
Nutrition Therapy Regulates Caffeine Metabolism with Relevance to NAFLD and Induction of Type 3 Diabetes
*Corresponding Author(s):
Ian J MartinsCentre Of Excellence In Alzheimers Disease Research And Care, School Of Medical Sciences, School Of Psychiatry And Clinical Neurosciences, McCusker Alzheimer's Research Foundation, The University Of Western Australia, Holywood Medical Centre, Edith Cowan University, Joondalup, Australia
Tel:+61 863042574,
Email:i.martins@ecu.edu.au
Abstract
In the developed and developing world nutritional interventions have become essential to prevent global Non Alcoholic Fatty Liver Disease (NAFLD) and to maintain the metabolism of glucose, fatty acids, cholesterol, amyloid beta, bile acids and xenobiotics. The World Health Organization (WHO) has reported that cardiovascular disease is the most prevalent global chronic disease that may be connected to NAFLD and the alarming death rate in various communities. Caffeine (appetite suppressant) may improve the adipose tissue-liver cross talk with the prevention of NAFLD in obese and Type 2 diabetic populations. Overeating may accelerate chronic diseases with repression of anti-aging genes linked to NAFLD and delayed caffeine clearance linked to the induction of Type 3 diabetes in global populations. Nutritional interventions to reverse NAFLD in the developing world are associated with accelerated caffeine clearance rates with prevention of caffeine induced mitochondrial apoptosis that is linked to early neuron loss and the development of Type 3 diabetes in these populations.
Keywords
ABBREVIATIONS
Sirt 1: Sirtuin 1;
T3D: Type 3 Diabetes;
NAFLD: Non Alcoholic Fatty Liver Disease;
SCN: Supra Chiasmatic Nucleus;
FOXO: Forkhead Box Protein;
Cytochrome A1, CYP 1A1: BDNF: Brain Derived Neurotrophic Factor;
NO: Nitric Oxide;
LPS: Bacterial Lipopolysaccharides
BACKGROUND
In the developing and developed world Non Alcoholic Fatty Liver Disease (NAFLD) and diabetes [1-7] has escalated in various communities with the essential need for nutritional interventions [8] to improve and control overeating and to accelerate the clearance of hepatic of glucose, fatty acids, bile acids, xenobiotics, amyloid beta and cholesterol that are associated with various chronic diseases. Appetite dysregulation is connected to NAFLD and the defective metabolism of various plasma components [4-6] that may accelerate chronic diseases that include obesity, diabetes, cardiovascular disease and neurodegenerative diseases. Nutrition therapy is essential to maintain appetite control [4]and determines the hepatic degradation of glucose, fatty acids and cholesterol that is connected to the toxic amyloid beta oligomers [8,9] and xenobiotics with relevance to programmed cell death in various chronic diseases.
Caffeine is an appetite suppressant [10-12] and with the aging process its delayed clearance can be related to the induction of NAFLD and Type 3 Diabetes (T3D) [6,7]. The beneficial effects of caffeine may be corrupted with toxic caffeine doses transported to the brain relevant to neurodegeneration and induction of T3D diabetes. In previous studies Alzheimer’s disease represents a form of T3D and related to extensive disturbances in brain insulin and insulin-like growth factor signalling pathways with relevance to critical abnormalities in AD [13].
T3D is associated with Supra Chiasmatic Nucleus (SCN) dysfunction with the abnormal maintenance of brain and whole body glucose in various species and man [14-16]. Nutritional interventions have become important to increase caffeine metabolism and prevent the induction of T3D [17-19] in global populations (Figure 1). Delayed caffeine metabolism may further induce myocardial infarction, pancreas and thyroid disorders relevant to Type 1 (T1D), Type 2 (T2D) and T3D [20] and various other chronic diseases [21-30] (Figure 1).
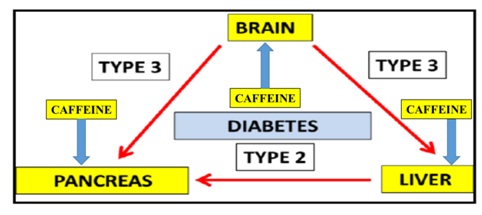
Figure 1: In the global epidemic for chronic diseases T3D and T2D are now connected to Non Alcoholic Fatty Liver Disease (NAFLD). Hepatic caffeine metabolism in T3D/T2D is corrupted with the induction of caffeine induced pancreatic disease and various chronic diseases.
Appetite control in diabetic populations is essential to prevent mitochondrial apoptosis that induce diseases such as NAFLD, myocardial infarction and neurodegenerative disease [21-30]. T3D diabetes and appetite dysregulation is a major concern with brain disorders and autonomic dysregulation [6] related to a defect in the cross talk between the adipose tissue and the liver [31,32]. Interests in the adipose tissue has escalated with relevance to the global obesity epidemic [5] with NAFLD as the major disease progression. Caffeine and prevention of adipogenesis [33-35] has been reported but in T3D caffeine toxicity [36,37] may be relevant to caffeine intake (dose) and the nature of food composition (fat/glucose) important to the induction of mitochondrial apoptosis and NAFLD in T3D.
ANTI-AGING GENES AND CAFFEINE METABOLISM WITH RELEVANCE TO NAFLD, DIABETES AND CHRONIC DISEASES
Interests in nutrition therapy to prevent NAFLD and diabetes now involve low calorie diets that identify a single anti-aging gene defect in the development of diabetes [38-40]. The gene defect involves the anti-aging gene Sirtuin 1 (Sirt 1) that regulates appetite, nuclear-mitochondria interaction, adipose tissue-liver crosstalk, synaptic plasticity and neuron proliferation in various populations in the developing and developed world [6,31,32,41-46]. Sirt 1 is important to SCN function that maintains synchrony between neurons that is critical to circadian rhythm and glucose clearance in the brain and periphery [14-16]. Metabolic disturbances in neurons may be linked to other anti-aging genes associated glucose dyshomeostasis and dyslipidemia. Interests in calorie restriction, appetite regulation and neurodegeneration that involve Sirt 1 mediated regulation of other anti-aging genes has accelerated [47-49] and involve p53 and FOXO deacetylation in relation to autonomous disease of the brain and liver. Over nutrition is associated with the repression of Sirt 1 and control the other anti-aging genes such as Klotho, p66shc (longevity protein) and FOXO1/FOXO3a that are connected to brain and liver with SCN disturbances. Nutritional interventions that maintain Sirt 1 activity are important to T3D, T2D and T1D with Sirt 1 down regulation in NAFLD relevant to the defective metabolism of caffeine, glucose, fatty acids, cholesterol, amyloid beta, bile acids, and xenobiotics.
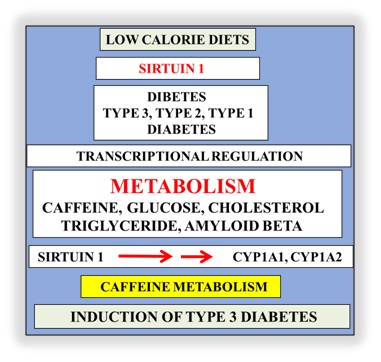
Figure 2: Defective hepatic metabolism of caffeine and induction of T3D has now become important to global chronic diseases. Unhealthy diets and Sirt 1 transcriptional dysregulation involve the inoperative cytochrome p450 enzymeswith defective caffeine associated with defective glucose, fatty acids, cholesterol and amyloid beta metabolism and induction of T3D.
