
Learning from Negative Results-Critical Incident Reporting System in Laboratory Animal Science (CIRS-LAS.de)
*Corresponding Author(s):
Sabine BischoffCentral Experimental Animal Facility, University Hospital Jena, 07747 Jena, Germany
Tel:+49 1577 4394628,
Email:sabine.bischoff@med.uni-jena.de
David Trietschel
Central Experimental Animal Facility, University Hospital Jena, 07747 Jena, Germany
Tel:+49 1577 4394628,
Email:david.trietschel@med.uni-jena.de
Abstract
In 2016, almost 3 million animals were used for scientific experimental purposes in Germany. In view of this large number, the aim of the project presented here is to contribute to the refinement of animal research practice and to the reduction of laboratory animals in accordance to the guiding 3R-Principles for ethical use as far as possible in the field of laboratory animal science. For this purpose, the Central Experimental Animal Facility of the University Hospital Jena established a "critical incident reporting system in laboratory animal science" (CIRS-LAS) in 2014, based on established CIRS in human medicine. The reporting system should help to clarify negative events and to enable the development of error avoidance strategies by means of a cause analysis. The aim is thus to contribute sustainably to an increase in quality and efficiency in the working environment of laboratory animal science in the sense of the 3R principles. The CIRS-LAS project is supported by the German Federal Ministry of Education and Research (BMBF) since 2017. The domain "www.cirs-las.de" currently exists as a functional beta version, over which 40 reports of critical incidents have been received and evaluated in the sense of descriptive statistics within a reporting period of approximately 18 months. The results show that a large number of different categories ("animal species" involved in incidents, "occupational group" involved, the "estimated degree of stress" for the respective laboratory animal species, the "context of the incident", the "usability of results from experiment" and also “visitor numbers” on the portal) are suitable for evaluation and statistical analysis. Based on the case reports evaluated in the present study, initial statements can be made regarding the underlying causes of the occurrence of critical events. However, reliable statements on observed effects necessitate further analysis and collection of case reports in this regard. Overall, it was possible to demonstrate the high potential of a CIRS for laboratory animal science with regard to the consideration of the 3R principles in daily scientific operations. This is also proven by an ever faster growing number of registrations of new portal users. Critical incident reporting in laboratory animal science can thus serve as a valuable tool to monitor the quality of animal care and give insight into the nature of critical incidents.
Keywords
INTRODUCTION
Both legislative texts were extended by the so-called 3R principles since 2010 [7,8]. In accordance with these principles, every scientist, veterinarian and animal keeper that works in the field of animal test should strives to reduce the amount of animal testing to a minimum and to improve the conditions for laboratory animals. As it is well known from experience in the field of human medicine, “Critical Incident Reporting Systems” (CIRS) can make an efficient contribution to clarify causal error chains in hospital operations and have the potential to minimize the risks to the patient's well-being [9-14]. The high relevance of this topic in general is underlined by currently 3881 hits for the keyword "critical incident reporting system" in the database pubmed. In contrast, up to this point, no CIRS had been implemented in veterinary medicine and only reports on panel meeting discussions existed [15]. In our opinion, a CIRS for laboratory animal science, with the aim to improve conditions for laboratory animals by reducing avoidable incidents as cause of suffering, is long overdue.
Considering the “Declaration of Helsinki” and the current state of medicine, the use of animals in experimental medicine cannot be avoided completely. The need for a CIRS in laboratory animal science is due to almost 3 million animals that were used for scientific experimental purposes in Germany in 2016 [7]. Every year, a large number of scientific articles based on animal experimental studies are published. In view of the high number of laboratory animals, the Swiss Cheese Model [16] predicts the occurrence of critical situations with a risk potential for animals and/or humans. There is therefore a great need to clarify the number of unreported cases and to evaluate dangerous situations in order to further improve the conditions and procedures in the field of laboratory animal science. However, negative experiences gained from experiments get lost or are not referred in publications. The objective of the CIRS-LAS.de reporting system is the collection of negative experiences in the entire range of laboratory animal science and to avoid them in the future. In accordance to the 3R principles the evolved portal will therefore improve animal safety and reduce the number of laboratory animals.
MATERIALS AND METHODS
General aspects
Case reports and user registration
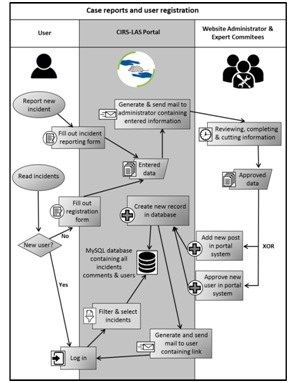 Figure 1: CIRS-LAS-Portal Data flow chart of reported incidents and user registrations (modified after Kobold M [22]).
