
The Incidence of Histopathological Lesions in Gastric Mucosa and Gastrointestinal Signs in Dogs Suffering from Canine Ehrlichiosis Undergoing Treatment with Doxycycline: A Descriptive Longitudinal Study
*Corresponding Author(s):
Gonzalez-Dominguez MSVeterinary Teaching Hospital And Zootechny Centre, Faculty Of Veterinary Medicine And Zootechny, Loma El Escobero Km 4 Envigado, CES University, Colombia
Tel:+57 43360260,
Email:mgonzalez@ces.edu.co
Abstract
Study background: Canine Ehrlichiosis is an infectious disease caused by bacteria from the Anaplasmataceae family, infecting monocytes, platelets, and polymorph nuclear cells. The disease can affect dogs at any age and causes a great variety of clinical signs and hematological changes. Treatment for canine Ehrlichiosis includes oral doxycycline for at least 28 days to several months, depending on each dog individual responses to the treatment. Prolonged treatments appear to be related to adverse collateral effects at the gastrointestinal level. We designed this study for evaluating the frequency of gastrointestinal tract injuries and clinical signs before and after treatment with oral doxycycline of dogs suffering from canine Ehrlichiosis.
Methods: In a descriptive longitudinal study, a group of working dogs that rested seropositive for canine Ehrlichiosis (n=19) received oral doxycycline (10mg/kg/24h/28days). Dogs were evaluated by clinical exam, endoscopic gastro-duodenoscopy performed under general anesthesia, a sampling of a biopsy and it’s processing of histopathological evaluation and detection of Helicobacter spp., and clinical follow up during treatment and at 30 days after treatment was finished.
Results: Diarrhea was the most frequent clinical sign, showing a positive correlation between the age of the dog (P<0.05). The most common histopathological finding was edema of the gastric and duodenal mucosa, which tended to increase at day 30 after treatment finished (P=0.12). The presence of Helicobacter spp., tended to increase in patients after treatment finished (P=0.12).
Conclusion: No significant changes of gastric mucosa were evidenced after oral treatment with doxycycline, whereas the presence of Helicobacter spp., in gastric mucosa trend to reduce at day 30 after treatment. Finally, the significant correlation between doxycycline treatment and age of patient suggest an adverse outcome of this treatment schedule on older patients.
Keywords
INTRODUCTION
MATERIALS AND METHODS
Sample selection
Treatment schedule of dogs seropositive for CE
Endoscopic evaluation, biopsy, sampling, and histopathological processing and evaluation
Microscopic findings were read according to the method of semi-quantitative estimate under the standardizing criteria for microscopic gastrointestinal evaluation proposed by WSAVA International. It makes a more accurate sample assessment taking into account the distribution of layers of the digestive tract and the relevant microscopic changes, for generating a common starting point for histopathological evaluation [23]. Similarly, the diagnosis and quantification of Helicobacter spp., in microscopic slides were conducted by the modified Sydney method [25]. The same Pathologist read and diagnosed all histopathological slides as mentioned above.
Clinical follow-up of the dogs
Statistical analysis
RESULTS
Retching, reflux, vomiting, or diarrhea, did not significantly differ between gender of dogs, nor between time zero and day 30 after antibiotic treatment. Seven out of 15 dogs (46.7%) developed adverse reactions to doxycycline treatment: Three dogs (20%) (One female and 2 males), two dogs (13,3%) (One female and one male), and four dogs (26,6%) (One female and 3 males), presented retching, vomiting, and diarrhea, respectively. Only a dog showed retching and vomiting concomitantly. Gastrointestinal signs did occur more frequently (although not statistically different) in patients with a Body Condition Score (BCS) of 3 (1 to 5 scale) compared to other BCS degrees.
The frequency of gastrointestinal signs was significantly higher in adult dogs: Three dogs presented retching (Two dogs between 3 to 6 years and one dog within 1 to 3 years old), and two dogs presented vomiting (Both dogs between 3 to 6 years old). Also, dysentery signs were significantly higher in patients from 1 to 3years old (One dog), and 6 to 9 years old (Two dogs) (P<0.05) (Figure 1).
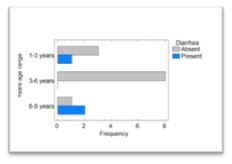 Figure 1: Frequency distribution of diarrhea in dogs suffering from CE treated with doxycycline, according to the group of age of the dogs.