Regulation of food intake involves Sirt 1 and is the gene that is linked to life span, obesity and cardiovascular disease with effects on NAFLD, inflammation, energy, cognition, mitochondrial biogenesis, neurogenesis, glucose/cholesterol levels and amyloidosis. Sirt 1 is a Nicotinamide Adenine Dinucleotide (NAD+) dependent class III Histone Deacetylase (HDAC) that targets transcription factors to adapt gene expression to metabolic activity and the deacetylation of the nuclear receptors with its critical involvement in insulin resistance. Sirt 1 is important to telomerase reverse transcriptase and genomic DNA repair with its involvement in telomere maintenance that maintains chromosome stability and cell proliferation [7]. Tissue nuclear receptors undergo deacetylation of histone and non-histone targets by Sirt 1 (NAD+ dependent class III histone deacetylase) that target transcription factors Peroxisome Proliferator-Activated Receptor-Gamma Coactivator (PGC-1 alpha), p53, Pregnane X Receptor (PXR) to adapt gene expression to metabolic activity, insulin resistance and inflammation. Sirt 1 is linked to glucose regulation with the involvement of Forkhead box protein O1 (FOXO1) deacetylation (apoptosis) that involve p53 transcriptional dysregulation and Peroxisome Proliferator Activated Receptor (PPAR) gamma nuclear receptor. Furthermore Sirt 1/p53 interactions may regulate adipocytokines and immune responses that may be important to NAFLD, obesity and neurodegeneration [31]. Sirt 1 is critical to caffeine metabolism with Sirt 1 regulation of Cytochrome A1 (CYP 1A1) and Cytochrome A1 (CYP 1A2) essential for caffeine [50] and xenobiotic clearance [51]. Sirt 1 regulation of Brain Derived Neurotrophic Factor (BDNF) has been shown [47] with caffeine linked to Sirt 1/BDNF regulation and synaptic plasticity [52,53]. Caffeine induces FOXO1 transport to the nucleus [54,55] with relevance to neuron apoptosis and neurodegeneration in diabetes. Nutrition therapy that involves coffee (composition) versus caffeine intake (dose) is critical to the regulation of Sirt 1 with the primary hepatic caffeine metabolism (Figure 2) that determines the secondary clearance of hepatic glucose, fatty acids, bile acids, xenobiotics and amyloid beta that are closely connected to various chronic diseases such as NAFLD, cardiovascular disease, diabetes, stroke and neurodegeneration [56-63]. Caffeine is essential for the prevention of blood brain barrier disruption [64-68] but with NAFLD excess caffeine transport to the brain is associated with neurodegeneration. Coffee may contain ochratoxin A that is a potent neurotoxin relevant to mitochondrial apoptosis and overrides Sirt‘s regulation of mitochondrial biogenesis with relevance to neurodegeneration [69].
The global death rate by the WHO has reported cardiovascular disease to be the most prevalent chronic disease and connected to appetite dysregulation with Sirt 1 defects relevant to myocardial infarction in various studies. Defective Sirt 1 [38-40,47-49] is associated with glucose dysregulation with inhibition of insulin signalling and acute mitochondrial apoptosis that may be relevant to myocardial infarction.Over nutrition that down regulates Sirt 1 interferes with hepatic caffeine and cellular Nitric Oxide (NO) homeostasis with implications to cardiovascular disease [70-76] and various global organ diseases [6]. Over nutrition that involves excessive NO consumption prevents mitochondrial biogenesis [4] with effects on mitochondria size, number and shape [77-80].
Mitochondrial function has been reported to be important to chronic disease with Sirt 1/p53 defects critical to mitophagy with relevance to NAFLD, cardiovascular disease, obesity and neurodegenerative diseases [7,20,31]. In the current obesity/diabetes epidemic caffeine metabolism is possibly defective [18] and effects of caffeine is via Sirt 1/p53 regulated mitochondria biogenesis with caffeine doses related to p53 mediated mitophagy relevant to cellular programmed cell death [17]. Sirt 1 is relevant to mitophagy [17-20] in myocardial infarction [81-86] and hepatic caffeine metabolism now important to endothelial death and interference of the endothelial NO synthase pathway with promotion of neurodegeneration and T3D [6]. Caffeine and its defective clearance in NAFLD may indicate that caffeine may reach a saturation point by binding to albumin [18,87] with primary saturation of albumin (Figure 3) in the plasma and secondary caffeine saturation of cell membranes. Caffeine can be converted to theophylline in cells but beneficial theophylline effects may be sensitive to toxic elevated caffeine levels in cells. Furthermore delayed caffeine clearance and increased theophylline levels [88-94] may interfere with magnesium and calcium binding to albumin (Figure 3). Saturation of albumin by caffeine or theophylline interferes with plasma magnesium and calcium corrections [95,96] that are related to plasma albumin contents (Figure 3). Caffeine excess with aging is relevant to magnesium deficiency [97-99] and relevant to neuron calcium dyshomeostasis [17,100] that is connected to SCN dysynchrony critical to the maintainance of circadian rhythms [7]. Disturbances in neurons by caffeine involve neuron calcium disturbances that involve the adenosine receptors, synapses and neuron networks [101,102] with detrimental effects on the central nervous system with irreversible T3D [36,37]. Sirt 1 regulation of SCN synchrony and neuron synapse plasticity is completely corrupted by caffeine as caffeine overload in the CNS occurs with aging process. Magnesium deficiency is connected to endothelial dysfunction [103-105], myocardial infarction [106-110], NAFLD [111,112] and the induction of T3D (magnesium/Sirt 1 activator) [113].
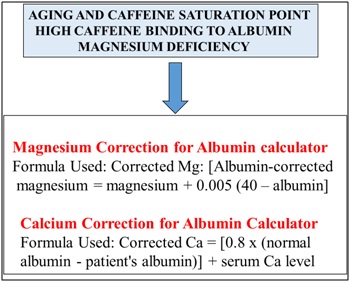
Figure 3: In the global chronic diseases the impact of defective caffeine metabolism effects cellular magnesium/calcum levels with magnesium deficiency (Sirt 1 activator) relevant to the induction of T3D and various chronic diseases.
The expression of four G-protein-coupled receptors referred to as adenosine receptors (AR) A1, A2A, A2B, A3 can be induced to regulate various mechanisms involved in the onset and progression of T1DM and T2DM [114,115]. Adenosine (signalling metabolite) and AR receptors are involved in cell growth and proliferation, apoptosis, immune response, and angiogenesis in various other diseases such as cardiovascular disease, asthma, Parkinson’s disease and Alzheimer’s disease. Under diet, stress or cell damage AR receptor genes may be induced [116] with adenosine released from tissues such as the liver, pancreas, muscle and adipose tissue. Connections between the stress sensitive Sirt 1 [6,7] and AR receptors [117] to hepatic glucose, fatty acids and cholesterol metabolism are relevant to hyperlipidemia and cardiovascular disease. High dietary fat that down regulates Sirt 1 and effects adenosine cell levels with relevance to glucose and lipid metabolism related to the induction of NAFLD [118]. Ethanol (Sirt 1 inhibitor) is involved with adenosine generation and with A1 and A2B receptors accelerate NAFLD [119]. Sirt 1 regulation of cell cholesterol may be related specifically to membrane cholesterol interactions that involve the A2A receptor [120,121] with critical relevance to reverse cholesterol transport [8,9].