Figure 1: CIRS-LAS-Portal Data flow chart of reported incidents and user registrations (modified after Kobold M [22]).Contact options
Public relations work
STATISTICS
RESULTS
Figure 2 shows the absolute number of visits to the portal over a period of one year. From June 2017 till December 2017, the number of visits remained relatively constant. An increase in the frequency of visits can be seen within the months January 2018 to May 2018. The maximum number of visits here in March 2018 was 1248 on the page “www.cirs-las.de”.
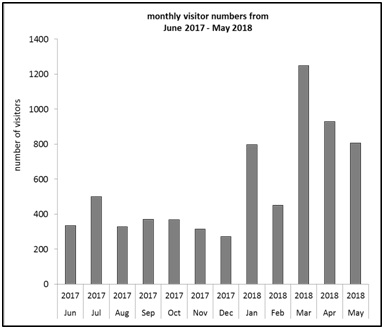
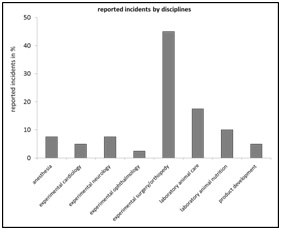 Figure 3: Reported incidents by “disciplines”, relative frequency in percent of n=40.
Figure 3: Reported incidents by “disciplines”, relative frequency in percent of n=40.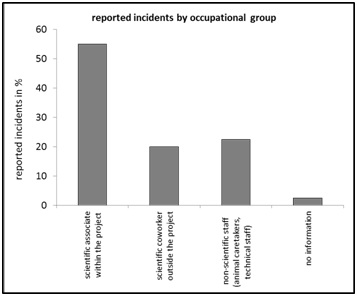 Figure 4: Reported incidents by “occupational groups”, relative frequency in percent of n=40.
Figure 4: Reported incidents by “occupational groups”, relative frequency in percent of n=40.Looking at the distribution of frequencies with respect to the parameter "estimated severity level", the highest values are found in a middle range of the estimation continuum. Relatively high values are also found at the respective ends of the continuum ("no": 20%; "no recovery": 20%) (Figure 5). A “moderate” level of stress for the animals due to a critical incident was reported in the majority of the cases, with a total of 47.5%. The case numbers for an estimated “mild” level of severity (5%) and “severe” (7.5%) occur considerably less frequently at cumulatively 12.5% (Figure 5).
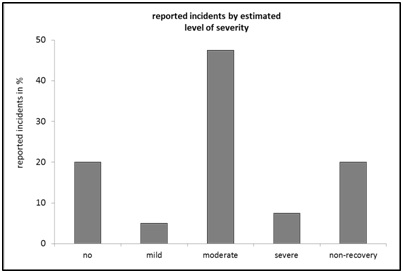 Figure 5: Reported incidents by “estimated severity level”, relative frequency in percent of n=40.
Figure 5: Reported incidents by “estimated severity level”, relative frequency in percent of n=40.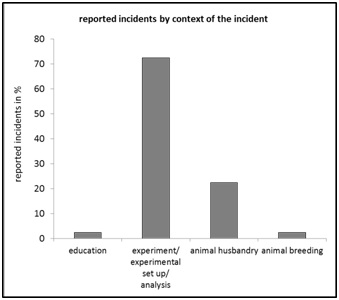 Figure 6: Reported incidents by “context of the incident”, relative frequency in percent of n=40.
Figure 6: Reported incidents by “context of the incident”, relative frequency in percent of n=40.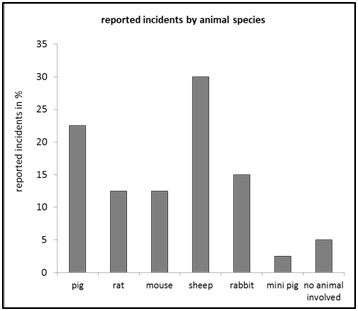 Figure 7: Reported incidents by “animal species”, relative frequency in percent of n=40.
Figure 7: Reported incidents by “animal species”, relative frequency in percent of n=40.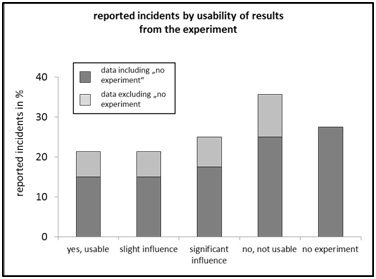 Figure 8: Reported incidents by “usability of results” relative frequency in percent.
Figure 8: Reported incidents by “usability of results” relative frequency in percent.Light gray tone: Relative frequency event reports where an experiment was conducted (n=28).
