, Tanwar S,et al. (2018) Combination of Follicular and Epidermal Cell Suspension as a Novel Surgical Approach in Difficult-to-Treat Vitiligo: A Randomized Clinical Trial. JAMA Dermatol 154: 301-308.
- Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, et al. (1994) Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 130: 489-493.
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, et al. (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15: 641-648.
- Ismail AM, Abdou SM, Aty HA, Kamhawy AH, Elhinedy M, et al. (2016) Autologous transplantation of CD34(+) bone marrow derived mononuclear cells in management of non-reconstructable critical lower limb ischemia. Cytotechnology 68: 771-781.
- Darinskas A, Paskevicius M, Apanavicius G, Vilkevicius G, Labanauskas L, et al. (2017) Stromal vascular fraction cells for the treatment of critical limb ischemia: a pilot study. J Transl Med 15: 143.
- Raposio E, Bertozzi N, Bonomini S, Bernuzzi G, Formentini A, et al. (2016) Adipose-derived Stem Cells Added to Platelet-rich Plasma for Chronic Skin Ulcer Therapy. Wounds Compend Clin Res Pract 28: 126-131.
Citation: Garg S, Saginatham H, Badheka A (2019) Use of Stem Cells in Intervention Dermatology and Trichology: A New Hope. J Stem Cell Res Dev Ther: S1004.
Copyright: © 2019 Suruchi Garg, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