The neuromodulatory role of adenosine is important to the control of brain disorders and modulation of AR receptors has become critical to the long-term burden of brain disorders in different neurodegenerative conditions [122-126] such as ischemia, epilepsy, Parkinson's or Alzheimer's disease. Adenosine acts as a neuromodulator [127] by activation of inhibitory A1 Receptors (A1R) and facilitatory A2A Receptors (A2AR) and controls the excitatory glutamatergic synapses that are engaged to promote synaptic plasticity. Sirt 1 dysfunction and connections to defective amyloid beta metabolism and synaptic plasticity [7,41,42] is now relevant to diabetes and various neurological diseases. The A1 receptor has long been known to mediate neuroprotection with amyloid beta regulation and by blockade of Ca2+ influx which results in inhibition of glutamate release and reduction of its excitatory effects at a postsynaptic level [128,129]. Astrocytic adenosine A2A receptors control the amyloid-β peptide metabolism and toxicity to neurons [130].
Caffeine neuroprotection involves blockade of adenosine A2A receptor to prevent amyloid beta induced neurotoxicity [131]. Caffeine via adenosine and AR receptors affects brain functions such as sleep, cognition, learning, and memory, and modify brain dysfunctions and diseases such as Alzheimer's disease, Parkinson’s disease, Huntington’s disease, epilepsy, pain/migraine, depression and schizophrenia [101,102,132-136]. Sirt 1 is critical to the regulation of the SCN circadian rhythm and NAFLD [7] that determine hepatic caffeine metabolism with excessive transport of caffeine to the brain. Caffeine has been shown to have major effects on the circadian rhythm in vitro and in vivo with sleep disrupting effects associated with delayed human circadian timekeeping [137-139]. Pharmacological modulation of the A2A and A3 receptors via selective ligands now represents a novel strategy for acute or chronic neurodegenerative events. Sirt 1 and AR receptors are connected to NO modulation [6,140] indicating their important role in cellular processes. Sirt 1 dyregulation with relevance to NO metabolism [6] may overide effects of NO modulation by adenosine with relevance to adenosine A2A and A1 receptors in neurons and adenosine and NO mediated vasodilation effects in arterial and endothelial cells [141-144].
BACTERIAL LPS AND CAFFEINE METABOLISM WITH RELEVANCE TO NAFLD AND CHRONIC DISEASES IN THE DEVELOPING WORLD
In the developing world the sensitivity to accelerated aging and induction of diabetes is now related to repression of the anti-aging gene Sirt 1 [145]. In the developing world alarm has been raised with the increase in plasma bacterial Lipopolysaccharides (LPS) levels with LPS [8,9,19,20,31,49] of relevance to Sirt 1 repression and associated with the development of insulin resistance and NAFLD. LPS may induce zinc [9] and magnesium [113] deficiency with LPS effects on zinc, magnesium and albumin levels relevant to increased free caffeine levels and linked to increased mitochondrial apoptosis in cells. The role of zinc/magnesium (Sirt 1 activators) deficiency in the developing world [146] may indicate Sirt 1 to be non-functional in the central nervous system and relevant to the induction of T3D. LPS dysregulation of nuclear Sirt 1 involve circadian rhythm abnormalities [7,147] with LPS associated with defective rhythmic release of various proteins such as albumin from cells [148,149]. The role of nutritional interventions to maintain zinc and albumin levels in developing world may be important with relevance to caffeine consumption. Caffeine, zinc and albumin levels determine the induction of various chronic diseases with albumin sensitivity to myocardial infarction [108,109].
In the developed world the aging process may allow caffeine to be metabolized faster when compared with individuals from the developing world with the slow caffeine clearance related to the hydrophobic caffeine deposition in cell membranes with relevance to cell membrane fluidity alterations [150]. The membrane interactions of caffeine may be completely abnormal with relevance to LPS induced membrane alterations. LPS and caffeine may interfere with hepatic cholesterol levels (Figure 4) with relevance to bile acid metabolism [151-155] and induction of NAFLD [156-158] in the developing world. Repression of Sirt 1 in cells is responsible for major intracellular defects and caffeine induced mitochondrial apoptosis relevant to adipogenesis defects [31,32] related to NAFLD with NAFLD to reach 30% of the developing world [159-162]. Defective hepatic caffeine clearance rates in the developing world may be connected to pancreatic [163-170] and thyroid disease [171-173] but xenobiotics may also be the inducing factor in these chronic diseases. LPS induced Sirt 1 repression may involve both caffeine and xenobiotic toxic effects with the induction of NAFLD, NAFLD linked to gall bladder disease [174-177] and cardiovascular disease [107-109].
In the developing world xenobiotic levels have increased in food and water [51] with relevance to caffeine and the induction of T3D [17,18]. Caffeine consumption should be assessed with regard to common metabolic pathways that involves Sirt 1/CYP450 enzymes (Figure 4) involved in both caffeine and xenobiotic clearance [17,51]. Caffeine as a Sirt 1 modulator [17,178] can interfere with the clearance of bile/cholesterol/xenobiotic with relevance to primary clearance of caffeine versus secondary clearance of xenobiotic and thirdly bile acid metabolism in these populations (Figure 4).
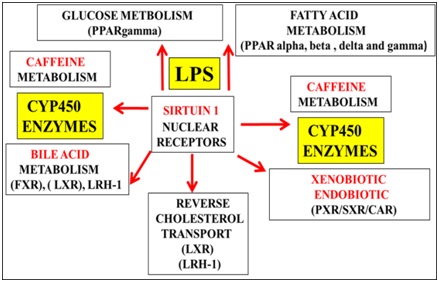
Figure 4: In the developing world rising xenobiotic levels in food and water may interfere with hepatic caffeine metabolism with relevance to the induction of T3D diabetes and various chronic diseases. Defective Sirt 1 regulation by LPS induces defective hepatic caffeine and xenobiotic metabolism with relevance to induction of NAFLD and T3D diabetes linked to various chronic diseases.
Nutritional diets that contain Sirt 1 activators (Figure 5) may reverse NAFLD with relevance to the metabolism of caffeine, glucose, fatty acids, cholesterol, amyloid beta, bile acids and xenobiotics. Consumption of Sirt 1 inhibitors (Figure 5) should be avoided that induce NAFLD that inactivate consumption of essential nutrients with relevance to the prevention of NAFLD. Delayed caffeine metabolism may induce mitophagy and impair various therapeutic drugs/insulin therapy [179] (Figure 5) that are essential for treatment of various chronic diseases. Diets that contain patulin [69] in the developing world should be avoided with relevance to Sirt 1 inactivation to NAFLD and induction of various chronic diseases. Vegetarian diets should be carefully checked with relevance to caffeine containing plants (coffee plant), tea leaves and herbs [180].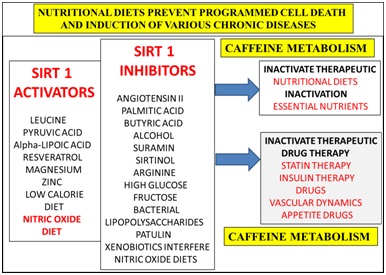
Figure 5: Nutritional interventions that involve the activation of the anti-aging genes have become important to the prevention of mitochondrial apoptosis that is connected to programmed cell death in various chronic diseases. Diets that do not contain Sirt 1 inhibitors promote Sirt 1 activators to accelerate hepatic caffeine metabolism to prevent T3D connected to various organ diseases in the developing world.