CONCLUSION
The rate of 17.5% of reported cases from the discipline "laboratory animal care" (Figure 3) underlines the importance of analytical clarification of the occurrence of errors that lead to critical events even outside the actual core project of experimental investigations. The importance of interdisciplinary use of CIRS-LAS is illustrated by case reporting rates of 22.5% generated by the occupational group: “Non-scientific staff” and a cumulative share of 42.5% of reports submitted by staff who were not directly involved in the experimental project ("scientific coworker outside the project" with 20% and "non-scientific staff" with 22.5%) (Figure 4). About half of all reported cases originate from "scientific associate within the project" (55%) (Figure 5), whereby 72.5% (Figure 6) of the cases are attributed to the supposedly corresponding context "experiment/ experimental set up/ analysis". A further comparative statistical study could answer the interesting question of which "occupational group" assigns a critical event to which "context". The contextual attribution of the occurrence of critical events in connection with an "occupational group", however, could have conflicting effects in terms of a less condemning error culture [24]. This, insofar as the results provide scope for interpretation in the sense of a mutual error attribution effect between the respective "occupational groups".
In relation to the “estimated severity level” on the experimental animal (Figure 5), normally-distributed data would be expected according to the gradations of severity. Although the majority of cases report a moderate degree of severity, this cannot be observed here. It therefore remains open until further notice whether this anomaly can be attributed to the relatively small data set or whether it is based on real effects. The result of the frequency analysis of the corresponding “animal species” in the reported cases therefore also leaves room for open questions (Figure 7). For example, “sheep” are reported to be involved in 30% of cases (Figure 7). Whether there is a connection with an increasing number of reports by a certain working group, which is increasingly experimenting on “sheep”, remains unclear within the scope of the anonymity agreement, since the corresponding data are not collected on the portal side. Independent interpretation possibilities, for example whether the sheep as an experimental animal is potentially more difficult to handle than comparatively other types of experimental animal, or whether the observed tendencies may also occur purely random, remain open here.
With regard to the usability of results from the cases with experimental context, the analysis of the data set evaluated here suggests a high potential for a significant increase in efficiency and quality through improved error detection in animal experimentation practice (Figure 8). Systematic error detection by a CIR system of laboratory animal science, combined with the development of a corresponding catalogue of measures for error prevention, thus form the cornerstones of a promising strategy for contributing to the refinement and reduction of laboratory animals.
However, to obtain more reliable information from the statistical analysis of the case report database, a higher number of reports are required. The aim is therefore to increase participation in the reporting system in the future. Within the framework of further education lectures, participation in congresses and conferences as well as online lectures, a different awareness of the prevailing error culture [24] is to be initiated. On the other hand, however, the aim is also to pursue concrete educational work with regard to the degree of awareness, the mode of operation and the benefits of CIRS-LAS. In order to create the possibility of receiving ad hoc feedback on an individual case report, the formation of an expert panel is planned in the medium term, which will provide information on troubleshooting and avoidance. Independent of statistical meta-analyses, the planned expert panel will act as an additional feedback tool and generate a direct benefit for reporting a critical incident in the form of a learning effect. Additionally, the comment section of the collected incidents serves as a basis for fundamental discussions and an exchange of ideas between scientific peers on the portal. A detailed error analysis from the concrete incidents with proposals for measures which should contribute to their avoidance will be carried out separately and after further reports have been collected. The resulting error avoidance strategies form the basis for two of the 3R principles (reduction and refinement) so that failed projects are not repeated and the conditions for experimental animals in experiments, animal husbandry and breeding can be improved. In addition to the plausibility check of reported cases by the experts of the CIRS-LAS team [17], a further revision by an expert committee, which is currently under construction, is planned in the future. In this way, it is to be ensured that only cases that have actually occurred are entered into the database.
Over all the results show that CIRS-LAS portal has a great potential to serve as an essential contribution towards the implementation of two of the 3R principles. Anonymously shared negative experiences can have an influence on the experimental setup and execution of new scientific projects. In conclusion the realization of 3R-a reduction of the number of used animals in experiments (reduction), diminution of stressful experiments (refinement)-can be achieved by using the CIRS-LAS portal. It is furthermore conceivable that the implementation of the CIRS-LAS could serve to enhance the trust in laboratory animal science, both of the public and the scientific community. The time to rethink has been achieved-to learn from negative results in animal based research!
REFERENCES
- Ullrich KJ, Creutzfeld OD (1985) Gesundheit Und Tierschutz. Wissenschaftler melden sich zu Wort, Econ Verlag, Dusseldorf and Vienna, Germany.
- World Medical Association (2013) World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310: 2191-2194.
- Schweitzer A (1959) Die Ethik der Ehrfurcht vor dem Leben. Kap. 26 In: Kulturphilosophie Bd. 2: Kultur und Ethik, München 1923. Zitiert nach der US-Amerikanischen Ausgabe: Philosophy of Civilisation. Mac Millan, New York, USA.