Figure 1: Frequency distribution of diarrhea in dogs suffering from CE treated with doxycycline, according to the group of age of the dogs.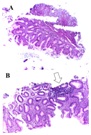
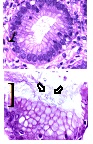 Figure 3: A. Corporal antral gastric mucosa with a diagnosis of inactive chronic superficial gastritis without intestinal metaplasia, atrophy or dysplasia, showing lymphocytes in the lamina propria and plasma cells 1/3 (mild). There were not found intraepithelial lymphocytes, eosinophils or neutrophils in the lamina propria and gastric lymphoid follicular hyperplasia. B. White arrow: Presence of Helicobacter1/3 (mild) (Hematoxylin & Eosin, A: 100X. B: 100X).
Figure 3: A. Corporal antral gastric mucosa with a diagnosis of inactive chronic superficial gastritis without intestinal metaplasia, atrophy or dysplasia, showing lymphocytes in the lamina propria and plasma cells 1/3 (mild). There were not found intraepithelial lymphocytes, eosinophils or neutrophils in the lamina propria and gastric lymphoid follicular hyperplasia. B. White arrow: Presence of Helicobacter1/3 (mild) (Hematoxylin & Eosin, A: 100X. B: 100X).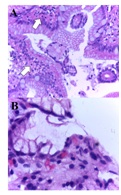 Figure 4: Microscopic findings in duodenal mucosa diagnosed as moderate chronic nonspecific inflammation (White arrows in figure A), negative for malignancy. In this figure, it can be observed a duodenal mucosa with no dysplasia although accompanied by a moderated lymphoplasmacytic infiltrate in the lamina propria (asterisks in figure B). Neither malignancy nor dysplasia was observed (Hematoxylin&Eosin, A: 40X. B: 100X).
Figure 4: Microscopic findings in duodenal mucosa diagnosed as moderate chronic nonspecific inflammation (White arrows in figure A), negative for malignancy. In this figure, it can be observed a duodenal mucosa with no dysplasia although accompanied by a moderated lymphoplasmacytic infiltrate in the lamina propria (asterisks in figure B). Neither malignancy nor dysplasia was observed (Hematoxylin&Eosin, A: 40X. B: 100X).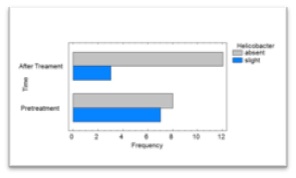 Figure 5: Bar chart for the presence of Helicobacter in the gastric mucosa of dogs suffering from CE treated with doxycycline, before and after treatment.
Figure 5: Bar chart for the presence of Helicobacter in the gastric mucosa of dogs suffering from CE treated with doxycycline, before and after treatment.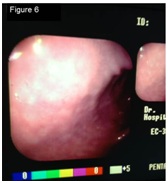 Figure 6: Previous-to-treatment Endoscopic assessment of dogs suffering from CE treated with doxycycline. It was not found edema, discoloring, friability, bleeding, erosion or ulceration. Slight gastric content and normal expansion were observed.
Figure 6: Previous-to-treatment Endoscopic assessment of dogs suffering from CE treated with doxycycline. It was not found edema, discoloring, friability, bleeding, erosion or ulceration. Slight gastric content and normal expansion were observed.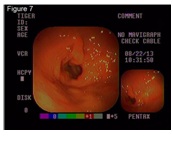 Figure 7: Post-treatment Endoscopic assessment of dogs suffering from CE treated with doxycycline. The following abnormal findings were observed: Edema, mild discoloring, friability, bleeding, absence of erosion or ulceration and moderate content with normal expansion.
Figure 7: Post-treatment Endoscopic assessment of dogs suffering from CE treated with doxycycline. The following abnormal findings were observed: Edema, mild discoloring, friability, bleeding, absence of erosion or ulceration and moderate content with normal expansion.DISCUSSION
Accordingly, in the current study it was observed that when administering doxycycline as a treatment for CE during 28 days, there were no statistically significant alterations of the gastric mucosa, although a tendency to present edema of the mucosa was observed between days 0 and 30 of treatment. Nevertheless, these changes were classified as mild. Although the aim of the present work was not to evaluate the sensitivity of Helicobacter to doxycycline, we incidentally found beneficial effects of treatment on diminishing the presence of the bacteria. For instance, we observed a trend in decreasing the presence of Helicobacter after treatment, with a reduction from 40 to 26.67% in these patients, in agreement with previous reports in which 20 mg/kg/day doxycycline treatment lasted 21 days [26,27].