Caffeine over consumption (Figure 5) in the developing world are critical to the induction of caffeine induced magnesium deficiency in myocardial infarction [107,113] and pancreatitis [181-184]. Caffeine can interfere with thyroid replacement therapy with effects on cardiovascular disease, hypothyroidism and gall bladder disease [185-186]. Other caffeine containing foods such as coffee, coca cola, energy drinks, caffeine tablets, dark chocolate, chocolate chips and energy mints should be assessed for caffeine content (mg) with NAFLD, gall bladder disease and various chronic diseases. Over nutrition with glucose and fatty acids such as palmitic acid [150] involved in Sirt 1 inhibition (Figure 5) should be avoided that are involved in the delay of hepatic caffeine metabolism. Controlled exercise regimes should be supervised to prevent magnesium deficiency [113] and the over ingestion of caffeine containing drinks that promote endurance and performance [187-190]. Nitric oxide foods [6] (Figure 5) should be restricted to prevent caffeine related endothelial death with caffeine interference of the Sirt 1 eNOS pathway [75,76] related to myocardial infarction [70-74].
CONCLUSION
Unhealthy western diets and lifestyles lead to circadian rhythm disorders with defective nutrient and caffeine metabolism associated with NAFLD, cardiovascular disease and T3D diabetes in the developed world (Figure 6). Nutritional interventions are required to prevent mitophagy that is linked to the induction of chronic disease. Caffeine doses in various global populations should be reassessed with relevance to NAFLD and induction of T3D diabetes and neurodegeneration (Figure 6). In the developing world LPS may induce NAFLD and associated with defective anti-aging genes and accelerated mitochondrial apoptosis. Delayed caffeine and xenobiotic metabolism with zinc/magnesium deficiency may be associated to other organ diseases such as gall bladder disease, cardiovascular disease and pancreatic disease in the developing world (Figure 6).
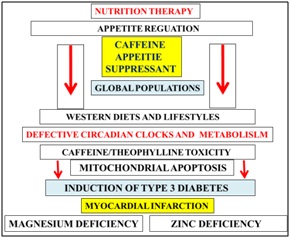
Figure 6: Nutrition therapy and circadian clock function has now become essential to prevent mitochondrial apoptosis that is relevant to the induction of Type 3 diabetes and cardiovascular diseases. Caffeine consumption should be reduced markedly in the developed and developing world with relevance to NAFLD and various global chronic diseases. Plasma magnesium/zinc/caffeine are important appetite control with overeating critical to the induction of diabetes, myocardial infarction and various organ diseases.
ACKNOWLEDGEMENT
This work was supported by grants from Edith Cowan University, the McCusker Alzheimer's Research Foundation and the National Health and Medical Research Council.
REFERENCES
- Hulbert AJ, Turner N, Storlien LH, Else PL (2005) Dietary fats and membrane function: implications for metabolism and disease. Biol Rev Camb Philos Soc 80: 155-169.
- WHO (2002) Diet, nutrition and the prevention of chronic diseases: Report of a joint WHO/FAO expert consultation, Geneva, 28 January - 1 February 2002. WHO Library Cataloguing-in-Publication Data Joint WHO/FAO Expert Consultation on Diet, Nutrition and the Prevention of Chronic Diseases, Geneva, Switzerland.
- Coulston AM, Rock CL, Monsen ER (2001) Nutrition in the Prevention and Treatment of Disease, Academic Press, London, UK.
- Martins IJ (2016) Appetite Control with Relevance to Mitochondrial Biogenesis and Activation of Post-Prandial Lipid Metabolism in Obesity Linked Diabetes. Ann Obes Disord 1: 1-3.
- Martins IJ (2013) Appetite dysregulation and obesity in Western Countries. LAP Lambert Academic Publishing, Saarbrücken, Germany.
- Martins IJ (2015) Nutritional diets accelerate amyloid beta metabolism and prevent the induction of chronic diseases and Alzheimer’s disease. Photon ebooks.
- Martins IJ (2015) Nutritional and Genotoxic Stress Contributes to Diabetes and Neurodegenerative Diseases such as Parkinson's and Alzheimer’s Diseases. In: Atta-ur-Rahman (ed.). Frontiers in Clinical Drug Research -CNS and Neurological Disorders. Bentham e Books 3: 158-192.
- Martins IJ (2015) Diabetes and Cholesterol Dyshomeostasis Involve Abnormal a-Synuclein and Amyloid Beta Transport in Neurodegenerative Diseases. Austin Alzheimers J Parkinsons Dis 2: 1020.
- Martins IJ (2015) Unhealthy Diets Determine Benign or Toxic Amyloid Beta States and Promote Brain Amyloid Beta Aggregation. Austin J Clin Neurol 2: 1060-1066.
- Greenberg JA, Geliebter A (2012) Coffee, hunger, and peptide YY. J Am Coll Nutr 31: 160-166.
- Schubert MM, Grant G, Horner K, King N, Leveritt M, et al. (2014) Coffee for morning hunger pangs. An examination of coffee and caffeine on appetite, gastric emptying, and energy intake. Appetite 83: 317-326.
- Jessen A, Buemann B, Toubro S, Skovgaard IM, Astrup A (2005) The appetite-suppressant effect of nicotine is enhanced by caffeine. Diabetes Obes Metab 7: 327-333.
- de la Monte SM, Wands JR (2008) Alzheimer's disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol 2: 1101-1113.
- Nakagawa H, Okumura N (2010) Coordinated regulation of circadian rhythms and homeostasis by the suprachiasmatic nucleus. Proc Ja Acad Ser B Phys Biol Sci 86: 391-409.
- Kalsbeek A, la Fleur S, Fliers E (2014) Circadian control of glucose metabolism. Mol Metab 3: 372-383.
- Bailey SM, Udoh US, Young ME (2014) Circadian regulation of metabolism. J Endocrinol 222: 75-96.
- Martins IJ (2016) Caffeine consumption with Relevance to Type 3 diabetes and accelerated brain aging. Research and Reveiws: Neuroscience.
- Martins IJ (2017) Caffeine Consumption and Induction of Obesity in the Developed World. Ann Obes Disord 2: 1018.
- Martins IJ (2016) Food intake and caffeine determine amyloid beta metabolism with relevance to mitophagy in brain aging and chronic disease. European Journal of Food Science and Technology.
- Martins IJ (2015) Diabetes and Organ Dysfunction in the Developing and Developed. Global Journal of Medical Research: F Diseases 15: 1-9.
- Manfredini R, Boari B, Bressan S, Gallerani M, Salmi R, Portaluppi F et al. (2004) Influence of circadian rhythm on mortality after myocardial infarction: data from a prospective cohort of emergency calls. Am J Emerg Med 22: 555-559.
- Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mähönen M, et al. (2011) World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epide miol 40: 139-146.
- Virag J, Lust R (2014) Circadian influences on myocardial infarction. Front Physiol 5: 422.
- Fabbrini E, Sullivan S, Klein S (2010) Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51: 679-689.
- Patell R, Dosi R, Joshi H, Sheth S, Shah P, et al. (2014) Non-Alcoholic Fatty Liver Disease (NAFLD) in Obesity. J Clin Diagn Res 8: 62-66.
- Wong RJ, Ahmed A (2014) Obesity and non-alcoholic fatty liver disease: Disparate associations among Asian populations. World J Hepatol 6: 263-273.