- Official Journal of the European Union (2010) Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union L 276/33.
- Federal Ministry of Justice and Consumer Protection (2006) Animal Protection Act. Article 141 of the Act of 29 March 2017 (BGBl. I p. 626), Federal Ministry of Justice and Consumer Protection, Germany.
- Federal Ministry of Food, Agriculture and Consumer Protection (2013) Regulation implementing Directive 2010/63 / EU of the European Parliament and of the Council of 22 September 2010 for the protection of animals used for scientific purposes. Federal Law Gazette Year 2013 Part I No. 47 issued to Bonn on 12 August 2013, Federal Ministry of Food, Agriculture and Consumer Protection, Germany.
- https://www.tierversuche-verstehen.de/
- Russell WMS, Burch RL (1959) The principles of humane experimental technique. Methuen , London, UK. Pg no: 238.
- Ahluwalia J, Marriott L (2005) Critical incident reporting systems. Seminars in Fetal & Neonatal 10: 31-37.
- Beyer M, Blazejewski T, Güthlin C, Klemp K, Wunder A, et al. (2015) The family medical error reporting and learning system, aller-fehler-zaehlt.de '- Report portfolio and usage prospects aller-fehler-zaehlt.de: Content of and prospective benefits from a critical incident reporting and learning system (CIRS) for primary care. Journal of Evidence, Training and Quality in Health Care 109: 62-68.
- Meyer-Massetti C, Krummenacher E, Hedinger-Grogg B, Luterbacher S, Hersberger KE (2016) Medication safety in the home care area - Development and piloting of a critical incident reporting system. Pflege 29: 247-255.
- Hohenstein C, Fleischmann T, Hempel D (2011) Critical events in emergency medicine. Notfallmedizin up2date, Georg ThiemeVerlag KG Stuttgart 6: 89-105.
- Hohenstein C, Hempel D, Schultheis K, Lotter O, Fleischmann T (2014) Critical incident reporting in emergency medicine: Results of the prehospital reports. Emerg Med J 31: 415-418.
- Hohenstein C, Schultheis K, Winning J, Rupp P, Fleischmann T (2013) Critical incidents in preclinical emergency airway management: Evaluation of the CIRS emergency medicine databank. Anaesthesist 62: 720-724, 726-727.
- Hartnack S, Bettschart-Wolfensberger R, Driessen B, Pang D, Wohlfender F (2013) Critical incidence reporting systems-an option in equine anaesthesia? Results from a panel meeting. Vet Anaesth Analg 40: 3-8.
- Reason J (2000) Human error: Models and management. BMJ 320: 768-770.
- University Hospital Jena Dornburger (2018) Task Force CIRS-LAS, Central Experimental Animal Facility, University Hospital Jena Dornburger, Jena, Germany.
- Staender S, Davies J, Helmreich B, Sexton B, Kaufmann M (1997) The anaesthesia critical incident reporting system: An experience based database. Int J Med Inform 47: 87-90.
- https://awstats.sourceforge.io/
- Webalizer (2014) Free web server log file analysis program.
- Official Journal of European Union (2016) Legislation. Official Journal of European Union 59: 117.
- Loesel R, Bischoff S (2015) Guideline for the Promotion of "Alternative Methods to Animal Testing". Federal Gazette dated 24.12.2015. Projektskizze zum Thema: Berichtsystem kritischer Ereignisse in der Versuchstierkunde/Critical Incident Reporting System in Laboratory Animal Science (CIRS-LAS), Projektskizze 48, Institut für Versuchstierkunde und Tierschutz, Universitätsklinikum Jena,Germany.
- Sendlhofer G, Leitgeb K, Kober B, Brunner G, Kamolz LP (2016) The evolution of the Critical Incident Reporting System in an Austrian University Hospital. Z Evid Fortbild Qual Gesundhwes 114: 48-57.
- Hubertus J, Piehlmeier W, Heinrich M (2016) Communicating the Improvements Developed from Critical Incident Reports is an Essential Part of CIRS. Klin Padiatr 228: 270-274.
- Köbberling J (2005) The critical incident reporting system (CIRS) as a measure to improve quality in medicine. Med Klin (Munich) 100: 143-148.
- GV-Solas (2013) Society for Laboratory Animal Science. Marburg, Germany.
- Felasa (2018) Federation of European Laboratory Animal Science Associations. Suffolk, UK.
- https://www.aaalac.org/
Citation: Bischoff S, Trietschel D, Enkelmann A, Schiffner R, Estrade P, et al. (2018) Learning from Negative Results-Critical Incident Reporting System in Laboratory Animal Science (CIRS-LAS.de). J Anim Res Vet Sci 2: 009.
Copyright: © 2018 Sabine Bischoff, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