Among the adverse signs evaluated in the present study, diarrhea was the only one that showed differences between age ranges: In dogs in the range of 6 to 9 years old and dogs in the group of 1 to 3 years old. On the contrary, we did not find these differences amongst dogs of the group from 3 to 6 years old. These findings are in agreement, at least partially, with previous research conducted by Schulz et al., [17], in which authors investigated the side effects associated to the use of doxycycline in 386 cases, where 7% of dogs developed diarrhea during treatment. Authors reported that age was a risk factor for the occurrence of the above mentioned intestinal effects [17].
In the present study,we reported for the first time in our city the incidence of doxycycline adverse effects in dogs. Considering that doxycycline is the choice treatment of CE, and the disease is one of the most common in our practice, it is essential to consider its adverse gastrointestinal effects in canine patients. This study confirms that, despite the absence of statistically significant alterations of gastric mucosa, the risk of adverse effects manifestation must be considered as reported elsewhere [17,19,28].
In this study, none of the patients showed reflux-related signs during treatment, contrary to the report by Tutuian et al., [29] in which authors found that oral doxycycline treatment promoted gastroesophageal reflux and led to esophagitis [29]. In our study, mucosal edema of mild intensity was observed during the endoscopy procedure, which showed a tendency to increase when comparing between pre- and post-treatment times, increasing from 13.3 to 30% of cases.
Treatment of dogs suffering from CE with doxycycline for prolonged periods causes microscopic and macroscopic effects of mild severity on the gastric mucosa, including a slight increase of mucosal edema. Moreover, treatment with doxycycline of working dogs suffering from Ehrlichia infection proved to be favorable for the elimination or reduction of Helicobacter spp., in agreement with the reports by Anacleto et al., [26] and Morales et al., [27]. On the other hand, diarrhea was the main adverse effect related to this treatment, directly correlated with the ageof the dog [26,27].
CONCLUSION
COMPETING INTEREST
ACKNOWLEDGMENT
REFERENCES
- Vinasco J, Li O, Alvarado A, Diaz D, Hoyos L, et al. (2007) Molecular evidence of a new strain of Ehrlichia canis from South America. J Clin Microbiol 45: 2716-2719.
- Yabsley MJ, Mc Kibben J, Macpherson CN, Cattan PF, Cherry NA, et al. (2008) Prevalence of Ehrlichia canis, Anaplasma platys, Babesia canis vogeli, Hepatozoon canis, Bartonella vinsonii berkhoffii, and Rickettsia spp., in dogs from Grenada. Vet Parasitol 151: 279-285.
- Santos F, Coppede JS, Pereira AL, Oliveira LP, Roberto PG, et al. (2009) Molecular evaluation of the incidence of Ehrlichia canis, Anaplasma platys and Babesia spp., in dogs from Ribeirão Preto, Brazil. Vet J 179: 145-148.
- Álvaro ME, Álvaro EG, Guillermo RC (2000) Prevalencia de Ehrlichia canis en la ciudad de Montería, Córdoba, Colombia. Rev MVZ Córdoba 5: 20.
- Parrado M, Vargas F, Hernandez G, Vergara H (2003) Asociación de los resultados de una prueba serológica (ELISA) Y frotis sanguíneo en caninos con sintomatología compatible de c. Orinoquia 7: 6-11.
- Silva-Molano RF, Sánchez-Ucrós N, Loaiza-Echeverri AM (2008) Reporte de presentacion de Ehrlichia canis en muestras sanguineas de caninos en la ciudad de Cali, Colombia. Vet Zootec 2: 27-31.
- González H, Loaiza J (2012) Medición de la concordancia en el diagnóstico entre la prueba de Elisa y el cuadro hemático mediante un estudio paraclínico-epidemiológico de la Ehrlichia canis. Rev Colomb Cienc Anim 5: 47-51.
- Vargas-Hernández G, André MR, Faria JL, Munhoz TD, Hernandez-Rodriguez M, et al. (2012) Molecular and serological detection of Ehrlichia canis and Babesia vogeli in dogs in Colombia. Vet Parasitol 186: 254-260.