- Targher G, Byrne CD (2016) Obesity: Metabolically healthy obesity and NAFLD. Nat Rev Gastroenterol Hepatol 13: 442-444.
- Ezzat W, Ragab S, Ismail N, Elhosary A, Nour Eldin Abd E, et al. (2012) Frequency of non-alcoholic fatty liver disease in overweight/obese children and adults: Clinical, sonographic picture and biochemical assessment. J Genet Eng Biotechnol 10: 221-227.
- Suano de Souza FI, Silverio Amancio OM, Saccardo Sarni RO, Sacchi Pitta T, Fernandes AP, et al. (2008) Non-alcoholic fatty liver disease in overweight children and its relationship with retinol serum levels. Int J Vitam Nutr Res 78: 27-32.
- Kim N, Kim J, Kim Y, Yoo H, Kim H, Seo JA, et al. (2014) Clinical and metabolic factors associated with development and regression of non alcoholic fatty liver disease in nonobese subjects. Liver Int. 34: 604-611.
- Martins IJ (2015) Unhealthy Nutrigenomic Diets Accelerate NAFLD and Adiposity in Global communities. J Mol Genet Med 9: 162.
- Martins IJ (2014) Induction of NAFLD with Increased Risk of Obesity and Chronic Diseases in Developed Countries. Open J of Endocr Metab Dis 4: 90-110.
- Su SH, Shyu HW, Yeh YT, Chen KM, Yeh H, et al. (2013) Caffeine inhibits adipogenic differentiation of primary adipose-derived stem cells and bone marrow stromal cells. Toxicol In Vitro. 27: 1830-7.
- Kim AR, Yoon BK, Park H, Seok JW, Choi H, et al. (2016) Caffeine inhibits adipogenesis through modulation of mitotic clonal expansion and the AKT/GSK3 pathway in 3T3-L1 adipocytes. BMB Rep 49: 111-115.
- Nordestgaard AT, Thomsen M, Nordestgaard BG (2015) Coffee intake and risk of obesity, metabolic syndrome and type 2 diabetes: a Mendelian randomization study. Int J Epidemiol 44: 551-565.
- Nehlig A, Daval JL, Debry G (1992) Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev 17: 139-170.
- 6 Caffeine Effects on the Central Nervous System and Behavioral Effects Associated with Caffeine Consumption (2017) Caffeine in Food and Dietary Supplements: Examining Safety: Workshop Summary, Planning Committee for a Workshop on Potential Health Hazards Associated with Consumption of Caffeine in Food and Dietary Supplements; Food and Nutrition Board; Board on Health Sciences Policy; Institute of Medicine. National Academies Press, Washington USA.
- Martins IJ (2016) Type 3 diabetes with links to NAFLD and Other Chronic Diseases in the Western World. Int J Diabetes 1: 1-5.
- Martins IJ (2016) Diet and Nutrition reverse Type 3 Diabetes and Accelerated Aging linked to Global chronic diseases. J Diab Res Ther 2: 1-6.
- Martins IJ (2016) Heat shock gene Sirtuin 1 regulates post-prandial lipid metabolism with relevance to nutrition and appetite regulation in diabetes. Int J Diab Clin Diagn 3: 1-3.
- Michán S, Li Y, Chou MM, Parrella E, Ge H, et al. (2010) SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci 30: 9695-9707.
- Ng F, Wijaya L, Tang BL (2015) SIRT1 in the brain-connections with aging-associated disorders and lifespan. Front Cell Neurosci 9: 64.
- Stranahan A, Norman E, Lee K, Cutler R, Telljohann R, et al. (2008) Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 18: 1085-1088.
- Gispen WH, Biessels GJ (2000) Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci 23: 542-549.
- Biessels G, Kamal A, Ramakers G, Urban I, Spruijt B, et al. (1996) Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes 45: 1259-1266.
- Colicos MA, Syed NI (2006) Neuronal networks and synaptic plasticity: understanding complex system dynamics by interfacing neurons with silicon technologies. J Exp Biol 209: 2312-2319.
- Martins IJ (2016) Anti-Aging Genes Improve Appetite Regulation and Reverse Cell Senescence and Apoptosis in Global Populations. Advances in Aging Research 5: 9-26.
- Martins IJ (2016) Drug Therapy for Obesity with Anti-Aging Genes Modification. Ann Obes Disord 1: 1001.
- Martins IJ (2015) Nutrition increases Survival and Reverses NAFLD and Alzheimer’s disease. LAP Lambert Academic Publishing, Saarbrücken, Germany.
- Yang A, Palmer AA, de Wit H (2010) Genetics of caffeine consumption and responses to caffeine. Psychopharmacology (Berl) 211: 245-257.
- Martins IJ (2016) Increased Risk for Obesity and Diabetes with Neurodegeneration in Developing Countries. J Molecular Genetic Medicine 1: 001.
- Lao-Pererín C, Ballesteros J, Fernández M, Zamora-Moratalla A, Saavedra A,Gómez Lázaro M, et al. (2016) Caffeine-mediated BDNF release regulates long-term synaptic plasticity through activation of IRS2 signaling. Addict Biol.
- Sallaberry C, Nunes F, Costa M, Fioreze G, Ardais A, et al. (2013) Chronic caffeine prevents changes in inhibitory avoidance memory and hippocampal BDNF immunocontent in middle-aged rats. Neuropharmacology 64: 153-159.
- Sun F, Han D, Cao B, Wang B, Dong N, et al. (2016) Caffeine-induced nuclear translocation of FoxO1 triggers Bim-mediated apoptosis in human glioblastoma cells. Tumour Biol 37: 3417-3423.
- Maiese K (2015) FoxO proteins in the nervous system. Anal Cell Pathol (Amst).
- Bhupathiraju SN, Pan A, Manson JE, Willett WC, van Dam RM, et al. (2014) Changes in coffee intake and subsequent risk of type 2 diabetes: three large cohorts of US men and women. Diabetologia 57: 1346-1354.
- Bhaktha G, Nayak BS, Mayya S, Shantaram M (2015) Relationship of Caffeine with Adiponectin and Blood Sugar Levels in Subjects with and without Diabetes. J Clin Diagn Res 9: 1-3.
- Shen H, Rodriguez A, Shiani A, Lipka S, Shahzad G, et al. (2016) Association between caffeine consumption and nonalcoholic fatty liver disease: a systemic review and meta-analysis. Therap Adv Gastroenterol 9: 113-120.
- Kim B, Nam Y, Kim J, Choi H, Won C (2012) Coffee Consumption and Stroke Risk: A Meta-analysis of Epidemiologic Studies. Korean J Fam Med 33: 356-365.
- Martins IJ (2014) The Global Obesity Epidemic is Related to Stroke, Dementia and Alzheimer’s disease. JSM Alzheimer’s Dis Related Dementia 1: 1-9.
- Neurologic Effects of Caffeine/Stimulants/Botanical Dermatology.
- Prediger RD (2010) Effects of caffeine in Parkinson’s disease: from neuroprotection to the management of motor and non-motor symptoms. J Alzheimers Dis 1: 205-220.
- Postuma RB, Lang AE, Munhoz RP, Charland K, Pelletier A, et al. (2012) Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology 79: 651-658.
- Stamatovic SM, Keep RF, Andjelkovic AV (2008) Brain endothelial cell-cell junctions: how to "open" the blood brain barrier. Curr Neuropharmacol 6: 179-192.
- Chen X, Gawryluk JW, Wagener JF, Ghribi O, Geiger JD (2008) Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer’s disease. J Neuroinflammation 5: 12.
- Luissint A, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO (2012) Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 9: 23.
- Chen X, Ghribi O, Geiger JD (2010) Caffeine protects against disruptions of the blood-brain barrier in animal models of Alzheimer’s and Parkinson’s diseases. J Alzheimers Dis 1: 127-141.
- McCall AL, Millington WR, Wurtman RJ (1982) Blood-brain barrier transport of caffeine: dose-related restriction of adenine transport. Life Sci 31: 2709-2715.
- Martins IJ (2015) Overnutrition Determines LPS Regulation of Mycotoxin Induced Neurotoxicity in Neurodegenerative Diseases. Int J Mol Sci 16: 29554-29573.
- Papamichael CM, Aznaouridis KA, Karatzis EN, Karatzi KN, Stamatelopoulos KS, et al. (2005) Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin Sci (Lond) 109: 55-60.
- Lopez-Garcia E, van Dam RM, Qi L, Hu FB (2006) Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr 84: 888-893.
- Ding M, Bhupathiraju S, Satija A, van Dam R, Hu F (2014) Long-Term Coffee Consumption and Risk of Cardiovascular Disease: A Systematic Review and a Dose-Response Meta-Analysis of Prospective Cohort Studies. Circulation 129: 643-659.
- JAMA (2006) Coffee Consumption Linked to Increased Risk of Heart Attack for Persons with Certain Gene Variation. JAMA 295: 1135-1141.
- Li H, Jin SY, Son HJ, Seo JH, Jeong GB (2013) Caffeine-induced endothelial cell death and the inhibition of angiogenesis. Anat Cell Biol 46: 57-67.
- Xia N, Strand S, Schlufter F, Siuda D, Reifenberg G, et al. (2013) Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide 32: 29-35.
- Ota H, Eto M, Ogawa S, Iijima K, Akishita M, et al. (2010) SIRT1/eNOS axis as a potential target against vascular senescence, dysfunction and atherosclerosis. J Atheroscler Thromb 17: 431-435.
- Nisoli E, Carruba MO (2006) Nitric oxide and mitochondrial biogenesis. J Cell Sci 119: 2855-2862.
- Erusalimsky JD, Moncada S (2007) Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol 27: 2524-2531.
- Bossy-Wetzel E, Lipton S (2003) Nitric oxide signaling regulates mitochondrial number and function. Death Differ 10: 757-760.
- Ghafourifar P, Cadenas E (2005) Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 26: 190-195.
- Andres AM, Stotland A, Queliconi BB, Gottlieb RA (2015) A time to reap, a time to sow: mitophagy and biogenesis in cardiac pathophysiology. J Mol Cell Cardiol 78: 62-72.
- Shires SE, Gustafsson ÅB (2015) Mitophagy and heart failure. J Mol Med (Berl) 93: 253-262.
- Cheng J, Cho M, Cen JM, Cai MY, Xu S, et al. (2015) A TagSNP in SIRT1 gene confers susceptibility to myocardial infarction in a Chinese Han population. PLoS One 10: 0115339.
- Vásquez-Trincado C, García-Carvajal I, Pennanen C, et al. (2016) Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol 594: 509-525.
- Doulamis I, Tzani A, Konstantopoulos P, Samanidis G, Georgiopoulos G, et al (2017) A sirtuin 1/MMP2 prognostic index for myocardial infarction in patients with advanced coronary artery disease. Int J Cardiol 230: 447-453.
- Xiao J, Sheng X, Zhang X, Guo M, Ji X (2016) Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Des Devel Ther 10: 1267-1277.
- Blanchard J (1982) Protein binding of caffeine in young and elderly males. J Pharm Sci 71: 1415-1418.
- Theophylline metabolism in human liver. Pathway, PharmGKB.
- Knutsen R, Bøhmer T, Falch J (1994) Intravenous theophylline-induced excretion of calcium, magnesium and sodium in patients with recurrent asthmatic attacks. Scand J Clin Lab Invest 54: 119-125.
- Nishijo J, Morita N (1985) Binding parameters of theophylline and aminophylline to bovine serum albumin. Chem Pharm Bull (Tokyo) 33: 2522-2524.
- Behbehani G, Saboury A, Sarvestani S, Mohebbian M, Payehghadr M, et al. (2010) A thermodynamic study on the binding of theophylline with human serum albumin. Journal of Thermal Analysis and Calorimetry 102: 793-798.
- Maci??ek-Jurczyk M, Su?kowska A, Bojko B, Równicka-Zubik J, Szkudlarek-Ha?nik A, et al. (2012) The influence of fatty acids on theophylline binding to human serum albumin. Comparative fluorescence study. Spectrochim Acta A Mol Biomol Spectrosc 89: 270-275.
- Theophylline and caffeine - Therapeutic uses and adverse effects. Pharmacorama.
- Tjia JF, Colbert J, Back DJ (1996) Theophylline metabolism in human liver microsomes: inhibition studies. J Pharmacol Exp Ther 276: 912-917.
- Kroll MH, Elin RJ (1985) Relationships between magnesium and protein concentrations in serum. Clin Chem 31: 244-246.
- Parent X, Spielmann C, Hanser AM (2009) [“Corrected” calcium: calcium status underestimation in non-hypoalbuminemic patients and in hypercalcemic patients]. Ann Biol Clin (Paris) 67: 411-418.
- Kynast-Gales SA, Massey LK (1994) Effect of caffeine on circadian excretion of urinary calcium and magnesium. J Am Coll Nutr 13: 467-472.
- Bergman EA, Massey LK, Wise KJ, Sherrard DJ (1990) Effects of dietary caffeine on renal handling of minerals in adult women. Life Sci 47: 557-564.
- Cunha AR, Umbelino B, Correia ML, Neves MF (2012) Magnesium and vascular changes in hypertension. Int J Hypertens 2012: 754250.
- Martins IJ, Creegan R (2014) Links between Insulin Resistance, Lipoprotein Metabolism and Amyloidosis in Alzheimer’s Disease. Health 6: 1549-1579.
- Krizaj D, Bao JX, Schmitz Y, Witkovsky P, Copenhagen DR (1999) Caffeine-Sensitive Calcium Stores Regulate Synaptic Transmission from Retinal Rod Photoreceptors. J Neurosci 19: 7249-7261.
- Costenla AR, Cunha RA, de Mendonça A (2010) Caffeine, adenosine receptors, and synaptic plasticity. J Alzheimers Dis 20: 25-34.
- Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, et al. (2000) Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 102: 2353-2358.
- Maier JA, Malpuech-Brugère C, Zimowska W, Rayssiguier Y, Mazur A (2004) Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta 1689: 13-21.
- Wolf F, Trapani V, Simonacci M, Ferré S, Maier JA (2008) Magnesium deficiency and endothelial dysfunction: is oxidative stress involved? Magnes Res 21: 58-64.
- Li J, Zhang Q, Zhang M, Egger M (2007) IIntravenous magnesium for acute myocardial infarction. Cochrane Database Syst Rev.
- Antman EM (1995) Magnesium in acute MI. Timing is critical. Circulation 92: 2367-2372.
- Djoussé L, Rothman KJ, Cupples LA, Levy D, Ellison RC (2002) Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation 106: 2919-2924.