- Rojas-Triviño A, Rueda-Hurtado A, Díaz-Molano DM, Mesa-Cobo NC, Benavides-Montaño JA, et al. (2013) Identificación de Ehrlichia canis (Donatien & Lestoquard) Moshkovski mediante PCR anidada. Veterinaria y Zootecnia 7: 37-48.
- Barber RM, Li Q, Diniz PP, Porter BF, Breitschwerdt EB, et al. (2010) Evaluation of brain tissue or cerebrospinal fluid with broadly reactive polymerase chain reaction for Ehrlichia, Anaplasma, spotted fever group Rickettsia, Bartonella, and Borrelia species in canine neurological diseases (109 cases). J Vet Intern Med 24: 372-378.
- Neelakanta G, Sultana H, Fish D, Anderson JF, Fikrig E (2010) Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. J Clin Invest 120: 3179-3190.
- Ravnik U, Tozon N, Smrdel KS, Zupanc TA (2011) Anaplasmosis in dogs: The relation of haematological, biochemical and clinical alterations to antibody titre and PCR confirmed infection. Vet Microbiol 149: 172-176.
- McClure JC, Crothers ML, Schaefer JJ, Stanley PD, Needham GR, et al. (2010) Efficacy of a doxycycline treatment regimen initiated during three different phases of experimental Ehrlichiosis. Antimicrob Agents Chemother 54: 5012-5020.
- Chambers P (2011) Rational use of medicines. Vet Rec 169: 226-227.
- Schulz BS, Hupfauer S, Ammer H, Sauter-Louis C, Hartmann K (2011) Suspected side effects of doxycycline use in dogs-a retrospective study of 386 cases. Vet Rec 169: 229.
- Neer TM, Breitschwerdt EB, Greene RT, Lappin MR (2002) Consensus statement on ehrlichial disease of small animals from the infectious disease study group of the ACVIM. American college of veterinary internal medicine. J Vet Intern Med 16: 309-315.
- Akbayir N, Alkim C, Erdem L, Sakiz D, Sökmen HM (2002) A case report of doxycycline-induced esophageal and gastric ulcer. Turk J Gastroenterol 13: 232-235.
- Botana-López LM, Landoni MF, Martín-Jiménez T (2002) Farmacología y terapéutica veterinaria. McGraw-Hill/Interamericana, Madrid, Spain. Pg no: 1-5.
- Maddison JE, Page SW, Church D (2008) Small animal clinical pharmacology. [2nd edition], Saunders/Elsevier, Edinburgh, New York, USA. Pg no: 584.
- Hernández CA, Gallón G, Restrepo LF (2007) Análisis de biopsias gástricas endoscópicas en caninos. Rev Colomb Cienc Pecua 20: 250-259.
- Washabau RJ, Day MJ, Willard MD, Hall EJ, Jergens AE, et al. (2010) Endoscopic, Biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 24: 10-26.
- Dixon MF, Genta RM, Yardley JH, Correa P (1996) Classification and grading of gastritis. The updated Sydney System. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol 20: 1161-1181.
- Anacleto TP, Lopes LR, Andreollo NA, Bernis-Filho WO, Resck MCC, et al. (2011) Studies of distribution and recurrence of Helicobacter spp., gastric mucosa of dogs after triple therapy. Acta Cirurgica Brasileira 26: 82-87.
- Morales A, Arrieta D (2012) Presence of Helicobacter like organisms after twenty-one days of treatment in asymptomatic dogs: A preliminary study. Arch Venezolanos Farmacol Terap 31: 34-36.
- Gutierrez L, Velazco ZH, Vazquez C, Vargas D, Sumano H (2012) Pharmacokinetics of an injectable long-acting formulation of doxycycline hyclate in dogs. Acta Vet Scand 54: 35.
- Tutuian R (2010) Adverse effects of drugs on the esophagus. Best Pract Res Clin Gastroenterol 24: 91-97.
Citation: Giraldo AM, Hernandez CA, Gonzalez-Dominguez MS (2018) The Incidence of Histopathological Lesions in Gastric Mucosa and Gastrointestinal Signs in Dogs Suffering from Canine Ehrlichiosis Undergoing Treatment with Doxycycline: A Descriptive Longitudinal Study. J Anim Res Vet Sci 2: 011.
Copyright: © 2017 Giraldo AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