- Berton G, Cordiano R, Mazzuco S, Katz E, De Toni R, et al. (2008) Albumin excretion in acute myocardial infarction: a guide for long-term prognosis. Am Heart J 156: 760-768.
- Matsui T (2012) [Magnesium and liver]. Clin Calcium 22: 1181-1187.
- Karandish M, Tamimi M, Shayesteh AA, Haghighizadeh MH, Jalali MT (2013) The effect of magnesium supplementation and weight loss on liver enzymes in patients with nonalcoholic fatty liver disease. J Res Med Sci 18: 573-579.
- Turecky L, Kupcova V, Szantova M, Uhlikova E, Viktorinova A, et al. (2006) Serum magnesium levels in patients with alcoholic and non-alcoholic fatty liver. Bratisl Lek Listy 107: 58-61.
- Martins IJ (2016) Magnesium Therapy Prevents Senescence with the Reversal of Diabetes and Alzheimer’s Disease. Health 8: 694-710.
- Antonioli L, Blandizzi C, Csóka B, Pacher P, Haskó G (2015) Adenosine signalling in diabetes mellitus--pathophysiology and therapeutic considerations. Nat Rev Endocrinol 11: 228-41.
- Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, et al. (2011) Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes 60: 669-679.
- St Hilaire C, Carroll SH, Chen H, Ravid K (2009) Mechanisms of induction of adenosine receptor genes and its functional significance. J Cell Physiol 218: 35-44.
- Koupenova M, Ravid K (2013) Adenosine, adenosine receptors and their role in glucose homeostasis and lipid metabolism. J Cell Physiol.
- Dhalla AK, Wong MY, Voshol PJ, Belardinelli L, Reaven GM (2007) A1 adenosine receptor partial agonist lowers plasma FFA and improves insulin resistance induced by high-fat diet in rodents. Am J Physiol Endocrinol Metab 292: 1358-1363.
- Robson SC, Schuppan D (2010) Adenosine: tipping the balance towards hepatic steatosis and fibrosis. J Hepatol 52: 941-943.
- Lee JY, Lyman E (2012) Predictions for cholesterol interaction sites on the A2A adenosine receptor. J Am Chem Soc 134: 16512-16515.
- Bingham TC, Parathath S, Tian H, Reiss A, Chan E, Fisher EA, et al. (2012) Cholesterol 27-hydroxylase but not apolipoprotein apoE contributes to A2A adenosine receptor stimulated reverse cholesterol transport. Inflammation 35: 49-57.
- Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA (2011) A Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta 1808: 1380-1399.
- Abbracchio MP, Cattabeni F (1999) Brain adenosine receptors as targets for therapeutic intervention in neurodegenerative diseases. Ann N Y Acad Sci 890: 79-92.
- Morelli M, Carta AR, Jenner P (2009) Adenosine A2A receptors and Parkinson's disease. Handb Exp Pharmacol 193: 589-615.
- Blum D, Hourez R, Galas MC, Popoli P, Schiffmann SN (2003) Adenosine receptors and Huntington's disease: implications for pathogenesis and therapeutics. Lancet Neurol 2: 366-374.
- Lee CF, Chern Y (2014) Adenosine receptors and Huntington's disease. Int Rev Neurobiol 119: 195-232.
- Cunha RA (2001) Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38: 107-125.
- Matos M, Augusto E, Machado NJ, dos Santos-Rodrigues A, Cunha RA, Agostinho P. Astrocytic adenosine A2A receptors control the amyloid-β peptide-induced decrease of glutamate uptake. J Alzheimers Dis 31: 555-567.
- Giunta S, Andriolo V, Castorina A (2014) Dual blockade of the A1 and A2A adenosine receptor prevents amyloid beta toxicity in neuroblastoma cells exposed to aluminum chloride. Int J Biochem Cell Biol 54: 122-136.
- Angulo E, Casadó V, Mallol J, Canela EI, Viñals F, et al. (2003) A1 adenosine receptors accumulate in neurodegenerative structures in Alzheimer disease and mediate both amyloid precursor protein processing and tau phosphorylation and translocation. Brain Pathol 13: 440-51.
- Dall'Igna OP, Porciúncula LO, Souza DO, Cunha RA, Lara DR (2003) Neuroprotection by caffeine and adenosine A2A receptor blockade of beta-amyloid neurotoxicity. Br J Pharmacol 138: 1207-1209.
- Ribeiro JA, Sebastião AM (2010) Caffeine and adenosine. J Alzheimers Dis 1: 3-15.
- Mukhopadhyay S, Poddar MK (1998) Is GABA involved in the development of caffeine tolerance? Neurochem Res 23: 63-68.
- Eskelinen MH, Kivipelto M (2010) Caffeine as a protective factor in dementia and Alzheimer's disease. J Alzheimers Dis 1: 167-174.
- Ross GW, Petrovitch H (2001) Current evidence for neuroprotective effects of nicotine and caffeine against Parkinson’s disease. Drugs Aging 18: 797-806.
- Seidl SE, Potashkin JA (2011) The Promise of Neuroprotective Agents in Parkinson’s Disease. Front Neurol 2: 68.
- Burke TM, Markwald RR, McHill AW, Chinoy ED, Snider JA, et al. (2015) Effects of caffeine on the human circadian clock in vivo and in vitro. Sci Transl Med 7.
- Antle MC, Steen NM, Mistlberger RE (2001) Adenosine and caffeine modulate circadian rhythms in the Syrian hamster. Neuroreport 12: 2901-2905.
- Rosati AM, Traversa U, Florio C, Vertua R (1993) Circadian rhythm of cortical and striatal adenosine receptors. Life Sci 52: 1677-1684.
- Li JM, Fenton RA, Cutler BS, Dobson JG Jr (1995) Adenosine enhances nitric oxide production by vascular endothelial cells. Am J Physiol 269: 519-523.
- Brodie C, Blumberg PM, Jacobson KA (1998) Activation of the A2A adenosine receptor inhibits nitric oxide production in glial cells. FEBS Lett 429: 139-142.
- Lawrence AJ, Krstew E, Jarrott B (1997) Complex interactions between nitric oxide and adenosine receptors in the rat isolated nodose ganglion. Eur J Pharmacol 328: 83-88.
- Olanrewaju HA, Mustafa SJ (2000) Adenosine A(2A) and A(2B) receptors mediated nitric oxide production in coronary artery endothelial cells. Gen Pharmacol 35: 171-177.
- Abebe W, Hussain T, Olanrewaju H, Mustafa SJ (1995) Role of nitric oxide in adenosine receptor-mediated relaxation of porcine coronary artery. Am J Physiol 269: 1672-1678.
- Martins IJ (2017) The Future of Genomic Medicine Involves the Maintenance of Sirtuin 1 in Global Populations. Int J Mol Biol 2: 1-4.
- Wessells KR, Brown KH (2012) Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One 7: 50568.
- Ma D, Li S, Molusky MM, Lin JD (2012) Circadian autophagy rhythm: a link between clock and metabolism? Trends Endocrinol Metab 23: 319-325.
- Mauvoisin D, Wang J, Jouffe C, Martin E, Atger F, et al. (2014) Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functionsin mouse liver. Proc Natl Acad Sci USA 111: 167-172.
- Ando D, Hirawa N, Yasuda G (2016) Relation between circadian blood pressure rhythm and serum albumin level in non-diabetic patients with proteinuria. Blood Press 25: 44-50.
- Martins IJ (2016) Bacterial Lipopolysaccharides Change Membrane Fluidity with Relevance to Phospholipid and Amyloid Beta Dynamics in Alzheimer’s Disease. J Microb Biochem Technol 8: 322-324.
- Ruhl CE, Everhart JE (2000) Association of coffee consumption with gallbladder disease. Am J Epidemiol 152: 1034-1038.
- Ishizuk H, Eguchi H, Oda T, Ogawa S, Nakagawa K, et al. (2003) Relation of coffee, green tea, and caffeine intake to gallstone disease in middle-aged Japanese men. Eur J Epidemiol 18: 401-405.
- Spady DK, Stange EF, Bilhartz LE, Dietschy JM (1986) Bile acids regulate hepatic low density lipoprotein receptor activity in the hamster by altering cholesterol flux across the liver. Proc Natl Acad Sci USA 83: 1916-1920.
- Lane JD, Pieper CF, Barefoot JC, Williams RB Jr, Siegler IC (1994) Caffeine and cholesterol: interactions with hostility. Psychosom Med 56: 260-266.
- Javitt NB (1994) Bile acid synthes is from cholesterol: regulatory and auxiliary pathways. FASEB J 8: 1308-1311.
- Fracanzani A, Valenti L, Russello M, Miele L, Bertelli C, et al. (2012) Gallstone disease is associated with more severe liver damage in patients with non-alcoholic fatty liver disease. PLoS One 7: 41183.
- Nervi F, Arrese M (2013) Cholecystectomy and NAFLD: does gallbladder removal have metabolic consequences? Am J Gastroenterol 108: 959-961.
- Loria P, Lonardo A, Lombardini S, Carulli L, Verrone A, et al. (2005) Gallstone disease in non-alcoholic fatty liver: prevalence and associated factors. J Gastroenterol Hepatol 20: 1176-1184.
- Bahrami H (2005) Nonalcoholic fatty liver disease in developing countries. World J Gastroenterol 11: 3808-3809.
- Maitra S (2010) “Nonalcoholic fatty liver disease” in a developing country: a different perspective. Hepatology 52: 797.
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, et al. (2016) Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64: 73-84.
- Misra A, Khurana L (2008) Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab 93: 9-30.
- Luu L, Dai F, Prentice K, Huang X, Hardy A, et al. (2013) The loss of Sirt1 in mouse pancreatic beta cells impairs insulin secretion by disrupting glucose sensing. Diabetologia 56: 2010-2020.
- Pinho AV, Bensellam M, Wauters E, Rees M, Giry-Laterriere M, et al. (2015) Pancreas-Specific Sirt1-Deficiency in Mice Compromises Beta-Cell Function without Development of Hyperglycemia. PLoS One 10: 0128012.
- Lee J, Song M, Song E, Kim E, Moon W, Han NK, et al. (2009) Over expression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes 58: 344-351.
- Mukherjee R, Criddle DN, Gukovskaya A, Pandol S, Petersen OH, et al. (2008) Mitochondrial injury in pancreatitis. Cell Calcium 44: 14-23.
- Maléth J, Rakonczay Z, Venglovecz V, Dolman NJ, Hegyi P (2013) Central role of mitochondrial injury in the pathogenesis of acute pancreatitis. Acta Physiol (Oxf) 207: 226-235.
- Maléth J, Hegyi P. (2016) Ca2+ toxicity and mitochondrial damage in acute pancreatitis: translational overview. Philos Trans R Soc Lond B Biol Sci 5: 371.
- Coffey RJ, Go VL, Zinsmeister AR, DiMagno EP (1986) The acute effects of coffee and caffeine on human interdigestive exocrine pancreatic secretion. Pancreas 1: 55-61.
- Dong J, Zou J, Yu XF (2011) Coffee drinking and pancreatic cancer risk: a meta-analysis of cohort studies. World J Gastroenterol 17: 1204-1210.
- Dong J, Zou J, Yu XF (2011) Coffee drinking and pancreatic cancer risk: a meta-analysis of cohort studies. World J Gastroenterol 17: 1204-1210.
- Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F, Galofré J (2014) The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab 99: 923-31.
- Bensenor IM, Olmos RD, Lotufo PA (2012) Hypothyroidism in the elderly: diagnosis and management. Clin Interv Aging 7: 97-111.
- Stinton LM, Shaffer EA (2012) Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 6: 172-187.
- Loria P, Lonardo A, Lombardini S, Carulli L, Verrone A, et al. (2005) Gallstone disease in non-alcoholic fatty liver: prevalence and associated factors. J Gastroenterol Hepatol 20: 1176-1184.
- Nervi F, Arrese M (2013) Cholecystectomy and NAFLD: does gallbladder removal have metabolic consequences? Am J Gastroenterol 108: 959-961.
- Fracanzani AL, Valenti L, Russello M, Miele L, Bertelli C, et al. (2012) Gallstone disease is associated with more severe liver damage in patients with non-alcoholic fatty liver disease. PLoS One 7: 41183.
- Seidel C, Schnekenburger M, Dicato M, Diederich M (2012) Histone deacetylase modulators provided by Mother Nature. Genes Nutr 7: 357-367.
- Martins IJ (2017) Drug-Drug Interactions with Relevance to Drug Induced Mitochondrial Toxicity and Accelerated Global Chronic Diseases. EC Pharmacol and Toxicol 3: 18-21.
- www.neurologycare.net/caffeine-and-caffeine-containing-herbs-and-supplements.html
- Papazachariou IM, Martinez-Isla A, Efthimiou E, Williamson RC, Girgis SI (2000) Magnesium deficiency in patients with chronic pancreatitis identified by an intravenous loading test. Clin Chim Acta 302: 145-154.
- Ahmed A, Azim A, Gurjar M, Baronia AK (2016) Hypocalcemia in acute pancreatitis revisited. Indian J Crit Care Med 20: 173-177.
- Ryzen E, Rude RK (1990) Low intracellular magnesium in patients with acute pancreatitis and hypocalcemia. West J Med 152: 145-148.
- Schick V, Scheiber JA, Mooren FC, Turi S, Ceyhan GO, et al. (2014) Effect of magnesium supplementation and depletion on the onset and course of acute experimental pancreatitis. Gut 63: 1469-1480.
- Biondi B (2012) Mechanisms in endocrinology: Heart failure and thyroid dysfunction. Eur J Endocrinol 167: 609-618.
- Völzke H, Robinson DM, John U (2005) Association between thyroid function and gallstone disease. World J Gastroenterol 11: 5530-5534.
- Persad LA (2011) Energy drinks and the neurophysiological impact of caffeine. Front Neurosci 5: 116.
- Graham TE, Hibbert E, Sathasivam P (1998) Metabolic and exercise endurance effects of coffee and caffeine ingestion. J Appl Physiol (1985) 85: 883-889.
- Graham TE (2001) Caffeine and exercise: metabolism, endurance and performance. Sports Med 31: 785-807.
- Graham TE, Rush JW, van Soeren MH (1994) Caffeine and exercise: metabolism and performance. Can J Appl Physiol 19: 111-138.
Citation: Martins IJ (2017) Nutrition Therapy Regulates Caffeine Metabolism with Relevance to NAFLD and Induction of Type 3 Diabetes. J Diabetes Metab Disord 4: 019.
Copyright: © 2017 Ian J Martins, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

